Abstract
INTRODUCTION
α1-AR stimulation produces smooth muscle contraction, vasoconstriction, and myocyte hypertrophy, suggesting a potential therapeutic role for α1-AR antagonists to reduce cardiac workload and myocardial hypertrophy. Preliminary reports suggest that vascular α1-ARs are desensitized in heart failure (HF) in a manner similar to myocardial β1-ARs. We examined α1-AR signal transduction by repeat phenylephrine (PE) infusions in HF patients receiving chronic carvedilol therapy.
METHODS
12 HF subjects not currently receiving β-blockers were up-titrated to maximum tolerable doses of carvedilol. Subjects underwent α1-AR stimulation testing at study baseline, 2-weeks after each dose titration, and 6-months after maintenance of maximum carvedilol dose. PE infusions began at 0.5 mcg/kg/min, with dose titrations every 10 minutes, to a maximum of 5 mcg/kg/min. PE dose response was evaluated by the PE rate required to elicit a 20 mmHg increase in systolic BP, designated PS20.
RESULTS
All doses of carvedilol significantly reduced pre-infusion measures of heart rate, systolic BP, diastolic BP, and mean arterial pressure. However, carvedilol also produced a paradoxical trend towards PS20 reduction (indicating increased PE response) that reached significance at the completion of carvedilol dose titration (PS20 ratio vs baseline = 0.78, p<0.001). All effects were maintained over a 6-month treatment period with no evidence of tolerance.
CONCLUSIONS
Increasing BP response to PE infusion suggests improvement in vascular α1-AR signal transduction with chronic carvedilol therapy. This effect is evident despite no detectable tolerance to pre-infusion BP reductions. The varying affinities of α1-AR subtypes for carvedilol and PE may have contributed to this finding.
Keywords: alpha-blocker, carvedilol, phenylephrine, chronic heart failure
INTRODUCTION
Adrenergic blockade is a cornerstone of therapy for patients with heart failure. Multiple landmark clinical trials demonstrate the benefit of β-adrenergic receptor (AR) antagonism on heart failure morbidity and mortality.1–3 The impact of α-AR antagonism on heart failure outcomes is less well understood. α1-AR stimulation produces smooth muscle contraction, vasoconstriction, and myocyte hypertrophy,4–6 suggesting a potential therapeutic role for α1-AR antagonists to reduce cardiac workload and myocardial hypertrophy. However, previous studies report rapid tolerance to the hemodynamic effects of α1-AR antagonists in heart failure patients.7
Carvedilol is a non-selective third-generation β-AR antagonist with additional antagonist activity at the α1-AR. Data from the Carvedilol or Metoprolol European Trial (COMET) support carvedilol as superior to metoprolol tartrate (a selective β–AR without α1-AR antagonist activity) in blood pressure reduction and heart failure mortality.3 These carvedilol benefits appeared within months of therapy initiation and persisted throughout a mean follow-up of 4.8 years with no evidence of tolerance. Such findings highlight the need for careful study of α1-AR signaling in heart failure patients during chronic carvedilol therapy.
We therefore examined vascular α1-AR signal transduction through measurement of blood pressure responses to repeat phenylephrine (PE) infusions in class C heart failure subjects receiving chronic carvedilol therapy.
METHODS
DESIGN
This two-year, prospective, controlled trial was conducted at the University of Utah Health Sciences Center under the approval of the Institutional Review Board for Human Subjects. Subjects were eligible for enrollment if they presented with symptomatic heart failure (New York Heart Association [NYHA] functional class II – III), left ventricular ejection fraction ≤ 0.40, patient age 18 – 85 years old, and were willing to provide written informed consent. Subjects were excluded from participation if any of the following criteria were met: pregnant or lactating women, secondary HF etiologies (active myocarditis, congenital heart disease, uncorrected, hemodynamically significant stenotic valvular disease, hypertrophic cardiomyopathy), asthma or other obstructive airway diseases requiring bronchodilators, heart rate < 60 beats/min, supine systolic blood pressure < 85 mm Hg, supine diastolic blood pressure > 90 mm Hg, uncontrolled hypertension (systolic BP >140 mmHg, diastolic BP > 90 mmHg), sick sinus syndrome, Mobitz type 2 second degree AV block or third degree AV block unless controlled with an artificial implantable pacemaker, NYHA functional class IV symptoms, myocardial infarction or coronary artery intervention within three months, acute coronary syndromes, uncorrected endocrine disorders including primary aldosteronism, pheochromcytoma, hyperthyroidism, hypothyroidism, type 1 diabetes mellitus, evidence of significant renal disease (serum creatinine > 2.5 mg/dl), or hepatic disease (transaminase level > three fold higher than laboratory normal), symptomatic peripheral vascular disease, presence of any progressive systemic disease that would be expected to impact the patient’s outcome over the time course of the study, or inability or unwillingness to cooperate with study or give written informed consent
PROCEDURE
Subjects meeting the entrance criteria of the study were admitted to the University of Utah Health Sciences Center General Clinical Research Center (GCRC) at study entry and 2 weeks after each carvedilol dose titration. During each GCRC admission, subjects received phenylephrine infusions (described below) to test α1-AR blockade and signal transduction. Following the end of the carvedilol dose-titration, subjects were seen for outpatient visits in the GCRC once a month for 5 months to ensure medication compliance. Subjects returned to the GCRC 6 months following the end of carvedilol titration for the final phenylephrine infusion. Informed consent was obtained from all subjects prior to study participation. Study recruitment and procedures were approved by the University of Utah Institutional Review Board.
