Abstract
Normal colon epithelial cells express and most colon cancers over-express M3 muscarinic receptors (M3R). In human colon cancer cells, post-M3R signaling stimulates cell proliferation. To explore the importance of M3R expression in vivo we used the azoxymethane (AOM)-induced colon neoplasia model. Wild type (WT) and M3R-deficient (M3R−/−) mice, treated with weekly intraperitoneal injection of phosphate-buffered saline (10 WT mice) or AOM (22 WT and 16 M3R−/− mice) for 6 weeks, were euthanized at 20 weeks. At week 20, AOM-treated WT mice weighed ~16% more than M3R−/− mice (33.4 ± 1.0 vs. 27.9 ± 0.5 g; mean ± SE, P < 0.001). In AOM-treated M3R−/− mice, cell proliferation (BrdU staining) was reduced 43% compared to AOM-treated WT mice (P < 0.05). Whereas control mice (both WT and M3R−/−) had no colon tumors, AOM-treated WT mice had 5.3 ± 0.5 tumors/animal. Strikingly, AOM-treated M3R−/− mice had only 3.2 ± 0.3 tumors/mouse (P < 0.05); a 40% reduction. Tumor volume in AOM-treated M3R−/− mice was reduced 60% compared to AOM-treated WT mice (8.1 ± 1.5 vs. 20.3 ± 4.1 mm3; P < 0.05). Compared to WT, fewer M3R−/− mice had adenomas (6 vs. 36%, P = 0.05), and M3R−/− mice had fewer adenocarcinomas/mouse (0.6 ± 0.1 vs. 1.7 ± 0.4, P < 0.05). Eleven of 22 WT but no M3R−/− mice had multiple adenocarcinomas (P < 0.001). In conclusion, compared to WT, AOM-treated M3R-deficient mice have attenuated epithelial cell proliferation, tumor number, and tumor size. These findings identify M3R and post-M3R signaling as novel therapeutic targets for colon cancer.
Keywords: cell proliferation, colon cancer, muscarinic receptors, acetylcholine, azoxymethane
Introduction
The muscarinic cholinergic family of G-protein coupled receptors includes five muscarinic receptor subtypes designated M1-M5 (1–3). M3R, expressed widely in the gastrointestinal tract, couple to Gq11, activate phospholipase C signaling and inositol phosphate formation and increase cell calcium, thereby altering cell function, including proliferation (4). In 1991, Gutkind et al. reported that muscarinic receptors linked to phosphatidylinositol hydrolysis (i.e. M1R, M3R, M5R) act as conditional oncogenes when expressed in cells capable of proliferation (5). Subsequently, Frucht and colleagues reported that human colon cancer cell lines and colon cancer tissue express M3R, and that M3R expression was increased up to 8-fold in cancer compared to normal tissue (6, 7). Collectively, these findings suggest that the M3R is an important player in colon neoplasia.
Work from our laboratory elucidated cellular mechanisms underlying acetylcholine-induced colon cancer cell proliferation. In H508 human colon cancer cells which express high levels of M3R (8), acetylcholine activates post-receptor signaling, including robust phosphorylation of ERK1/2 and p90RSK, a nuclear response protein that regulates gene expression and the cell cycle (9). These actions of acetylcholine are blocked by muscarinic receptor inverse agonists, calcium chelators, and inhibitors of MEK (MAPK kinase; the regulatory protein just upstream of ERK), and they do not occur in SNU-C4 human colon cancer cells that do not express M3R (9). Moreover, recent studies support the hypothesis that luminal bile acids, long associated with the development of colon cancer (10), also stimulate colon cancer cell proliferation by M3R- and EGFR-dependent mechanisms (11–13). In these in vitro cell systems, proliferative actions of muscarinic agonists require both M3R expression and activation of post-M3R signaling.
Given the key role of M3R expression in proliferation of human colon cancer cells, it is important to determine whether genetic ablation of M3R reduces colon tumor formation in vivo. In rodents, azoxymethane (AOM; 10 mg/kg body weight), an intermediate in the metabolism of 1,2-dimethylhydrazine to the alkylating ion methyldiazonium, is a colorectal-specific procarcinogen (14). AOM-induced rodent tumors mimic human colon cancer; most lesions arise in the colon, form grossly visible exophytic polypoid or plaque-like tumors, and have a microscopic appearance similar to that of human adenomas and adenocarcinomas. Molecular changes in AOM-induced tumors, including β-catenin and p53 mutations, also mimic those in human colon cancer (15, 16). We used the AOM colon cancer model in M3R-deficient mice to determine whether M3R expression is required for AOM-induced cell proliferation and neoplasia (14). As reported herein, our findings strongly support the hypothesis that M3R plays a key role in colon epithelial cell proliferation and neoplasia.
