Abstract
Mn porphyrins are among the most efficient SOD mimics with potency approaching that of SOD enzymes. The most potent ones, Mn(III) N-alkylpyridylporphyrins bear positive charges in a close proximity to the metal site, affording thermodynamic and kinetic facilitation for the reaction with negatively charged superoxide. The addition of electron-withdrawing bromines onto β-pyrrolic positions dramatically improves thermodynamic facilitation for the O2•− dismutation. We have previously characterized the para isomer, MnIIBr8TM-4-PyP4+ [Mn(II) β-octabromo-meso-tetrakis(N-methylpyridinium-4-yl)porphyrin]. Herein we fully characterized its meta analogue, MnIIBr8TM-3-PyP4+ with respect to uv/vis spectroscopy, electron spray mass spectrometry, electrochemistry, O2•− dismutation, metal-ligand stability, and the ability to protect SOD-deficient E. coli in comparison with its para analogue. The increased electron-deficiency of the metal center stabilizes Mn in its +2 oxidation state. The metal-centered MnIII/MnII reduction potential, E½ = + 468 mV vs NHE, is increased by 416 mV with respect to non-brominated analogue, MnIIITM-3-PyP5+ and is only 12 mV less positive than for para isomer. Yet, the complex is significantly more stable towards the loss of metal than its para analogue. As expected, based on the structure-activity relationships, a large increase in E½ results in exceptionally high catalytic rate constant for the O2•− dismutation, log kcat ≥ 8.85; 1.5-fold increase with respect to the para isomer. The IC50 was calculated to be ≤ 3.7 nM. Manipulation of the electron-deficiency of a cationic porphyrin resulted, therefore, in the highest kcat ever reported for a metalloporphyrin, being essentially identical to the kcat of superoxide dismutases (log kcat = 8.84 – 9.30). The positive kinetic salt effect points to the unexpected, unique and first time recorded behavior of Mn β-octabrominated porphyrins when compared to other Mn porphyrins studied thus far. When species of opposing charges react, the increase in ionic strength invariably results in the decreased rate constant; with brominated porphyrins the opposite was found to be true. The effect is 3.5 - fold greater with meta than with para isomer, which is discussed with respect to the closer proximity of the quaternary nitrogens of the meta isomer to the metal center than that of the para isomer. The potency of MnIIBr8TM-3-PyP4+ was corroborated by in vivo studies, where 500 nM allows SOD-deficient E. coli to grow ≥ 60% of the growth of wild type; at concentrations ≥5 μM it exhibits toxicity. Our work shows that exceptionally high kcat for the O2•− disproportionation can be achieved not only with an N5-type coordination motif, as rationalized previously for aza crown ether (cyclic polyamines) complexes, but also with a N4-type motif as in the Mn porphyrin case; both motifs sharing “up-down-up-down” steric arrangement.
Keywords: SOD-mimics, Mn porphyrin, Mn(II) β-octabromo-meso-tetrakis-(N-methylpyridinium-3-yl)porphyrin, MnIIBr8TM-3-PyP4+, SOD-deficient E. coli
Introduction
Superoxide (O2•−) is involved in a variety of pathological and physiological processes. Under pathological conditions the endogenous superoxide dismutases may not efficiently remove the excessive O2•− produced; therefore there is a need for low-molecular weight SOD mimics. We have been developing Mn porphyrin-based SOD mimics for over a decade. We have established structure activity relationship (SAR) for both Mn and Fe porphyrins [1] between the metal-centered redox potential and the rate constant for O2•− dismutation and refined it recently to account for the differently charged/sized Mn porphyrins [2–5]. The introduction of both electron-withdrawing groups and positive charges onto the porphyrin core affords thermodynamic and kinetic/electrostatic facilitation for the approach of superoxide to the redox-cycling metal site [1–4]. The higher the metal-centered reduction potential the higher is the ability of the metalloporphyrin to catalyze O2•− dismutation. Additionally, the ability of Mn porphyrins to eliminate peroxynitrite was found to parallel the O2•− dismuting activity [6] and is, therefore, governed by closely related SAR. Moreover, as a result of the easy reducibility of the potent SOD mimics, their action in vivo may be coupled to cellular reductants (ascorbic acid, tetrahydrobiopterin, less efficiently with glutathione), converting their function to superoxide or ONOO− reductases [6–11].
In addition to the SAR we used SOD-deficient E. coli as a very convenient and valuable tool for evaluating the in vivo ability of the metalloporphyrin to substitute for the native SOD enzyme. Based on E. coli studies we can then predict the therapeutic usefulness of metalloporphyrins. In all cases tested Fe porphyrins killed E. coli therefore in our further studies we focused predominantly on Mn porphyrins.
Our very first compound that proved the validity of our approach was the electron-deficient Mn(II) β-octabromo-meso-tetrakis(N-methylpyridinium-4-yl)porphyrin, MnIIBr8TM-4-PyP4+ (Fig. 1), that has the log kcat = 8.34 and E½ = +480 mV vs NHE [12] and is protective to E. coli at nM levels. Our further synthetic efforts in searching for a potential therapeutic based on the SAR led us to Mn(III) ortho N-alkylpyridylporphyrins as the most potent candidates for forwarding them into animal models where superoxide-mediated damage is involved. Thus remarkable effects were observed in cancer, radiation, diabetes, ALS, Alzheimer’s disease etc [13–23]. Further, along with our mechanistic studies we observed a dramatic impact of the positive charges of the Mn porphyrin on the O2•− dismutation; the rate constants are more than two orders of magnitude higher when compared to the neutral or negatively charged porphyrins [3,4]. Thus, MnIIITE-2-PyP5+ is 130-fold more potent SOD mimic than its singly charged MnIIIBr8T-2-PyP+ [3], and 400-fold more potent than its negatively charged analogue [MnIIIBr8TSPP]3− [4]. Charge distribution had also remarkable impact [2]. In summary both metal-centered redox potential and charges in close proximity to the metal site have a major impact on the antioxidant potency of the Mn porphyrin. As our knowledge on porphyrins increases it became obvious that another major factor is their in vivo availability [2,4,24,25]. The optimal combination of thermodynamics, kinetics, bioavailability and low toxicity will determine the best drug candidate.