Phenylephrine Infusion
Subjects were admitted the evening prior to phenylephrine infusion to ensure NPO status (including tobacco products) for 8 hours prior to infusion. The morning of Day 2, an 18-gauge peripheral line was placed for (IV) access and an oral carvedilol dose (when on the second and subsequent visit) was administered 2–4 hours before the start of the infusion. Subjects were placed supine in a quiet room for 30 minutes prior to establishment of baseline parameters. Supine blood pressure (BP) was measured using an automated blood pressure cuff on the right arm with two consecutive readings averaged together. Heart rate (HR) was calculated from lead II of 3-lead telemetry connected to the subject (five consecutive R-R intervals on 3-lead telemetry).
Once sufficiently rested, patients received phenylephrine hydrochloride (0.9% NaCl) 0.5 mcg kg−1 min−1 via peripheral IV line.8 The phenylephrine infusion was titrated every 10 minutes to infusion rates of 1.0, 2.0, 3.5, and 5.0 mcg kg−1 min−1 until the systolic blood pressure (SBP) increased by 30 mm Hg from the baseline SBP at visit 1, the DBP exceeded 110 mm Hg, or the subject could not tolerate the infusion.8–10 Upon completion of each phenylephrine infusion, subjects remained under GCRC care for one hour after termination of phenylephrine infusion or until BP returned to pre-treatment baseline levels to ensure patient safety. Subjects were discharged with their next titration carvedilol dose, to be taken twice daily for two weeks prior to the next phenylephrine infusion or until 6 months of dosing was completed. Carvedilol was initiated at 3.125 mg twice daily, with doses titrated to 25 mg twice daily or 50 mg twice daily (for patients >85 kg).
STATISTICAL ANALYSIS
Individual dose-response curves for phenylephrine infusions were constructed using a non-linear quadratic fit (Microsoft Excel 2003 sp3). Each quadratic fit was used to calculate the dose of phenylephrine required to increase systolic blood pressure by 20 mm Hg (PS20). Dose ratios were calculated at each dose and normalized to pre-carvedilol values. All statistical analyses were preformed using Stata 9.3 (College Station, TX). Blood pressure, heart rate, and PS20 ratios were compared to baseline by way of students t test, with two-sided significance values set at p<0.05. No multiplicity adjustments were employed as each point in time (or carvedilol dose) constituted an individual test hypothesis.11, 12
RESULTS
15 subjects were enrolled in the study, 12 of which completed all visits. Two patients were dropped from the study due to inability to attend scheduled visits, and one patient was incarcerated in federal prison following up-titration of carvedilol. All 12 subjects who completed the study were able to tolerate at least 12.5 mg twice daily (average dose = 32 ± 15 mg). On average, our patients represent a middle-aged (50 – 60 year old) Caucasian population with Class C HF symptoms (NYHA FC II – III) and LV dysfunction (ejection fraction = 23 ± 6%).
PRE-INFUSION HEMODYNAMICS
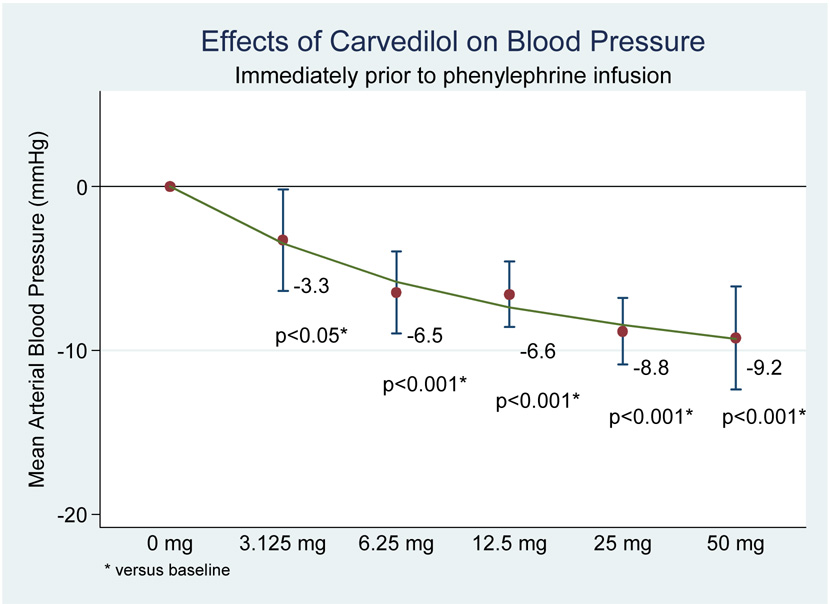
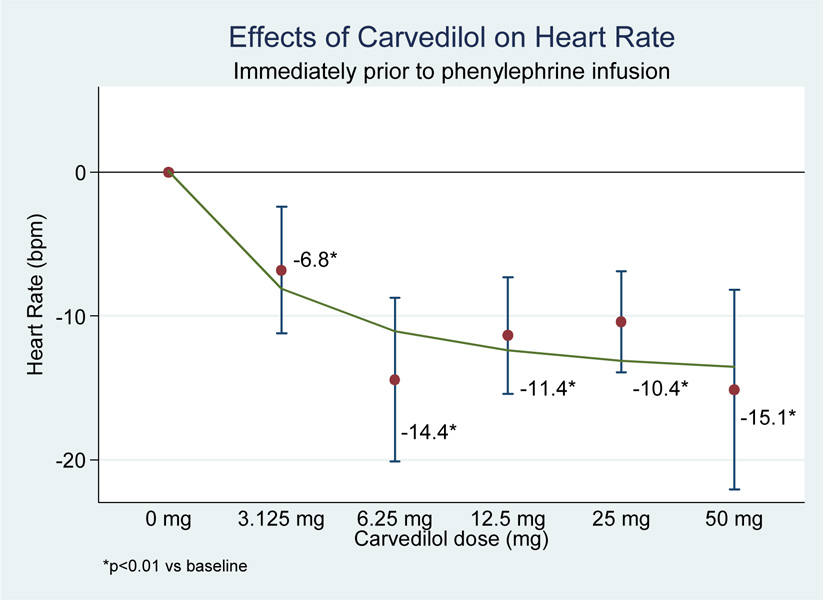
All doses of carvedilol produced statistically significant reductions in pre-infusion values for heat rate, systolic BP, diastolic BP, and mean arterial pressure (MAP) compared to pre-infusion values (see figure 1 and figure 2).