Materials and Methods
Animals
The generation of M3R−/− and WT mice of the same mixed genetic background (129S6/SvEvTac x CF1; 50%/50%) has been described previously (17). For all experiments, male mice were used. Mice were housed under identical conditions in a pathogen-free room, had free access to commercial rodent chow and water, and were allowed to acclimatize in the vivarium for 2 weeks before initiating treatments. Mice were weighed weekly. These studies were approved by the University of Maryland, Baltimore Institutional Animal Care and Use Committee (IACUC).
In situ hybridization
As described previously (18), digoxigenin-labeled antisense RNA probes were prepared from riboprobe plasmids containing M1R and M3R inserts. M1R riboprobe, synthesized from a 0.28-kb KpnI-SacI genomic fragment cloned into a pBluescript vector, corresponds to the M1R sequence lacking in the genome of M1R−/− mice. M3R riboprobe, synthesized from a 1.6-kb XbaI-Sse8337I genomic fragment, corresponds to M3R sequence absent in the genome of M3R−/− mice. M1R and M3R riboprobes were digested with KpnI and NotI, respectively. After purification of linearized plasmids, in vitro transcriptions for M1R and M3R RNA probes, 280 bp and 1.6 kb in length, respectively, were performed using the Digoxigenin RNA labeling kit (Roche Applied Sciences) with T7 and T3 RNA polymerases, respectively. The length of M3R digoxigenin-labeled RNA was shortened by alkaline hydrolysis to ~300 bp. The yield of transcripts was estimated using dot blots with control digoxigenin-labeled RNA (Roche Applied Science).
AOM treatment
For the initial 6 weeks of treatment, 48 animals received weekly intraperitoneal AOM (Midwest Research Institute, Kansas City, MO; 10 mg/kg body weight) (22 WT and M3R−/− 16 mice) or an equal volume of vehicle (phosphate-buffered saline) (10 WT mice). To determine tumor incidence, mice were observed for evidence of tumor formation (e.g. anal bleeding) and euthanized at 20 weeks. Colon length was measured, segments opened longitudinally, and placed flat on microscope slides. Tumors were identified by visual inspection and photographed using a dissecting microscope (Nikon SMZ1500). Tumor size was measured using calipers and confirmed using Nikon Image-Pro software. Tumor volume was calculated using the equation: volume = ½ (length x width2) (19).
Histological analysis
Adenomas and adenocarcinomas were defined according to consensus recommendations [Mouse Models of Human Cancers Consortium (14)]. Tumor number was counted in the colon from each mouse. Tumors were photographed, resected and bisected. Tissues were fixed in 4% paraformaldehyde and paraffin-embedded. Five-micrometer sections were stained with hematoxylin and eosin, and examined using a Nikon Eclipse 80i microscope. Investigators masked to mouse genotype and treatment performed gross and microscopic tumor counts and determined tumor size.
Immunohistological analysis
Two hours before euthanasia, mice received intraperitoneal injection of BrdU (Sigma-Aldrich) (50 mg/kg) to label S-phase cells, a marker of proliferation. BrdU labeling was determined after immunostaining with anti-BrdU antibody (BD Bioscience) by counting BrdU-positive nuclei in 1000 cells (data expressed as percent of total cells that were BrdU-positive). As a marker of apoptosis, we used immunostaining with anti-activated caspase-3 antibody (Cell Signaling Technology) (20). Only complete crypts were evaluated and investigators were masked to treatment group. For analysis of both BrdU and activated caspase-3 staining, only tissue from the distal half of the colon was examined.
Statistical analysis
Based on distribution of data, Student’s unpaired t-test (normally distributed data) or the Mann-Whitney U test (non-parametric data) were used to determine significance. Nominal data were analyzed using chi square with Fisher’s exact test. Values of P < 0.05 were considered significant. Statistical analysis was performed using StatView (SAS, version 5.0.1, Cary, NC).