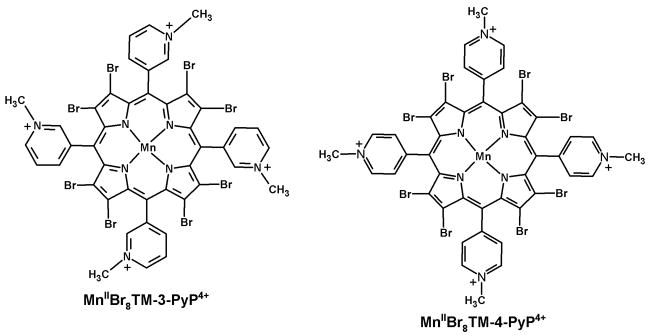
Figure 1.
Schematic structures of the MnIIBr8TM-3-PyP4+ and MnIIBr8TM-4-PyP4+.
Our first report on a potent ortho isomer, MnIIITM-2-PyP5+, in protecting SOD-deficient E. coli indicated that the meta isomer, MnIIITM-3-PyP5+, although ~10-fold inferior with respect to in vitro antioxidant potency [1] may still be effective in vivo [26]. Indeed, meta isomer was comparable to the ortho analogue in providing protection to SOD-deficient E. coli to grow aerobically in restricted five amino acid-medium while para isomer, MnTM-4-PyP5+, was toxic [26]. The reason likely lies in lesser bulkiness of the meta isomer, which allows it to accumulate within E. coli at higher levels than the ortho analogue [26]. Para isomer was toxic due to its interactions with nucleic acids [26]. Guided by these collective in vitro, in vivo, and SAR results [1–5,12,24–26], we resumed herein our studies on the meta Mn(III) N-alkylpyridylporphyrins by preparing a brominated derivative of MnIIITM-3-PyP5+. This is the first report on the synthesis and characterization of MnIIBr8TM-3-PyP4+ (Fig. 1). We also compared its in vitro and in vivo properties to those of the para analogue, MnIIBr8TM-4-PyP4+ (Fig. 1) [12]. The synthesis of an ortho analogue, for mechanistic purposes, has not been achieved as its electron-deficiency would likely preclude the existence of a Mn complex stable enough for isolation and characterization.
Experimental
Materials
Meso-tetrakis(N-methylpyridinium-3-yl)porphyrin (chloride salt) was purchased from MidCentury Chemicals (Chicago, IL) and used as received. MnIIITM-2-PyP5+ (chloride salt) [26] and MnIIITM-3-PyP5+ (chloride salt) [26] were prepared and characterized as previously described.
Xanthine and equine ferricytochrome c (lot no. 7752 [27,28]) were from Sigma, whereas xanthine oxidase was prepared by R. Wiley [29] and was a gift from K.V. Rajagopalan.
MnCl2·4H2O and CuCl2·2H2O were purchased from J. T. Baker and Aldrich, respectively. Anhydrous N,N-dimethylformamide (DMF, Sigma-Aldrich Chemical Co) was kept over 4 Å molecular sieves. Bromine and NaCl were from Aldrich, whereas ethyl ether (anhydrous), acetone, and 2-propanol were from EMD. Methanol was from Mallinckrodt. All other chemicals and solvents were of analytical grade and used without further purification.
Methods
Spectral analyses
The uv/vis measurements were performed using a Shimadzu UV-2501PC spectrophotometer at 0.5 nm resolution. The elemental analyses were conducted by Atlantic Microlabs (Norcross, GA, USA).
The mass spectrometric (ESI: electrospray ionization) measurements were performed on an Applied Biosystems MDS Sciex 3200 Q Trap liquid chromatography/MS/MS spectrometer at the Duke Comprehensive Cancer Center, Shared Resources. All porphyrins were analyzed using 1:1 acetonitrile:water solutions, at 20 and 30 V cone voltages as described previously [11,27].
Electrochemistry
The cyclic voltammetry was carried out on a CH Instruments Model 600 Voltammetry Analyzer [1]. A three-electrode system consisted of a 3 mm-diameter glassy carbon button working electrode (Bioanalytical Systems), the Ag/AgCl reference electrode, and a Pt auxiliary electrode was used in a small volume cell (3.0 mL). Helium-purged solutions contained 0.05 M phosphate buffer (pH 7.8), 0.1 M NaCl, and 0.5 mM metal-free porphyrin or Mn porphyrin. The scan rates were 0.01–0.5 V s−1, typically 0.1 V s−1. The potentials were standardized against MnIIITE-2-PyP5+ (chloride salt) (E½ = +228 mV vs NHE [1]). Also another experiment was done where the voltammogram was measured first in 0.09 M phosphate buffer only, then solid NaCl was added to yield a 0.1 M Cl− concentration and the voltammogram was recorded again. This was devised to assess the possible chloride binding to the Mn porphyrin axial site.
Synthesis of β-octabromo-meso-tetrakis(N-methylpyridinium-3-yl)porphyrinato-manganese(II) chloride, MnIIBr8TM-3-PyPCl4
This fully β-brominated complex was prepared from H2TM-3-PyP4+ in four steps. The first three steps were based on the synthetic protocol reported by Richards et al [30] for the synthesis of para-isomer (H2TM-4-PyP4+) derivatives.