Figure 1.
Figure 2.
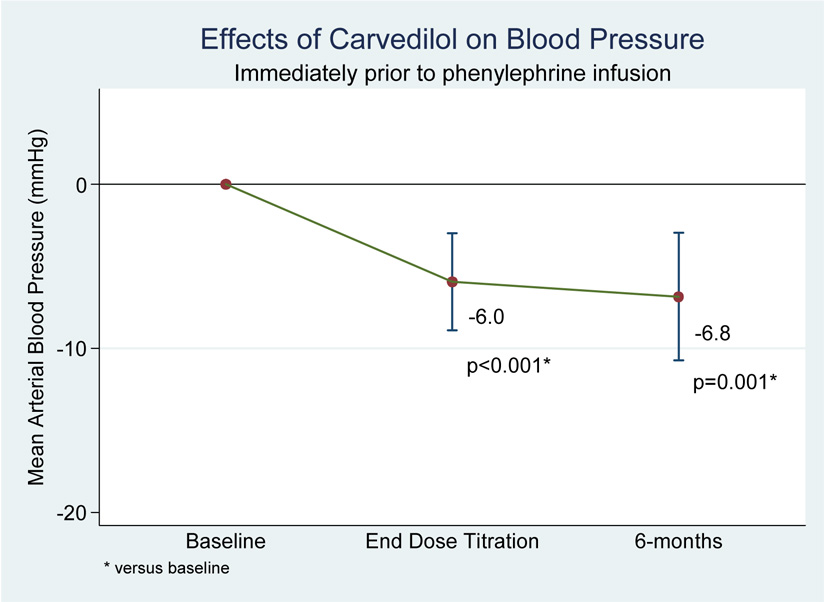
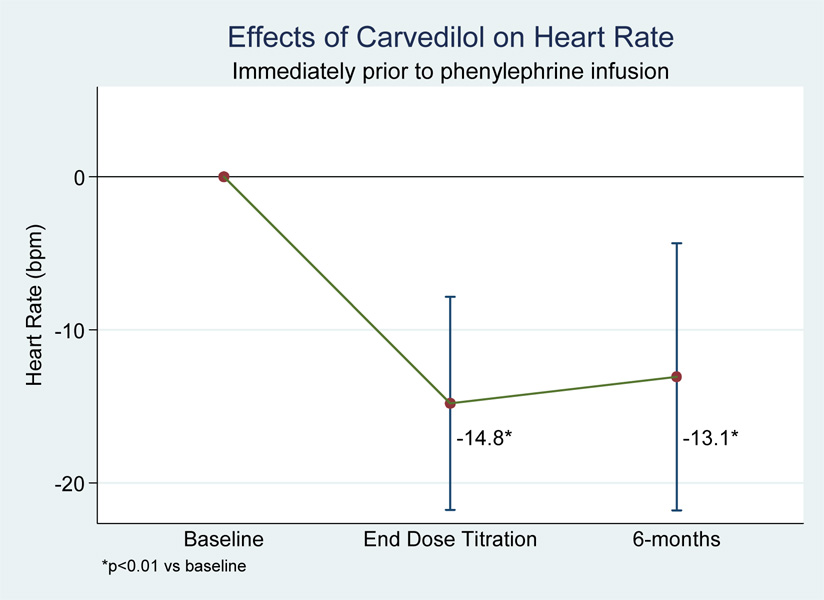
When analyzed by maximum tolerated carvedilol dose, subjects also experienced significant end-of-titration reductions in pre-infusion values for heart rate (−14.8 bpm, p<0.01), systolic BP (−10.0 mmHg, p<0.001), diastolic BP (−3.9 mmHg, p<0.05), and MAP (−6.0 mmHg, p<0.01). There was no difference in any of these values from end-of-titration to the 6-month follow-up visit, suggesting no tolerance to the heart rate and blood pressure effects of carvedilol (see figure 3 and figure 4).
Figure 3.
Figure 4.