Results
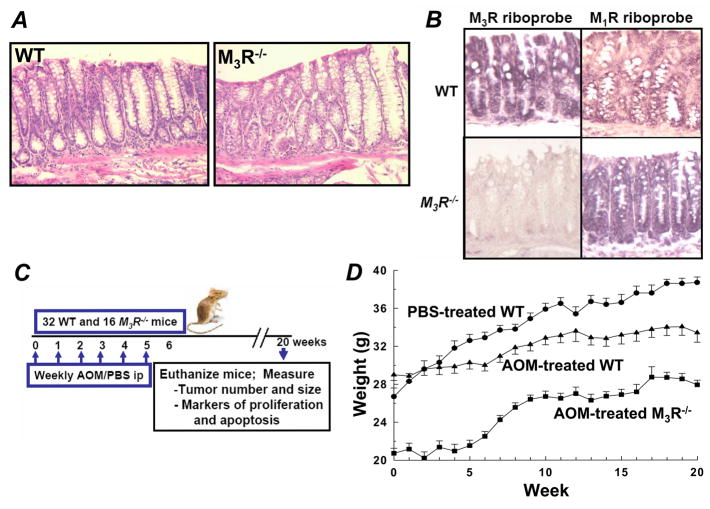
Deletion of M3R does not perturb normal gastrointestinal development (17). As shown in Fig. 1A, there was no difference in microscopic anatomy in hematoxylin and eosin-stained colon sections from WT compared to those from M3R−/− mice. To confirm expression of M3R and identify possible co-expression of M1R in colon epithelial cells we used in situ hybridization with muscarinic receptor-specific riboprobes. Co-expression of M1R and M3R was previously detected in WT murine gastric mucosa (18). As expected, whereas signals for both M3R and M1R were evident in colon epithelial cells from WT mice, only M1R signal was detected in M3R−/− animals (Fig. 1B). To determine the effect of M3R deficiency on colon tumor formation, 48 animals were allocated to weekly intraperitoneal injection of AOM (22 WT and 16 M3R−/− mice) or phosphate-buffered saline (PBS) (10 WT mice) for a total of 6 doses each (Fig. 1C). Animals were euthanized at 20 weeks to measure tumor number and size, and markers of proliferation and apoptosis (Fig. 1C). As reported previously (17), at baseline M3R−/− weighed less than WT mice (20.7 ± 0.5 vs. 28.3 ± 0.5 g; mean ± SE; P < 0.001). Over the course of the 20-week study, WT mice treated with AOM gained less weight than WT mice treated with PBS (33.4 ± 1.0 vs. 38.7 ± 0.6 g at 20 weeks; P < 0.01) (Fig. 1D). Whereas animals treated with PBS gained weight progressively over the 20-week study, animals treated with AOM did not gain weight during the initial 6 weeks but gained weight once the series of AOM injections was completed (Fig. 1D). Moreover, although both groups gained weight, AOM-treated WT mice persistently weighed ~16% more than M3R−/− mice (33.4 ± 1.0 vs. 27.9 ± 0.5 g at week 20; P < 0.001) (Fig. 1D). Colons were modestly, but significantly, shorter in M3R−/− compared to WT mice (10.7±0.2 vs. 11.6±0.2 cm for M3R−/− compared to WT mice treated with AOM; P = 0.002). Prior to euthanasia, no mice developed rectal bleeding or other clinical evidence of colon tumor formation.
Figure 1.
WT and M3R−/− mouse colon mucosal histology and muscarinic receptor expression, study protocol and animal weights. A, representative hematoxylin and eosin-stained colon sections from WT and M3R−/− mice. B, in situ hybridization using specific M1R and M3R riboprobes in colon epithelium from WT and M3R−/− mice. C, schematic of study design; WT and M3R−/− mice were treated with intraperitoneal injection of PBS (10 WT mice) or AOM (22 WT and 16 M3R−/− mice) (weekly for 6 weeks) and followed for a total of 20 weeks. At 20 weeks, animals were euthanized and colon tumor number and size, and mucosal markers of proliferation and apoptosis were measured. D, weights of PBS-treated WT, and AOM-treated WT and M3R−/− mice during the 20-week study (mean ± SE).