First, CuIITM-3-PyPCl4 was prepared by the metallation of H2TM-3-PyPCl4 (67.1 mg, 0.0818 mmol) with CuCl2:2H2O (109 mg, 0.638 mmol) in a CH3OH:H2O mixture (10:1, 12 mL) at 45°C. After 1 hour, the volume was reduced to approximately 5 mL in a rotavapor and 5 mL of 2-propanol was added to precipitate the copper porphyrin. The solid was filtrated, washed with 2-propanol until the washings were colorless, and dried under vacuum at room temperature. This compound was carried forward to bromination (second step) without further purification. A Br2 (0.2 mL, 4 mmol) solution in DMF (2 mL) was added dropwise over 30 minutes to a well-stirred solution of CuIITM-3-PyP4+ (72.1 mg, 0.0818 mmol) in DMF (8 mL) at room temperature. The color of the solution changed immediately from red to green. The reaction course was monitored by uv/vis and by TLC (silica gel, 1:1:8 saturated aqueous KNO3:H2O:MeCN mixture as eluent). After 48 hours the reaction was quenched by the addition of approximately 5 mL of water, which precipitated the brominated compound. The copper brominated porphyrin was collected by filtration and washed first with water (3 × 3 mL) then with 3 mL of a 1:1 etanol:2-propanol mixture (excess Br2 in the filtrate can be quenched by sodium metabisulfite prior to disposal). The green powder was air-dried and then dissolved in a minimal amount of water. The copper porphyrin was precipitated as the PF6− salt by the addition of a concentrated aqueous solution of NH4PF6. The precipitate was filtered off, thoroughly washed with diethyl ether, and air-dried. The resulting solid was then dissolved in acetone and re-precipitated as the chloride salt by the addition of saturated tetrabutylammonium chloride solution in acetone. The CuIIBr8TM-3-PyPCl4 complex was washed thoroughly with acetone and dried under vacuum at room temperature. Yield: 65 mg (53 % based on starting H2TM-3-PyP4+). Uv/vis (0.05 M phosphate buffer, pH 7.8): 266.0 nm (ε/M−1 cm−1, 15,239), 378.5 (13,686), 447.0 (55,239), 472.5 (sh; 35,405), 588.0 (7,526). ESI-MS (1:1, H2O:MeCN): clusters centered at m/z 343.2 (CuIIBr8TM-3-PyP4+/4) and m/z 469.4 ([CuIIBr8TM-3-PyP4+ + Cl−]3+/3).
In the third step, the CuIIBr8TM-3-PyP4+ complex (45.6 mg, 0.0301 mmol) was demetallated by the slow addition of a concentrated sulfuric acid (6.0 mL, 111 mmol) while cooled to 10°C. Upon dissolution, the color of the reaction mixture changed immediately from green to greenish orange; cooling was discontinued and the reaction mixture was allowed to reach the room temperature. The acid solution was left under stirring at room temperature for 4 hours, after which it was carefully poured over the ice (~64 g). Once the ice melted the octabrominated free-base porphyrin was precipitated as the PF6− salt by the addition of a concentrated NH4PF6 (aq) solution. The precipitate was recovered by filtration, washed first with water (2 × 3 mL) then with a 1:1 diethyl ether:2-propanol mixture (5 mL), and air-dried. The resulting solid was then dissolved in acetone and precipitated as the chloride salt by the addition of saturated tetrabutylammonium chloride solution in acetone as with CuIIBr8TM-3-PyP4+ to yield the corresponding free-base. Yield: 41.5 mg (95 % based on starting CuIIBr8TM-3-PyP4+). Anal. Calcd for H2Br8TM-3-PyPCl4·9H2O, C44H48Br8N8O9Cl4: C, 32.74; H, 3.00; N, 6.94. Found: C, 32.66; H, 3.03; N, 6.84. Uv/vis (0.05 M phosphate buffer, pH 7.8): 270.5 nm (ε/M−1 cm−1, 26,351), 508.0 (95,663), 646.0 (6,375), 729.0 (8,104). ESI-MS (1:1, H2O:MeCN): clusters centered at m/z 328.0 (H2Br8TM-3-PyP4+/4) and m/z 436.8 ([H2Br8TM-3-PyP4+ + e−]3+/3).
In the last step the MnIIBr8TM-3-PyP4+ was synthesized using a procedure adapted from that described for the para-isomer MnIIBr8TM-4-PyP4+ [12]. H2Br8TM-3-PyP4+ (38.3 mg, 0.0264 mmol) was dissolved in 10 mL of water and the pH was then raised to 11.2 by the addition of ~30μL of 1 M NaOH. This increase in pH was accompanied by a color change from green to reddish pink. A 2-fold excess of MnCl2·4H2O (10.5 mg, 0.0528 mmol) was added followed by a drop in pH from 11.2 to ~9 along with instantaneous color change from reddish pink to green. Metathesis of the anions of this Mn porphyrin with NH4PF6 to yield the PF6− salt, and subsequently with tetrabutylammonium chloride in acetone to yield the chloride salt of MnIIBr8TM-3-PyP4+ was carried out as with CuIIBr8TM-3-PyP4+. Yield: 36.6 mg (92 % based on starting H2Br8TM-3-PyP4+). Anal. Calcd for MnIIBr8TM-3-PyPCl4·5H2O·NH4PF6, C44H42Br8N9O5Cl4PF6Mn: C, 30.06; H, 2.41; N, 7.17. Found: C, 29.82; H, 2.86; N, 7.43. Uv/vis (0.05 M phosphate buffer, pH 7.8): 265.5 nm (ε/M−1 cm−1, 31,689), 415.5 (34,248), 483.0 (84,693), 599.0 (10,053). ESI-MS (1:1, H2O:MeCN): clusters centered at m/z 340.7 (MnIIBr8TM-3-PyP4+/4) and at m/z 465.5 ([MnIIBr8TM-3-PyP4+ + Cl−]3+/3). Of note, when a 20-fold excess of Mn was used, as in the standard metallation procedure for the non-brominated analogues [26], the pH dropped too much (to ~6.5), due to the extensive hydrolysis of Mn, and prevented a completion of metallation.