RESPONSE TO PHENYLEPHRINE INFUSION
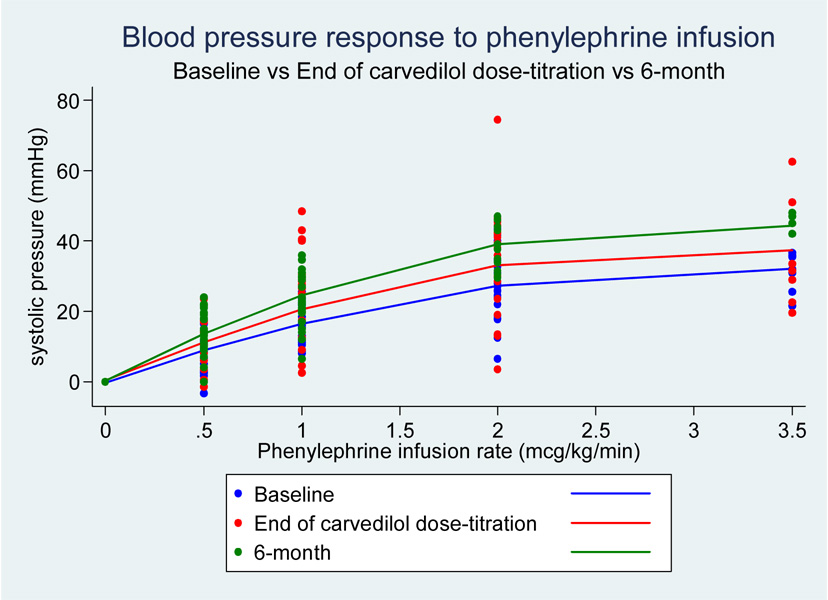
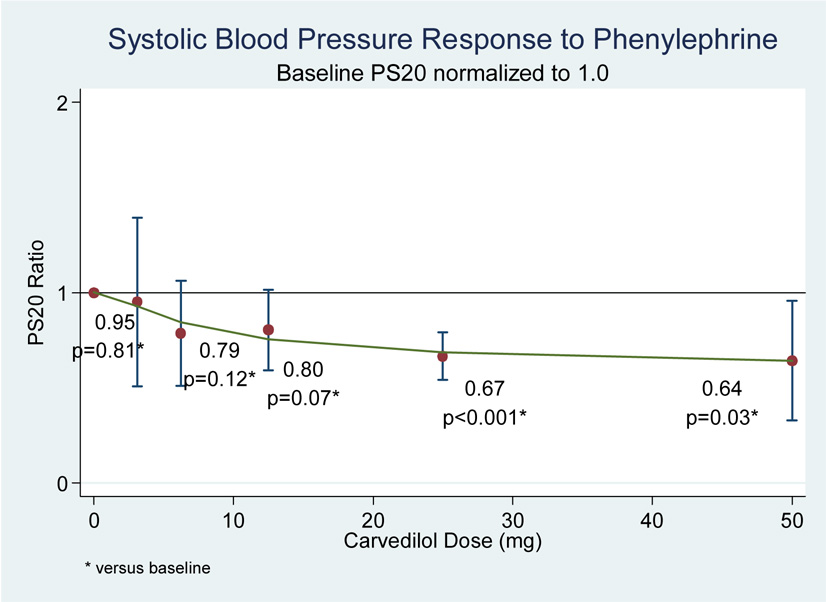
Despite the well-described α1-AR antagonistic effects of carvedilol, subjects demonstrated a trend towards increasing responsiveness to phenylephrine infusions (as demonstrated by reduced PS20 ratios) with increasing carvedilol doses (see figure 5 and 6).
Figure 5.
Figure 6.
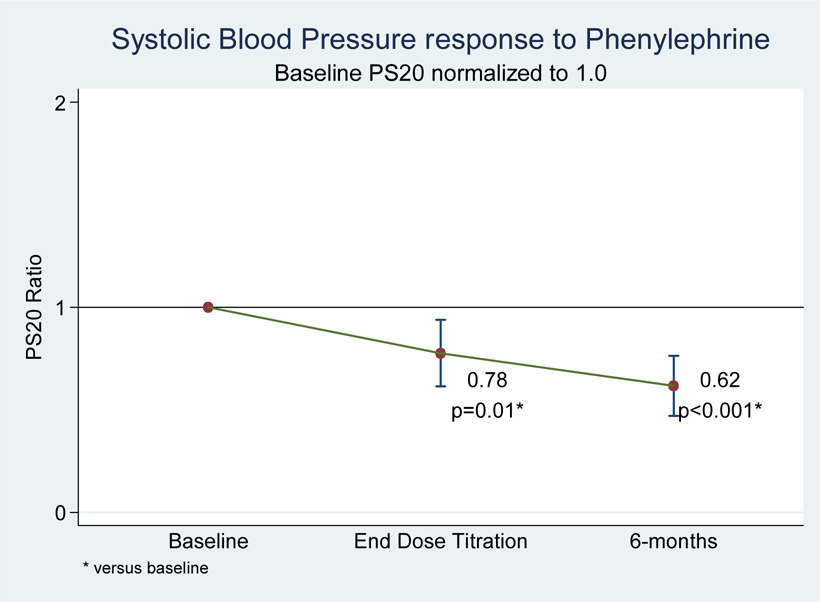
This effect achieved statistical significance at carvedilol doses of 25 –50 mg twice daily. When analyzed by maximum tolerated carvedilol dose, subjects experienced significantly increased responsiveness to phenylephrine infusions (reduced PS20 ratios) at the end-of-titration (PS20 ratio = 0.78, p=0.01) which remained at the 6-month follow-up visit (PS20 ratio = 0.62, p<0.001) (see figure 7). No significant arrhythmias were observed during the course of the study.
Figure 7.
DISCUSSION
To our knowledge, ours is the first study to systematically characterize α1-AR signal transduction during chronic therapy in HF patients. As expected, all subjects exhibited a significant reduction in pre-infusion heart rate with any dose of carvedilol. This effect served as a positive control for this protocol, due to the well described carvedilol effects at the myocardial β1-AR,3, 13 and showed no tolerance or deterioration with time (6 months).