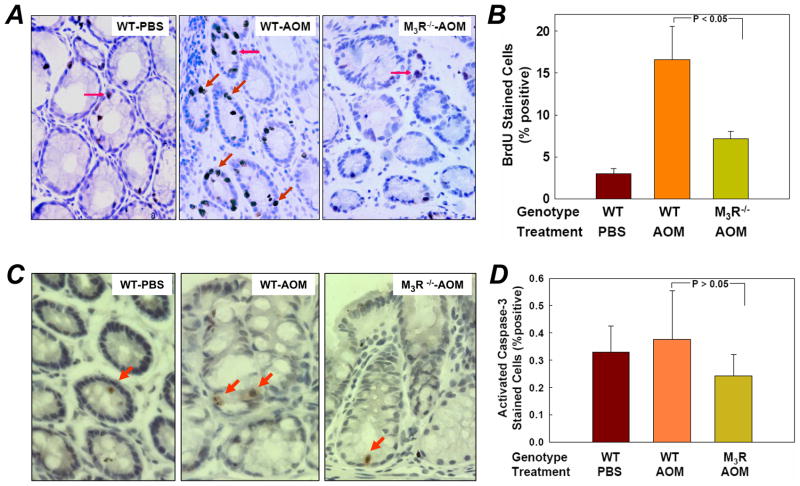
As a marker of cell proliferation, BrdU (50 mg/kg) was administered by intraperitoneal injection 2 hours before euthanasia. Fig. 2A shows representative micrographs of BrdU incorporation in colon mucosa from PBS-treated WT, and AOM-treated WT and M3R−/− mice. AOM treatment induced BrdU incorporation in normal colon crypts (Fig. 2A). A 5-fold increase in BrdU-positive epithelial cells was detected in tissue from AOM-treated WT mice and this was reduced in M3R−/− animals (Fig. 2A and B). Fig. 2B demonstrates an ~70% reduction in BrdU-positive cells in tissue from M3R−/− compared to WT mice (7.2 ± 0.9% vs. 16.6 ± 4.0%, P < 0.05). Clearly, AOM treatment stimulates proliferation of normal colon epithelial cells and this effect is attenuated in M3R-deficient mice (Fig. 2B).
Figure 2.
Markers of cell proliferation and apoptosis in colon epithelial cells from PBS- and AOM-treated WT and M3R−/− mice. A, representative BrdU-stained sections from PBS-treated WT, AOM-treated WT, and AOM-treated M3R−/− mice. Arrows indicate representative BrdU-stained nuclei. B, percentage of BrdU-positive cells in colon epithelial cells in the three treatment groups. Columns, mean percent BrdU-stained cells; bars, SE. N = 5 animals/group. Unpaired Student’s t-test. C, representative activated caspase-3-stained sections from PBS-treated WT, AOM-treated WT, and AOM-treated M3R−/− mice. Arrows indicate activated caspase-3-stained cells. D, percentage of activated caspase-3-positive cells in colon epithelial cells in the three treatment groups. Columns, mean percent activated caspase-3-stained cells; bars, SE. N = 5 animals/group. Unpaired Student’s t-test.
As a marker of apoptosis, sections of colon mucosa from PBS-treated WT, and AOM-treated WT and M3R−/− mice were examined for activated caspase-3 immunostaining (representative micrographs shown in Fig. 2C). As depicted in Fig. 2D, the number of apoptotic cells in the three treatment groups was an order of magnitude lower than observed with BrdU staining. There was no significant difference in the number of apoptotic cells between groups (P > 0.05). These findings indicate that AOM primarily stimulates epithelial cell proliferation and that genetic ablation of M3R reduces cell proliferation with no appreciable effect on apoptosis.
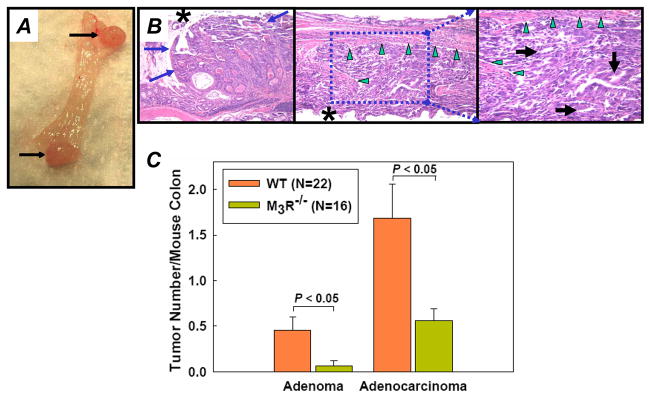
Representative colon sections from AOM-treated animals shown in Fig. 3A indicate reduced tumor number in AOM-treated M3R−/− compared to WT mice. At 20 weeks, no colon tumors were observed in WT mice that had not been treated with AOM. Likewise, colon tumors were not observed in M3R−/− mice that were not treated with AOM (not shown). As illustrated in Fig. 3B, WT mice treated with AOM had 5.3 ± 0.5 tumors/colon whereas M3R−/− mice had 3.2 ± 0.3 tumors/colon; a 40% reduction (P < 0.05). In both WT and M3R-deficient mice, all tumors were in the distal half of the colon. Tumor volume was reduced by 60% in M3R−/− compared to WT mice (8.1 ± 1.5 vs. 20.3 ± 4.1 mm3; P < 0.05) (Fig. 3C). These findings provide strong evidence that M3R gene ablation decreases both colon tumor number and size.