Superoxide Dismutase Activity
The catalytic rate constants for the O2•− disproportionation were determined by the cytochrome c (cyt c) assay [31,32]. We have previously compared the kcat determined by pulse radiolysis and with cyt c assay for several of our Mn porphyrins and MnCl2 [27,32]. The kcat determined by both methods were the same, which justified using a convenient cyt c assay for testing our prospective SOD mimics. The xanthine/xanthine oxidase (40 μM xanthine, ~2 nM xanthine oxidase) was the source of O2•−and ferricytochrome c was used as the indicating scavenger of O2•−. The reduction of cyt c was followed at 550 nm. Assays were conducted in 0.05 M phosphate buffer, pH 7.8 in the presence and absence of 0.1 mM EDTA to account for any metal impurity. A 2μM Mn porphyrin stock solution (in 0.05 M phosphate buffer, pH 7.8) was made without EDTA and allowed to reach the metal/ligand equilibrium (~10 min), after which the solution was used for cyt c assay (see Fig. 2 bellow). Rate constants for the reactions of Mn porphyrins with O2•− were based upon the competition with 10 μM cyt c, kcyt c = 2.6 × 105 M−1 s−1 [31,32]. The O2•− was produced at 1.2 μM min−1. Any possible interference of Mn porphyrins with production of O2•− was examined following urate formation at 295 nm in the absence of cyt c. No reoxidation of cytochrome c by metalloporphyrins was observed. The effect of ionic strength upon the rate constant of dismutation was determined as above using either NaCl (in the range of 0 to 0.10 M) or phosphate (in the range of 0.05 to 0.087 M) as ionic strength modifier. All measurements were done at (25 ± 1)°C and unaffected by the presence or absence of 15 μg mL−1 catalase.
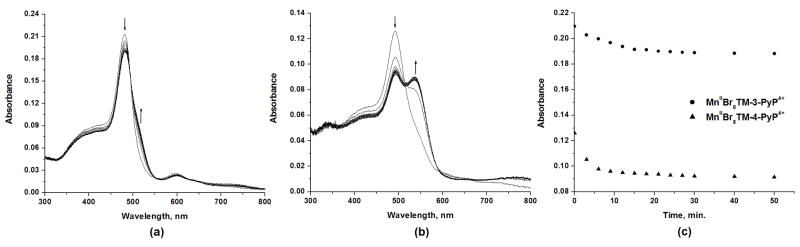
Figure 2.
Partial demetallation of ~2 μM solutions of (a) MnIIBr8TM-3-PyP4+ and (b) MnIIBr8TM-4-PyP4+ in 0.05 M phosphate buffer (pH 7.8). (c) Decay of the Soret bands of MnIIBr8TM-3-PyP4+ (•, 484 nm) and MnIIBr8TM-4-PyP4+ (▲, 492 nm) during the demetallation process.
We have previously observed that the SOD-like activity of the para isomer MnIIBr8TM-4-PyP4+ was the same with or without EDTA [12]. We confirmed such an observation in this work and verified that the SOD activity of MnIIBr8TM-3-PyP4+ is also unaffected by the presence or absence of EDTA in the assay, if EDTA was added into the cuvette about the time the cyt c reduction was to be followed at 550 nm. When EDTA was added to the MnIIBr8TM-3-PyP4+ stock solution a considerable loss of Mn during the collection of data affected significantly the consistency in kcat determination.
The SOD activity of metal-free ligand was assessed also. The 5 μM stock solution was made with 1 mM EDTA in the 0.05 M phosphate buffer; EDTA was added to prevent any metallation that could otherwise occur with traces of metals present in the solution.
Based on the linear relationship between the E1/2 (MnIII/MnII) and the acidity of the inner pyrrolic nitrogens [1], the slightly less positive E1/2 of the β-octabrominated Mn porphyrin (MnIIBr8TM-3-PyP4+, see below) indicates that the pKa2 of the free base meta isomer is only slightly higher than the pKa2 = 6.5 of the para isomer [30] (pKa2 relates to the equilibrium: H2P4+ = HP3+ + H+). Consequently, there may be < 10% of the diprotonated species (H2Br8TM-3-PyP4+) present at pH 7.8. Thus, in all experiments performed at pH 7.8, we assumed that the HBr8TM-3-PyP3+ is the major species in solution.
E. coli growth experiments
Escherichia coli strains used in this study were AB1157, wild type (F-thr-1; leuB6; proA2; his-4; thi-1; argE2; lacY1; galK2; rpsL; supE44; ara-14; xyl-15; mtl-1; tsx-33), and JI132, SOD-deficient, sodA−sodB− (same as AB1157 plus (sodA::mudPR13)25 (sodB-kan)1-Δ2). Both strains were obtained from J. A. Imlay [33]. The experiments were carried out as described in detail by Rebouças et al [2]. Briefly, cultures were grown aerobically in a five-amino acid medium in 96-well flat-bottom microtiter plates [34] on a thermostatic shaker at 37°C and 200 rpm. Approximately 1 mM stock solutions of brominated porphyrins were prepared immediately before the start of the experiment, and the exact concentration determined by uv/vis spectroscopy. They were then diluted to μM solutions which were added to the medium. The effect of metal-free porphyrin, Mn porphyrins, and MnCl2 on the growth of these strains was followed turbidimetrically at 700 nm (to minimize the interference of the compounds studied) and compared to the growth curves of both strains in the absence of these compounds (controls). Three experiments were performed and the data from a representative one are shown herein.
Results and Discussion
In order for a Mn porphyrin to be an efficient SOD mimic, its reduction potential should be around the midway between the potential for the reduction and oxidation of O2•−, i.e. ~ +300 mV as is the case with the enzymes themselves [35–37]. Unsubstituted Mn(III) porphyrins are stable in Mn +3 oxidation state thus can not oxidize O2•− in the first step of a catalytic cycle of a O2•− dismutation process. The increase in the electron-deficiency of the Mn(III) porphyrin by introducing electron-withdrawing groups on its core results in an increased metal-centered reduction potential, stabilizes the Mn +2 oxidation state, and thus makes Mn(III) porphyrins more readily reducible by O2•−. That in turn leads to the increase in catalytic rate constants for O2•−dismutation as described by structure-activity relationships [1,2,4]. The most potent porphyrins that have the E1/2 around the midway potential for the O2•− reduction and oxidation have equal thermodynamic facilitation for both half-reactions (Equations 1 and 2) of the catalytic cycle.
| (1) |
| (2) |
Consequently, the kox and kred are nearly identical as is the case with SOD enzymes [38,39] themselves, and with our porphyrins [3,40]. As the redox potential increases further the oxidation of Mn(II) porphyrin becomes rate-limiting step, as is the cases with octabrominated porphyrins, leading eventually to a drop in kcat; a bell-shaped curve in the kcat vs E½ plot is observed [2,4,41].