Carvedilol also produced significant reductions in pre-infusion blood pressure (systolic BP, diastolic BP, and MAP) that were sustained over a 6 month follow-up period. While this effect may be attributed to actions at both the α1-AR and β1-AR, the significance of carvedilol α1-AR blockade on blood pressure was highlighted in the COMET trial, in which carvedilol produced superior blood pressure reductions versus metoprolol (selective β1-AR antagonist) despite similar reductions in heart rate.3, 14 Our study did not enroll patients with diabetes, limiting potential applications in this patient population. Following initial documentation of reduced LV ejection fraction (obtained from patient medical record), no follow-up echocardiography was preformed.
Published data show conflicting results regarding the development of tolerance to carvedilol α1-AR blocking effects. The acute administration of carvedilol (and subsequent α1-AR blockade) resulting in lowered blood pressure is well documented and many patients experience orthostatic hypotension during the first weeks of treatment.15, 16 However, with chronic carvedilol therapy, this orthostatic hypotension disappears, suggesting that tolerance may develop to the α1-AR blockade. Krum et al found that in patients with severe heart failure given carvedilol for 14 weeks, systemic vascular resistance (SVR) was significantly reduced compared with placebo.17 Similarly, Metra et al found that although there was no significant difference in SVR with short-term administration of carvedilol compared to placebo, after 16 weeks of continuous carvedilol therapy, SVR was significantly lower in the carvedilol group than the placebo group.18 In contrast to these findings, our group failed to demonstrate a reduction in SVR with chronic carvedilol therapy in previous studies.19, 20
Giannattasio et al treated eight patients with mild essential hypertension with carvedilol.21 The investigators infused each subject with phenylephrine and measured blood pressure and heart rate responses after a single dose of carvedilol and three weeks of continuous therapy of carvedilol. The authors reported no significant differences in the heart rate or blood pressure response to phenylephrine between single dose therapy and short-term (3 weeks) treatment.
Kubo et al randomized 36 patients with CHF to either carvedilol or metoprolol and measured the α1-AR mediated calf vasoconstrictor response to isometric handgrip exercise.22 After 16 weeks of continuous β-blocker therapy, treatment with carvedilol or metoprolol did not affect the calf vasoconstrictor response to handgrip exercise. The results of this study are difficult to interpret, because baroreceptor function is altered in HF and responses to handgrip exercises may involve multiple mechanisms beyond α1-AR activation.23
Packer et al followed cardiovascular hemodynamics in of 27 HF subjects receiving prazosin (pure α1-AR antagonist) over a 3 – 12 week follow-up period.7 Initial prazosin treatment produced a significant increase in cardiac index, accompanied by significant reductions in MAP, left ventricular filling pressure, and SVR. After completion of the follow-up period, complete tolerance had developed to the increase in cardiac index, while MAP, left ventricular filling pressure, and SVR remained significantly reduced (returned to baseline following discontinuation). Of interest, Packer et al hypothesized that the tolerance to “alpha-adrenergic responsiveness in vascular tissue may be regulated in both animals and humans at a post-receptor site, which may undergo significant modification during sustained alpha-blockade.”7
The most unexpected finding of this study was the increasing BP response to PE infusion during carvedilol treatment. We noted increased α1-AR agonist activity in the presence of a known α1-AR antagonist (carvedilol). This increased BP response was evident despite sustained reductions in pre-infusion BP, suggesting a previously undescribed pharmacologic consequence of carvedilol in HF subjects: the potential up-regulation of impaired vascular α1-AR signal transduction in human heart failure.
Goldsmith et al first reported evidence for desensitization of vascular α1-ARs in HF subjects.24 In this study, supra-physiologic doses of norepinephrine failed to elicit a blood pressure or heart rate response, suggesting some defect or impairment of the α1-AR signaling pathway in HF patients. Other evidence of impaired α1-AR signal transduction may be clinically manifest in HF patients with orthostatic hypotension. In healthy patients, postural changes in blood pressure are mitigated by baroreceptor activation of the sympathetic nervous system leading to increased heart rate and peripheral vasoconstriction. Impaired (down-regulated) α1-AR signal transduction in HF may limit the peripheral vasoconstriction response necessary to maintain blood pressure requisite for cerebral perfusion, thus leading to symptomatic orthostatic hypotension. Additional symptoms of orthostatic hypotension may be precipitated by initiation of pharmacologic agents, such as carvedilol, that block the α1-AR. However, patients frequently develop tolerance to orthostasis within weeks of treatment initiation, suggesting some level of improved α1-AR signal transduction to relay the sympathetic impulse for peripheral vasoconstriction. It should be noted that patients frequently develop tolerance to orthostatic hypotension without attenuation of anti-hypertensive effects,25, 26 suggesting that improved α1-AR signal transduction may develop independently of resting blood pressure. Our current report, in which carvedilol elicited increasing BP responses to phenylephrine despite sustained reductions in pre-infusion BP, supports these clinical observations.