Figure 3.
Macroscopic colon tumors in PBS- and AOM-treated WT and M3R−/− mice. A, representative dissecting microscope images of colon mucosa from PBS-treated WT (top), AOM-treated WT (middle) and AOM-treated M3R−/− mice (bottom). Macroscopic tumors > 0.5 mm diameter indicated by dashed circles, respectively. Number of tumors/section indicated at bottom of each image. size bars, 1 mm. B, reduced tumor number in M3R−/− compared to WT mice treated with AOM (mean ± SE; P < 0.05, Mann-Whitney U test). N, number of mice/treatment group. C, reduced tumor volume in M3R−/− compared to WT mice (mean ± SE, P < 0.05, Mann-Whitney U test). Columns, mean tumor number per mouse colon; bars, SE. N, number of mice/treatment group.
AOM-induced colon tumors mimicked the gross (Fig. 4A) and microscopic (Fig. 4B) appearance of human adenomas and adenocarcinomas. Tumors observed in AOM-treated mice included adenomas (Fig. 4B, left panel), well differentiated adenocarcinomas (not shown), and poorly differentiated adenocarcinomas (Fig. 4B, middle and right panels). To determine the relative number of adenomas and adenocarcinomas, hematoxylin and eosin-stained colon sections were reviewed by a pathologist masked to treatment group (22 WT and 16 M3R−/− mice treated with AOM). This analysis revealed that, compared to WT, fewer M3R−/− mice had adenomas (6 vs. 36%, P = 0.05) and M3R−/− mice had fewer adenocarcinomas/mouse (0.6 ± 0.1 vs. 1.7 ± 0.4, P < 0.05) (Fig. 4C). Eleven of 22 WT but no M3R−/− mice had multiple adenocarcinomas (P < 0.001). Collectively, these findings indicate that in this murine model of colon cancer, M3R-deficiency attenuates the overall number of colon tumors and, more specifically, the number of adenomas and adenocarcinomas.
Figure 4.
AOM-induced tumors mimic human adenomas and adenocarcinomas, and are reduced in M3R-deficient mice. A, photograph of two representative adenocarcinomas in the distal colon of an AOM-treated WT mouse. B, representative hematoxylin and eosin-stained microscopic images of adenoma and adenocarcinoma in AOM-treated WT mice. Left, adenoma (X20 magnification). Middle, poorly differentiated adenocarcinoma (X20 magnification). Right, higher magnification of indicated area of adenocarcinoma (X40 magnification). * indicates luminal surface. Green arrowheads delineate muscularis mucosa. Black arrows indicate poorly formed glands. C, number of adenomas and adenocarcinomas per mouse colon are reduced in M3R-deficient mice. Columns, mean number of adenomas and adenocarcinomas per mouse colon; bars, SE. N = number of mice/treatment group. Unpaired Student’s t-test.
Discussion
In the United States, colon cancer is a common disease that kills approximately 50,000 people each year (www.cancer.org). Prevention and early detection by screening, with endoscopic removal of colon adenomas, is effective. However, advanced, non-resectable lesions do not respond well to chemotherapy or radiation. Hence, targeting growth factors and growth factor receptors (e.g. M3R) remains an important therapeutic strategy. The present study was designed to evaluate the role of M3R in colon neoplasia, and to determine whether M3R is a potential target for colon cancer treatment.