We have further shown that the placement and distribution of charges close to the metal site are other major factors contributing to the SOD-like potency of Mn porphyrins [2–4]. Finally, the appropriate lipophilicity and the size would greatly impact the in vivo activity of these compounds [11,24].
We have shown that ortho isomers of Mn(III) alkylpyridylporphyrins have thus far been the most potent porphyrinic compounds in vitro, with log kcat being as high as 8.60 [42], and have proven remarkably effective in vivo [41]. Whereas ortho, meta, and para N-alkylpyridylporphyrins share the similar hydrophilicity, the ortho isomers are relatively more bulky (when compared to the para and meta analogues), which may limit to some extent their in vivo efficacy. In an E. coli experiment [26], the meta isomer MnIIITM-3-PyP5+, which is significantly less potent in vitro than its ortho analogue, showed in vivo efficiency comparable to the ortho isomer in protecting SOD-deficient E. coli to grow aerobically. The planar para isomer, while equally able antioxidant as meta in vitro, is inferior to the meta analogue in vivo; we have seen in our E. coli model of oxidative stress that the increased interactions of the para isomers with nucleic acids introduces toxicity [26]. At the concentration levels where no toxicity of the meta isomer is observed, equimolar levels of the para isomer did not allow for the aerobic growth of SOD-deficient E. coli in five amino acid medium.
We have shown already that the introduction of bromines onto β-pyrrolic positions of MnIIITM-4-PyP5+ increases the E½ from +60 to 480 mV [12]. That is accompanied by ca two orders of magnitude increase in kcat [12]. Herein we synthesized the meta analogue, MnIIBr8TM-3-PyP4+, which was fully characterized by uv/vis spectroscopy, elemental analysis, electron spray ionization mass spectrometry, and electrochemistry.
Uv/vis spectroscopy
Spectral properties of MnIIBr8TM-3-PyP4+ given in Experimental section are very similar to those of its para analogue. The addition of 8 bromines increases electron-deficiency in both isomers, which in turn stabilizes the Mn in its +2 oxidation state. This, in association with the non-planar conformation imposed by the bromines, affects significantly the metal/ligand stability of these brominated compounds (Fig. 2). Both the extent and the rate of demetallation of these complexes are concentration-dependent. At concentrations greater than 10 μM in phosphate buffer (pH 7.8) the complexes are stable for hours, whereas at lower concentrations (e.g., 2 μM; Fig. 2) both complexes (MnIIBr8TM-3-PyP4+ and MnIIBr8TM-4-PyP4+) show an initial rapid loss of Mn (~10 min), after which they reach metal/ligand equilibrium and no significant loss of the metal is further observed within hours (Fig. 2c); the demetallation is much faster with para than with meta isomer (Fig. 2b and 2a, respectively). This behavior is not altered by the presence or absence of excess chloride, which is of importance for the measurements of the kinetic salt effects on kcat. Of note, both Mn porphyrins are stable for days at mM concentration in the phosphate buffer (pH 7.8). Slightly less positive E1/2 of the meta isomer than of the para analogue (Table 1) indicates a less electron-deficient metal center, resulting in a more stable meta metal/ligand complex.
Table 1.
Electrochemical data for free-base porphyrins and their Mn complexes in aqueous solutionsa
| Porphyrins | E½, mV vs NHE | Ref. |
|---|---|---|
| H2TM-3-PyP4+ | −212b (268)c | this work |
| MnIIITM-3-PyP5+ | +52 | [26] |
| HBr8TM-3-PyP3+ | +122b (233)c | this work |
| MnII Br8TM-3-PyP4+ | +468 (93)c | this work |
| MnIIITM-4-PyP5+ | +60d | [26] |
| MnII Br8TM-4-PyP4+ | +480 | [12] |
| Cu,Zn-SOD | ca. +300 | [35–37] |
Conditions: 0.5 mM porphyrin, 0.05 M phosphate buffer, pH 7.8, 0.1 M NaCl, scan rate 0.1 V s−1.
Irreversible wave.
Cathodic/anodic peak-to-peak separation.
We are routinely studying the reducibility of Mn porphyrins with cellular reductants because they can possibly act in vivo as superoxide reductases, rather than superoxide dismutases [2,11,27]. While the stabilization of the Mn +2 oxidation state in β-octabrominated meso N-methylpyridylporphyrins precludes the assessment of their reducibility, the highly positive E1/2 indicates that outstandingly electron-deficient systems will be much more readily reduced (once oxidized) that Mn porphyrins that are stable in +3 oxidation state, as is MnTE-2-PyP5+.