Evidence of altered vascular α1-AR signal transduction mirrors that of the myocardial β1-AR, which undergoes down-regulation, uncoupling, and desensitization in HF following long-standing elevations in sympathetic tone.27, 28 Use of a β1-AR antagonist in HF interrupts the long-standing sympathetic over-stimulation and allows for gradual restoration of β1-ARs to their previous functional state, effectively restoring myocardial β1-AR signal transduction.29–33 For example, Heilbrunn et al reported increased ventricular response to dobutamine (peak positive left ventricular dP/dt) following chronic treatment (6 months) with metoprolol in heart failure patients. This increased β1-AR responsiveness occurred in conjunction with increased β1-AR density despite a significant reduction in resting heart rate. Our data suggest that a similar “restorative” biologic process occurs at the vascular α1-AR with chronic (6 months) carvedilol treatment. It is worth noting that desensitization of α1-AR and β1-AR may share a common etiology, as patients carrying the largest risks of orthostasis are typically those exposed to high levels of sympathetic signaling, whether chronologically (elderly) or pathologically (heart failure).34–36
The apparent inability of carvedilol to blunt PE agonist activity may also be attributable to recently described α1-AR subtypes, α1A-AR, α1B-AR, and α1D-AR, of which the effects of α1A-AR typically predominate under normal physiologic conditions. Koshimizu et al reported higher carvedilol affinity for α1B-AR and α1D-AR subtypes versus the α1A-AR subtype in human embryonic kidney cells.37 In these cells, carvedilol induced significant reductions in oscillatory intracellular calcium signaling at both the α1B-AR and α1D-AR, but not at the α1A-AR (p<0.01). These α1-AR subtypes also undergo differential regulation in laboratory models. Yang et al reported down-regulation of α1A-AR and α1B-AR in animal models following a 24-hour phenylephrine exposure, while the same conditions produced up-regulation of α1D-AR in a time-dependent and concentration-dependent manner.38 Lei et al followed a similar procedure in human embryonic kidney cells, reporting down-regulation of α1A-AR and α1D-AR following a 24-hour exposure to norepinephrine, accompanied by up-regulation of α1B-AR.39 Currently, we are aware of no studies reporting the relative prevalence of α1-AR subtypes in human HF. However, the combined work of Yang et al and Lei et al suggest that long-standing elevations in sympathetic tone may produce an altered population of α1-AR subtypes in HF patients, which may yield the responses to carvedilol observed in our study. The clinical impact of differential α1-AR subtype regulation in HF may depend on future development of α1-AR antagonists with subtype selectivity.
CONCLUSIONS
Increasing BP response to α1-AR stimulation in the presence of a known α1-AR antagonist is highly suggestive of improved in α1-AR signal transduction. This effect was observed in HF subjects (class C) with LVEF<0.40 during up-titration with carvedilol and maintained over a 6 month follow-up period. Differential regulation and carvedilol selectivity for α1-AR subtypes may have contributed to this finding. The clinical implication of improved α1-AR signaling remains undetermined, but may correlate with a resolution of orthostatic hypotension. Despite the apparent up-regulation of α1-AR signaling, we observed no tolerance to the anti-hypertensive effects of carvedilol on resting (pre-infusion) blood pressure.
Table 1.
Baseline demographics
| Mean | Range | SD | |
|---|---|---|---|
| Age (years) | 58.5 | 42 – 81 | 11.3 |
| Height (cm) | 177.2 | 160 – 193 | 10.8 |
| Weight (kg) | 88.7 | 56.9 – 131.8 | 19.8 |
| Male gender (%) | 58.3 | ||
| Caucasian (%) | 83.3 | ||
| NYHA FC | 2.2 | 2 – 3 | 0.4 |
| Left ventricular EF (%) | 23.4 | 15 – 35 | 5.8 |
| LVIDS (cm) | 5.9 | 4.6 – 7.9 | 1.0 |
| LVIDD (cm) | 6.5 | 3.9 – 8.7 | 1.3 |
| Fractional shortening (%) | 13.1 | 5 – 25 | 6.1 |
| BNP (pg/mL) | 243* | 117 – 3684 | 1300.1 |
| SCr (mg/dL) | 1.0 | 0.8 – 1.3 | 0.2 |
| Blood Pressure (mmHg) | |||
| Systolic | 114.1 | 98 – 138 | 12.5 |
| Diastolic | 67.2 | 55 – 87 | 8.1 |
| Heart rate (bpm) | 75.0 | 53 – 101 | 17.0 |
| Co-morbidities (%) | |||
| CAD | 33.3% | ||
| HTN | 33.3% | ||
| DM | 0.0% |
Median value listed due to irregular distribution
Acknowledgments
Funding:GlaxoSmithKline Investigator Initiated Trials
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest:
Benjamin W. Van Tassell: None
Matthew Rondina: None
Franklin Huggins: None
Mark A. Munger: GlaxoSmithKline Speakers Bureau and Research Grant Support
Edward M. Gilbert: GlaxoSmithKline Speakers Bureau and Research Grant Support
REFERENCES