Using a well established in vivo model of colon neoplasia and M3R-null mice, we provide strong evidence that M3R, which are commonly expressed in human colon cancer (7), play a key role in colon neoplasia. In both gross and microscopic appearance, colon tumors in AOM-treated animals mimicked human disease (Fig. 4). Our findings reveal that in AOM-treated mice, genetic ablation of M3R attenuates epithelial cell proliferation, and the number of adenomas and adenocarcinomas per mouse colon (65% reduction in the number of adenocarcinomas/colon). Whereas 50% of AOM-treated WT animals had multiple adenocarcinomas/colon, this was not the case with any M3R-deficient animal. Moreover, in M3R-deficient mice the overall colon tumor volume was reduced by 60% compared to that in WT animals. In the colon, potential muscarinic receptor agonists include acetylcholine that is released by enteric neurons or produced by colon cancer cells as autocrine growth factors (unpublished observations). Bile acids which are reported to increase colon cancer risk also interact functionally with M3R (11–13). Collectively, these observations suggest that M3R may play a role in both tumor initiation and promotion; that is, both the number and size of tumors was reduced in M3R-deficient animals. Hence, in addition to being a growth factor receptor, M3R may play an important role in initiation of colon neoplasia.
In general, treatment with AOM stimulated a robust increase in epithelial cell proliferation (Fig. 2A and B). In contrast, there was little apoptosis in colon sections obtained from animals in any treatment group (Fig. 2C and D). Neither treatment with AOM nor ablation of M3R impacted epithelial cell apoptosis as measured by the appearance of activated caspase-3. Therefore, the role of M3R in promoting colon neoplasia appears to depend primarily, if not solely, on pro-proliferative signaling. This observation is consistent with our in vitro observations in human colon cancer cells that post-M3R/EGFR signaling is mediated primarily by the pro-proliferative ERK pathway and that blocking ERK signaling abolishes M3R-agonist-induced cell proliferation (8, 11–13).
From the present data, we cannot comment on the role that co-expression of M1R and M3R in colon epithelial cells plays in colon neoplasia. Previous work indicates that expression of M1R does not increase in M3R-deficient mice, at least not in those tissues studied (17). Since M1R and M3R have a similar G protein coupling profile (2, 3), it is possible that M1R have a similar functional role as M3R in colon epithelial cells (18). This issue can be addressed in future studies by using mice that are deficient in both muscarinic receptor subtypes (18).
Finally, our findings have potentially important implications regarding reducing expression or activation of M3R to prevent or treat colon cancer in humans. Nonetheless, practical translation to clinical trials of the observations presented herein requires demonstration that targeting M3R and/or downstream signaling with chemical inhibitors mimics genetic ablation of M3R, thereby attenuating colon neoplasia.
Acknowledgments
Grant support This work was supported by the Office of Research and Development, Medical Research Service, Department of Veterans Affairs (JPR) and by NIH grants CA107345 (JPR). Nirish Shah and Guofeng Xie were supported by NIH grant T32 DK067872 (JPR).
References
- 1.Bonner T, Buckley N, Young A, Brann M. Identification of a family of muscarinic acetylcholine receptor genes. Science. 1987;237:527–32. doi: 10.1126/science.3037705. [DOI] [PubMed] [Google Scholar]
- 2.Brann MR, Ellis J, Jorgensen H, Hill-Eubanks D, Jones SV. Muscarinic acetylcholine receptor subtypes: localization and structure/function. Prog Brain Res. 1993;98:121–7. doi: 10.1016/s0079-6123(08)62388-2. [DOI] [PubMed] [Google Scholar]
- 3.Wess J. Muscarinic acetylcholine receptor knockout mice: novel phenotypes and clinical implications. Annu Rev Pharmacol Toxicol. 2004;44:423–50. doi: 10.1146/annurev.pharmtox.44.101802.121622. [DOI] [PubMed] [Google Scholar]