Electrochemistry
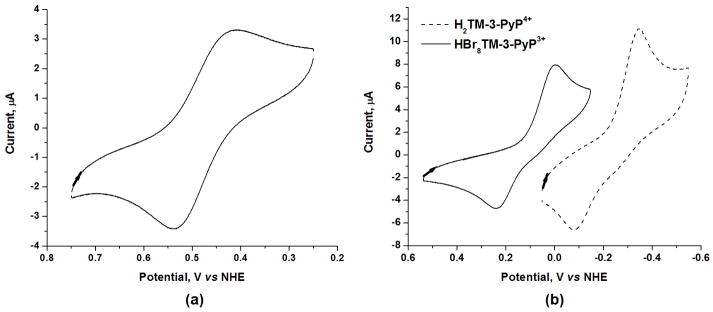
The voltammogram of the Mn complex MnIIBr8TM-3-PyP4+ shows a reversible process associated with the MnIII/MnII redox couple with E½ = +468 mV vs NHE (Fig. 3a), whereas the irreversible process with E½ = +122 mV vs NHE (Fig. 3b) was observed in the voltammogram of the brominated free-base porphyrin. The metal-centered reduction potential of the Mn porphyrin complex and the ring-centered reduction potential of the free-base porphyrin are similarly affected by the bromination of the pyrrolic positions. An anodic shift of 418 mV for the metal-centered process in MnIIBr8TM-3-PyP4+ (as compared to that of MnIIITM-3-PyP5+) was observed (Table 1) and such shift of 52 mV/Br is comparable to that reported for other β-brominated Mn porphyrins [12,43-45]. Likewise, the introduction of bromine atoms on the β-pyrrole positions of metal-free ligand, H2TM-3-PyP4+ resulted in an anodic shift in the reduction potential by 334 mV (i.e., 42 mV/Br, Table 1). Worth noting is that the reduction potential of HBr8TM-3-PyP3+ is 70 mV more positive than that of the non-brominated Mn complex, MnIII/IITM-3-PyP5+/4+ (E½ = +52 mV vs NHE, Table 1). That suggests that the brominated free-base porphyrin itself may be able to catalyze O2•− dismutation. No change in the reduction potential of MnIIBr8TM-3-PyP4+ in 0.05 M phosphate buffer (pH 7.8) was observed by increasing the ionic strength with either NaCl or phosphate buffer, which is of relevance to the kinetic salt effect measurements (see below); this suggested no chloride binding in 0.05 M phosphate buffer.
Figure 3.
Cyclic voltamogram of (a) 0.5 mM MnIIBr8TM-3-PyP 4+ and (b) 0.5 mM HBr8TM-3-PyP3+ and 0.5 mM H2TM-3-PyP4+. Conditions: 0.05 M phosphate buffer (pH 7.8), 0.1 M NaCl, scan rate 0.1 V s−1.
SOD activity (cytochrome c assay)
The higher metal/ligand stability of the brominated meta isomer MnIIBr8TM-3-PyP4+ (Fig. 2) allowed an easier assessment of its SOD-like activity as compared to its para analogue. In our previous work on MnIIBr8TM-4-PyP4+, however, the initial rapid loss of Mn (reported herein) to yield an “equilibrated” solution was not accounted for. Given the extent of demetallation and the rapid nature of this process with brominated para isomer, it is essentially very difficult under our experimental conditions to obtain a kcat that could accurately reflect the actual concentration of the Mn complex at nM conditions. The previously reported rate constant of log kcat = 8.34 corresponds to the situation where no demetallation was assumed; it represents, thus, the lower limit for kcat for MnIIBr8TM-4-PyP4+. If a minimum 30% loss of Mn is value for MnII considered, as suggested by the data in Fig. 2, an estimated log kcat Br8TM-4-PyP4+ is > 8.67.
With the brominated meta isomer the situation is better defined as this compound is considerably more stable towards demetallation than its para analogue (Fig. 2). Assuming that no demetallation occurs, the log kcat measured for MnIIBr8TM-3-PyP4+ is 8.80. Given that the loss of Mn2+ is ~10% during first several minutes (Fig. 2c), the log kcat is estimated to be at least 8.85 which is essentially identical to the kcat for the SOD enzymes [25–27] (Table 2). Thus, the bromination of MnIIITM-3-PyP5+ to yield MnIIBr8TM-3-PyP4+ increased the catalytic rate constant by ~160-fold. The data are summarized in Table 2.
Table 2.
SOD-like activity of the porphyrins studied.a
| Porphyrin | IC50, Mb | log kcatc | Ref. |
|---|---|---|---|
| MnIIITM-3-PyP5+ | 6.5 × 10−7 | 6.61 | [26] |
| MnIIBr8TM-3-PyP3+ | ≤3.7 × 10−9 | ≥8.85 | this work |
| MnIIITM-4-PyP5+ | 6.7 × 10−7 | 6.58 | [26] |
| MnIIBr8TM-4-PyP4+ | ≤5.6 × 10−9 | ≥8.67 | this work |
| Cu,Zn-SOD | 1.3 × 10−9 | ca. 9 | [35–37] |
Conditions: 0.05 M phosphate buffer, 0.10 mM EDTA, pH 7.8, 40 μM xanthine, ~ 10 μM cytochrome c.
IC50 is the concentration of compound that causes a 50% inhibition of the cyt c reduction by O•−. The errors in IC50 are within 10%.
Error of ± 10 %.