- 1.The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet. 1999 Jan 2;353(9146):9–13. [PubMed] [Google Scholar]
- 2.Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF) Lancet. 1999 Jun 12;353(9169):2001–2007. [PubMed] [Google Scholar]
- 3.Poole-Wilson PA, Swedberg K, Cleland JG, et al. Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol Or Metoprolol European Trial (COMET): randomised controlled trial. Lancet. 2003 Jul 5;362(9377):7–13. doi: 10.1016/S0140-6736(03)13800-7. [DOI] [PubMed] [Google Scholar]
- 4.Yoshikawa T, Port JD, Asano K, et al. Cardiac adrenergic receptor effects of carvedilol. Eur Heart J. 1996 Apr;17 Suppl B:8–16. doi: 10.1093/eurheartj/17.suppl_b.8. [DOI] [PubMed] [Google Scholar]
- 5.Ruffolo RR, Jr, Gellai M, Hieble JP, Willette RN, Nichols AJ. The pharmacology of carvedilol. Eur J Clin Pharmacol. 1990;38 Suppl 2:S82–S88. doi: 10.1007/BF01409471. [DOI] [PubMed] [Google Scholar]
- 6.Woodcock EA. Roles of alpha1A- and alpha1B-adrenoceptors in heart: insights from studies of genetically modified mice. Clin Exp Pharmacol Physiol. 2007 Sep;34(9):884–888. doi: 10.1111/j.1440-1681.2007.04707.x. [DOI] [PubMed] [Google Scholar]
- 7.Packer M, Medina N, Yushak M. Role of the renin-angiotensin system in the development of hemodynamic and clinical tolerance to long-term prazosin therapy in patients with severe chronic heart failure. J Am Coll Cardiol. 1986 Mar;7(3):671–680. doi: 10.1016/s0735-1097(86)80479-x. [DOI] [PubMed] [Google Scholar]
- 8.Sumner DJ, Elliott HL, Reid JL. Analysis of the pressor dose response. Clin Pharmacol Ther. 1982 Oct;32(4):450–458. doi: 10.1038/clpt.1982.188. [DOI] [PubMed] [Google Scholar]
- 9.Semplicini A, Pessina AC, Rossi GP, Hlede M, Morandin F. Alpha-adrenoceptor blockade by labetalol during long-term dosing. Clin Pharmacol Ther. 1983 Mar;33(3):278–282. doi: 10.1038/clpt.1983.33. [DOI] [PubMed] [Google Scholar]
- 10.Tham TC, Guy S, McDermott BJ, Shanks RG, Riddell JG. The dose dependency of the alpha- and beta-adrenoceptor antagonist activity of carvedilol in man. Br J Clin Pharmacol. 1995 Jul;40(1):19–23. doi: 10.1111/j.1365-2125.1995.tb04529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dunnett C, Goldsmith C. When and how to do multiple comparisons. In: Buncher C, Tsay J, editors. Statistics in the Pharmaceutical Industry. 3rd ed. New York: Chapman & Hall; 2006. pp. 421–452. [Google Scholar]
- 12.Ting N. Dose finding in drug development. Springer; 2006. [Google Scholar]
- 13.Packer M, Fowler MB, Roecker EB, et al. Effect of carvedilol on the morbidity of patients with severe chronic heart failure: results of the carvedilol prospective randomized cumulative survival (COPERNICUS) study. Circulation. 2002 Oct 22;106(17):2194–2199. doi: 10.1161/01.cir.0000035653.72855.bf. [DOI] [PubMed] [Google Scholar]
- 14.Metra M, Torp-Pedersen C, Swedberg K, et al. Influence of heart rate, blood pressure, and beta-blocker dose on outcome and the differences in outcome between carvedilol and metoprolol tartrate in patients with chronic heart failure: results from the COMET trial. Eur Heart J. 2005 Nov;26(21):2259–2268. doi: 10.1093/eurheartj/ehi386. [DOI] [PubMed] [Google Scholar]
- 15.Hansson L, Himmelmann A. Carvedilol in the treatment of hypertension--a review of the clinical data base. Scand Cardiovasc J Suppl. 1998;47:67–80. doi: 10.1080/140174398428072. [DOI] [PubMed] [Google Scholar]
- 16.Lund-Johansen P, Omvik P, Nordrehaug JE, White W. Carvedilol in hypertension: effects on hemodynamics and 24-hour blood pressure. J Cardiovasc Pharmacol. 1992;19 Suppl 1:S27–S34. [PubMed] [Google Scholar]
- 17.Krum H, Sackner-Bernstein JD, Goldsmith RL, et al. Double-blind, placebo-controlled study of the long-term efficacy of carvedilol in patients with severe chronic heart failure. Circulation. 1995 Sep 15;92(6):1499–1506. doi: 10.1161/01.cir.92.6.1499. [DOI] [PubMed] [Google Scholar]
- 18.Metra M, Nardi M, Giubbini R, Dei Cas L. Effects of short- and long-term carvedilol administration on rest and exercise hemodynamic variables, exercise capacity and clinical conditions in patients with idiopathic dilated cardiomyopathy. J Am Coll Cardiol. 1994 Dec;24(7):1678–1687. doi: 10.1016/0735-1097(94)90174-0. [DOI] [PubMed] [Google Scholar]
- 19.Olsen SL, Gilbert EM, Renlund DG, Taylor DO, Yanowitz FD, Bristow MR. Carvedilol improves left ventricular function and symptoms in chronic heart failure: a double-blind randomized study. J Am Coll Cardiol. 1995 May;25(6):1225–1231. doi: 10.1016/0735-1097(95)00012-S. [DOI] [PubMed] [Google Scholar]
- 20.Gilbert EM, Abraham WT, Olsen S, et al. Comparative hemodynamic, left ventricular functional, and antiadrenergic effects of chronic treatment with metoprolol versus carvedilol in the failing heart. Circulation. 1996 Dec 1;94(11):2817–2825. doi: 10.1161/01.cir.94.11.2817. [DOI] [PubMed] [Google Scholar]