- 4.Slack BE. The m3 muscarinic acetylcholine receptor is coupled to mitogen-activated protein kinase via protein kinase C and epidermal growth factor receptor kinase. Biochem J. 2000;348:381–7. [PMC free article] [PubMed] [Google Scholar]
- 5.Gutkind JS, Novotny EA, Brann MR, Robbins KC. Muscarinic acetylcholine receptor subtypes as agonist-dependent oncogenes. Proc Natl Acad Sci USA. 1991;88:4703–7. doi: 10.1073/pnas.88.11.4703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frucht H, Gazdar AF, Park J-A, Oie H, Jensen RT. Characterization of functional receptors for gastrointestinal hormones on human colon cancer cells. Cancer Res. 1992;52:1114–22. [PubMed] [Google Scholar]
- 7.Yang WL, Frucht H. Cholinergic receptor up-regulates COX-2 expression and prostaglandin E(2) production in colon cancer cells. Carcinogenesis. 2000;21:1789–93. doi: 10.1093/carcin/21.10.1789. [DOI] [PubMed] [Google Scholar]
- 8.Frucht H, Jensen RT, Dexter D, Yang W-L, Xiao Y. Human colon cancer cell proliferation mediated by the M3 muscarinic cholinergic receptor. Clin Cancer Res. 1999;5:2532–9. [PubMed] [Google Scholar]
- 9.Cheng K, Zimniak P, Raufman JP. Transactivation of the epidermal growth factor receptor mediates cholinergic agonist-induced proliferation of H508 human colon cancer cells. Cancer Res. 2003;63:6744–50. [PubMed] [Google Scholar]
- 10.Glinghammar B, Rafter J. Carcinogenesis in the colon: interaction between luminal factors and genetic factors. Eur J Cancer Prev. 1999;(Suppl 1):S87–94. [PubMed] [Google Scholar]
- 11.Cheng K, Chen Y, Zimniak P, Raufman JP, Xiao Y, Frucht H. Functional interaction of lithocholic acid conjugates with M3 muscarinic receptors on a human colon cancer cell line. Biochim Biophys Acta. 2002;1588:48–55. doi: 10.1016/s0925-4439(02)00115-1. [DOI] [PubMed] [Google Scholar]
- 12.Cheng K, Raufman JP. Bile acid-induced proliferation of a human colon cancer cell line is mediated by transactivation of epidermal growth factor receptors. Biochem Pharmacol. 2005;70:1035–47. doi: 10.1016/j.bcp.2005.07.023. [DOI] [PubMed] [Google Scholar]
- 13.Cheng K, Xie G, Raufman JP. Matrix metalloproteinase-7-catalyzed release of HB-EGF mediates deoxycholyltaurine-induced proliferation of a human colon cancer cell line. Biochem Pharmacol. 2007;73:1001–12. doi: 10.1016/j.bcp.2006.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boivin GP, Washington K, Yang K, et al. Pathology of mouse models of intestinal cancer: consensus report and recommendations. Gastroenterology. 2003;124:762–77. doi: 10.1053/gast.2003.50094. [DOI] [PubMed] [Google Scholar]
- 15.Takahashi M, Nakatsugi S, Sugimura T, Wakabayashi K. Frequent mutations of the beta-catenin gene in mouse colon tumors induced by azoxymethane. Carcinogenesis. 2000;21:1117–20. [PubMed] [Google Scholar]
- 16.Yamada Y, Yoshimi N, Hirose Y, et al. Frequent beta-catenin gene mutations and accumulations of the protein in the putative preneoplastic lesions lacking macroscopic aberrant crypt foci appearance, in rat colon carcinogenesis. Cancer Res. 2000;60:3323–7. [PubMed] [Google Scholar]
- 17.Yamada M, Miyakawa T, Duttaroy A, et al. Mice lacking the M3 muscarinic acetylcholine receptor are hypophagic and lean. Nature. 2001;410:207–12. doi: 10.1038/35065604. [DOI] [PubMed] [Google Scholar]
- 18.Xie G, Drachenberg C, Yamada M, Wess J, Raufman JP. Cholinergic agonist-induced pepsinogen secretion from murine gastric chief cells is mediated by M1 and M3 muscarinic receptors. Am J Physiol Gastrointest Liver Physiol. 2005;289:G521–9. doi: 10.1152/ajpgi.00105.2004. [DOI] [PubMed] [Google Scholar]
- 19.Inaba M, Kobayashi T, Tashiro T, et al. Evaluation of antitumor activity in a human breast tumor/nude mouse model with a special emphasis on treatment dose. Cancer. 1989;64:1577–82. doi: 10.1002/1097-0142(19891015)64:8<1577::aid-cncr2820640803>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 20.Yan F, John SK, Wilson G, Jones DS, Washington MK, Polk DB. Kinase suppressor of Ras-1 protects intestinal epithelium from cytokine-mediated apoptosis during inflammation. J Clin Invest. 2004;114:1272–80. doi: 10.1172/JCI21022. [DOI] [PMC free article] [PubMed] [Google Scholar]