Kinetic salt effect
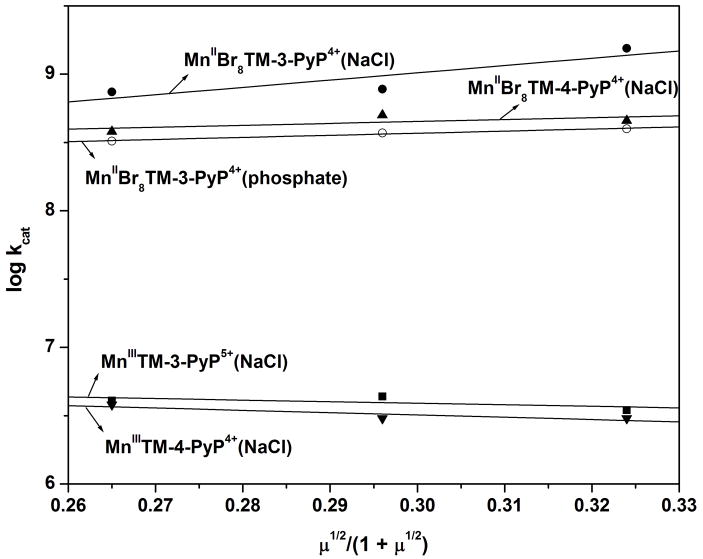
The kinetic salt effect (KSE) was determined as previously described [2,3]. In all cases where the reaction of cationic porphyrins with anionic superoxide was studied an increase in ionic strength resulted in a decrease in kcat, the effect being much less pronounced with meta and para isomers than with ortho isomers [2,3]. In a phosphate buffer of different concentrations (i.e., ionic strength), a small positive kinetic salt effect on the kcat values for MnIIBr8TM-3-PyP4+ was observed. Surprisingly, however, when NaCl was used to adjust the ionic strength, an increase in ionic strength resulted in a significant increase in kcat for this meta isomer (Fig. 4). For the brominated para isomer MnIIBr8TM-4-PyP4+, a positive KSE was also observed, but to a smaller extent than that found for its meta counterpart. For the non-brominated meta and para Mn porphyrins [2,3] (Fig. 4), a negative KSE indicated that the species of opposite charge are involved in the dismuting rate-limiting step; the small value of KSE shows that the electrostatics contributes very little to the catalytic rate constant of these complexes. This implies that the charges of the meta and para pyridinium moieties are considerably far away from the active site (Mn center) to provide suitable electrostatic facilitation for the superoxide dismutation. With brominated complexes, however, the positive KSE observed implies that species of similar charges are involved in the dismuting rate-limiting step, i.e. there is an overall negative electronic density nearby the Mn center in the brominated Mn complexes. A first hypothesis considered to explain the increase in electronic density near the Mn site was the one that invokes an axial coordination of chloride/phosphate to the Mn center, which would increase the formal charge on the Mn. The binding of Cl− would be expected to affect both E½ and the stability of the Mn complex, but neither was corroborated experimentally, i.e., no change in E½ or rate of Mn loss was observed as the chloride concentration increased. This suggests that the KSE for the brominated Mn complexes relates not to a change in the first but in the second coordination sphere, which has been documented for the free-base H2TM-4-PyP4+ [46]. An increased ion-pairing of the anion (phosphate and/or chloride) in a close proximity to the brominated porphyrin core [46] would result in an overall negative charge density near the Mn center and, thus, in a positive KSE. It is worth noting that the KSE for MnIIBr8TM-3-PyP4+ is considerably greater than that of MnIIBr8TM-4-PyP4+, which is consistent with the closer proximity of the quaternary nitrogens of the meta isomer to the metal center than that of the para isomer. To the best of our knowledge this is the first report on the positive KSE when cationic porphyrins encountered anionic species.
Figure 4.
Kinetic salt effect on the SOD activity of Mn porphyrins. The kcat values were determined by the cyt c assay in 0.05 M phosphate buffer, 0.1 mM EDTA at 25 ± 1°C. Ionic strength (μ) adjusted with NaCl (•, ▲, ■, ▼) or phosphate (○).
Protection of aerobic growth of SOD-deficient E. coli
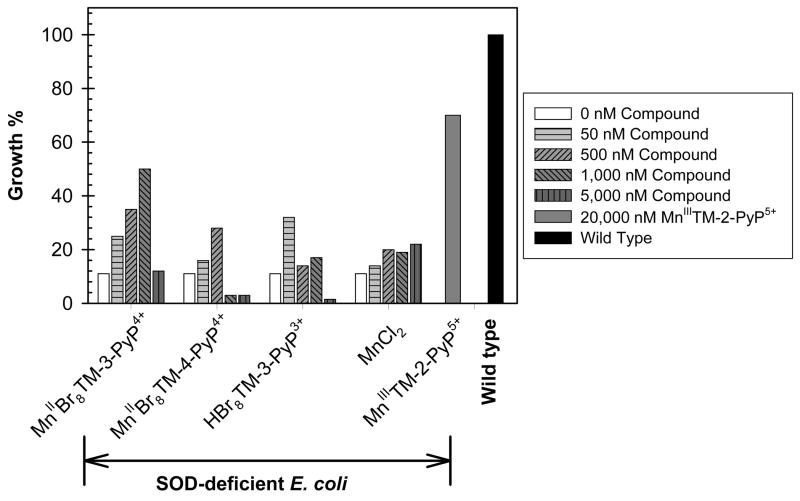
We have adopted the growth of SOD-deficient E. coli in the restricted five amino acids-medium as a reliable tool for evaluating the ability of a MnP to substitute for SOD. In all previous studies the data obtained with E. coli parallel the trends obtained in in vivo animal model studies [1–5,11–28,37]. Here we compare the effect of MnIIBr8TM-3-PyP4+, its metal-free porphyrin HBr8TM-3-PyP3+, and the para analogue MnIIBr8TM-4-PyP4+ on the aerobic growth of an SOD-deficient E. coli strain. The fairly low metal-ligand stability of these Mn porphyrins allow for metal loss during E. coli growth. To account for such an event we have compared the effect of Mn porphyrins on E. coli growth to that of MnCl2. MnIIITM-2-PyP5+ (or ethyl analogue, MnIIITE-2-PyP5+) is usually used as a positive SOD-active control compound in most of our studies [2,4]. We show here (Fig. 5) that the high kcat of the meta β-brominated Mn(II) porphyrin MnIIBr8TM-3-PyP4+ allows it to be extremely effective at nM levels and offer full protection up to 1 μM concentration. At the concentration range used, MnCl2 is hardly protective, indicating that MnIIBr8TM-3-PyP4+ indeed acts in its own right as an SOD mimic or as a carrier of Mn into the cell in a similar fashion as suggested for MnIIIBr8TSPP3− [4]. The same may be true for the brominated metal-free ligand HBr8TM-3-PyP3+. It is less efficient as an SOD mimic in its own right, due to the lower kcat; however β-octabrominated metal-free porphyrins are severely distorted and well known for their ability to coordinate metals promptly at room temperature under neutral pH [12,30,44]. HBr8TM-3-PyP3+ can thus readily complex metal from medium in situ, whereby acting in the same way as MnII Br8TM-3-PyP 4+. Both MnII Br8TM-3-PyP 4+ and HBr8TM-3-PyP3+ are toxic at high levels, which may be due to the presence of bromines and/or the scavenging of metals that are essential for E. coli growth. The para β-brominated Mn(II) porphyrin MnIIBr8TM-4-PyP4+ is less effective at protecting SOD-deficient E. coli than its meta isomer, which is consistent with the lower metal/ligand stability of the para isomer. We further showed that the para isomer is more toxic than its meta analogue which is presumably due to the lesser hindrance of the peripheral para N-methylpyridyl groups, resulting in increased, yet unfavorable/toxic interactions with nucleic acids [26]. It may thus be that the lower efficacy of MnIIBr8TM-4-PyP4+ at ≤ 500 nM compared to its meta analogue, MnIIBr8TM-3-PyP4+ is at least in part due to its higher toxicity. At concentration levels where brominated porphyrins are protective MnIIITM-2-PyP5+ is ineffective, which is consistent with our previous data that full protection is obtained at ~ 20 μM [26]. Our data indicate that the size of the molecule did not have a significant impact on the ability of the compound to substitute for SOD in E. coli. The same has been observed with fairly big lipophilic hexyl porphyrin, MnIIITnHex-2-PyP5+ (log kcat = 7.48) [24], which was also able to protect SOD-deficient E. coli at submicromolar levels.