- 21.Giannattasio C, Cattaneo BM, Seravalle G, et al. Alpha 1-blocking properties of carvedilol during acute and chronic administration. J Cardiovasc Pharmacol. 1992;19 Suppl 1:S18–S22. doi: 10.1097/00005344-199219001-00005. [DOI] [PubMed] [Google Scholar]
- 22.Kubo T, Azevedo ER, Newton GE, Parker JD, Floras JS. Lack of evidence for peripheral alpha(1)- adrenoceptor blockade during long-term treatment of heart failure with carvedilol. J Am Coll Cardiol. 2001 Nov 1;38(5):1463–1469. doi: 10.1016/s0735-1097(01)01577-7. [DOI] [PubMed] [Google Scholar]
- 23.Creager MA, Creager SJ. Arterial baroreflex regulation of blood pressure in patients with congestive heart failure. J Am Coll Cardiol. 1994 Feb;23(2):401–405. doi: 10.1016/0735-1097(94)90427-8. [DOI] [PubMed] [Google Scholar]
- 24.Goldsmith SR, Francis GS, Cohn JN. Norepinephrine infusions in congestive heart failure. Am J Cardiol. 1985 Nov 1;56(12):802–804. doi: 10.1016/0002-9149(85)91146-4. [DOI] [PubMed] [Google Scholar]
- 25.Graham RM, Thornell IR, Gain JM, Bagnoli C, Oates HF, Stokes GS. Prazosin: the first-dose phenomenon. Br Med J. 1976 Nov 27;2(6047):1293–1294. doi: 10.1136/bmj.2.6047.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krum H, Roecker EB, Mohacsi P, et al. Effects of initiating carvedilol in patients with severe chronic heart failure: results from the COPERNICUS Study. JAMA. 2003 Feb 12;289(6):712–718. doi: 10.1001/jama.289.6.712. [DOI] [PubMed] [Google Scholar]
- 27.Movsesian MA, Bristow MR. Alterations in cAMP-mediated signaling and their role in the pathophysiology of dilated cardiomyopathy. Curr Top Dev Biol. 2005;68:25–48. doi: 10.1016/S0070-2153(05)68002-7. [DOI] [PubMed] [Google Scholar]
- 28.Vatner DE, Asai K, Iwase M, et al. Beta-adrenergic receptor-G protein-adenylyl cyclase signal transduction in the failing heart. Am J Cardiol. 1999 Jun 17;83(12A):80H–85H. doi: 10.1016/s0002-9149(99)00266-0. [DOI] [PubMed] [Google Scholar]
- 29.Sigmund M, Jakob H, Becker H, et al. Effects of metoprolol on myocardial beta-adrenoceptors and Gi alpha-proteins in patients with congestive heart failure. Eur J Clin Pharmacol. 1996 51 2;:127–132. doi: 10.1007/s002280050172. [DOI] [PubMed] [Google Scholar]
- 30.Gilbert EM, Olsen SL, Renlund DG, Bristow MR. beta-adrenergic receptor regulation and left ventricular function in idiopathic dilated cardiomyopathy. Am J Cardiol. 1993 Mar 25;71(9):23C–29C. doi: 10.1016/0002-9149(93)90083-o. [DOI] [PubMed] [Google Scholar]
- 31.Kubo H, Margulies KB, Piacentino V, 3rd, Gaughan JP, Houser SR. Patients with end-stage congestive heart failure treated with beta-adrenergic receptor antagonists have improved ventricular myocyte calcium regulatory protein abundance. Circulation. 2001 Aug 28;104(9):1012–1018. doi: 10.1161/hc3401.095073. [DOI] [PubMed] [Google Scholar]
- 32.Lowes BD, Gilbert EM, Abraham WT, et al. Myocardial gene expression in dilated cardiomyopathy treated with beta-blocking agents. N Engl J Med. 2002 May 2;346(18):1357–1365. doi: 10.1056/NEJMoa012630. [DOI] [PubMed] [Google Scholar]
- 33.Heilbrunn SM, Shah P, Bristow MR, Valantine HA, Ginsburg R, Fowler MB. Increased beta-receptor density and improved hemodynamic response to catecholamine stimulation during long-term metoprolol therapy in heart failure from dilated cardiomyopathy. Circulation. 1989 Mar;79(3):483–490. doi: 10.1161/01.cir.79.3.483. [DOI] [PubMed] [Google Scholar]
- 34.Bradley JG, Davis KA. Orthostatic hypotension. Am Fam Physician. 2003 Dec 15;68(12):2393–2398. [PubMed] [Google Scholar]
- 35.Lipsitz LA. Orthostatic hypotension in the elderly. N Engl J Med. 1989 Oct 5;321(14):952–957. doi: 10.1056/NEJM198910053211407. [DOI] [PubMed] [Google Scholar]
- 36.Ooi WL, Barrett S, Hossain M, Kelley-Gagnon M, Lipsitz LA. Patterns of orthostatic blood pressure change and their clinical correlates in a frail, elderly population. JAMA. 1997 Apr 23–30;277(16):1299–1304. [PubMed] [Google Scholar]
- 37.Koshimizu TA, Tsujimoto G, Hirasawa A, Kitagawa Y, Tanoue A. Carvedilol selectively inhibits oscillatory intracellular calcium changes evoked by human alpha1D- and alpha1B-adrenergic receptors. Cardiovasc Res. 2004 Sep 1;63(4):662–672. doi: 10.1016/j.cardiores.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 38.Yang M, Ruan J, Voller M, Schalken J, Michel MC. Differential regulation of human alpha1-adrenoceptor subtypes. Naunyn Schmiedebergs Arch Pharmacol. 1999 Jun;359(6):439–446. doi: 10.1007/pl00005373. [DOI] [PubMed] [Google Scholar]
- 39.Lei B, Zhang Y, Han C. Sustained norepinephrine stimulation induces different regulation of expression in three alpha1-adrenoceptor subtypes. Life Sci. 2001 Jun 8;69(3):301–308. doi: 10.1016/s0024-3205(01)01115-8. [DOI] [PubMed] [Google Scholar]