Figure 5.
Aerobic growth of SOD-deficient E. coli JI132 strain in the presence or absence of porphyrins or MnCl2 (at 50, 500, 1000 or 5000 nM) in 5AA medium at the 13th hour. Readings were taken at 700 nm. Although “HBr8TM-3-PyP3+” was added to the medium as the metal-free porphyrin, the protective effect of this compound maybe at least in part due to its ability to complex Mn from the medium (see text). Aerobic growth of the wild type E. coli AB1157 was used as control.
In conclusions, a manipulation of the electron-deficiency of a cationic porphyrin resulted in the highest kcat ever reported for a metalloporphyrin, being essentially identical to the kcat of superoxide dismutases. Our data show that such high thermodynamic and kinetic facilitation, which leads to an exceptionally high kcat for the O2•− disproportionation, can be achieved not only with an N5-type coordination motif, as rationalized previously for aza crown ether (cyclic polyamines) complexes [47-49], but also with a N4-type motif as in the Mn porphyrin case. Interestingly, both aza crown ethers and β-octabrominated porphyrins [12] share a common alternating “up-down-up-down” steric arrangement of the nitrogens around the Mn center, which is presumably critical for the dismutation process [47–49]. Thus, future work on molecules that possess appropriate geometries for efficacious catalysis is highly justifiable.
Acknowledgments
JSR and IBH acknowledge the support by the National Institutes of Health (IR21-ESO/3682) and the National Institutes for Allergy and Infectious Diseases (U19AI067798) grants. GDS and YMI acknowledge the research grants of the Brazilian Research Council (CNPq) and the Fundação do Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG). IS thanks NIH/NCI Duke Comprehensive Cancer Center Core Grant (5-P30-CA14236-29). LB acknowledges grant MB07/04 from Kuwait University. The excellent technical assistance of Fatima Sequeira and Milini Thomas is acknowledged.
Abbreviations
- Meso
refers to the substituents at the 5,10,15, and 20 positions (meso carbon) of the porphyrin
- β
refers to the substituents at the 2,3,7,8,12,13,17, and 18 positions (β-pyrrole carbons) of the porphyrin
- cyt c
cytochrome c
- DMF
N,N-dimethylformamide
- EDTA
sodium salt of ethylenediaminetetracetic acid
- ESI
electrospray ionization
- H2P4+ and HP3+
any metal free porphyrin (di and monoprotonated)
- H2TM-3-PyP4+
meso-tetrakis(N-methylpyridinium-3-yl)porphyrin
- H2TM-4-PyP4+
meso-tetrakis(N-methylpyridinium-4-yl)porphyrin
- CuIITM-3-PyP4+
copper(II) meso-tetrakis(N-methylpyridinium-3-yl)porphyrin
- CuIIBr8TM-3-PyP4+
copper(II) β-octabromo-meso-tetrakis(N-methylpyridinium-3-yl)porphyrin
- HBr8TM-3-PyP3+
β-octabromo-meso-tetrakis(N-methylpyridinium-3-yl)porphyrin
- MnIIBr8TM-3-PyP4+
manganese(II) β-octabromo-meso-tetrakis(N-methylpyridinium-3-yl)porphyrin
- MnIIBr8TM-4-PyP4+
manganese(II) β-octabromo-meso-tetrakis(N-methylpyridinium-4-yl)porphyrin
- MnIIIBr8T-2-PyP+
manganese(III) β-octabromo-meso-tetrakis(2-pyridyl)porphyrin
- MnIIITE-2-PyP5+
manganese(III) meso-tetrakis(N-ethylpyridinium-2-yl)porphyrin
- MnIIITM-2-PyP5+
manganese(III) meso-tetrakis(N-methylpyridinium-2-yl)porphyrin
- MnIIITM-3-PyP5+
manganese(III) meso-tetrakis(N-methylpyridinium-3-yl)porphyrin
- MnIIITM-4-PyP5+
manganese(III) meso-tetrakis(N-methylpyridinium-4-yl)porphyrin
- MnIIITnHex-2-PyP5+
manganese(III) meso-tetrakis(N-n-hexylpyridinium-2-yl)porphyrin
- [MnIIIBr8TSPP]3−
manganese(III) β-octabromo-meso-tetrakis(4-sulfonatophenyl)porphyrin; metal-free ligand denotes same species as free-base porphyrin
- IC50
concentration that causes 50% inhibition of cytochrome c reduction by O2•−
- MS
mass spectrometry
- NHE
normal hydrogen electrode
- SAR
structure-activity relationship
- SOD
superoxide dismutase
References
- 1.Batini-Haberle I, Benov L, Spasojevi I, Hambright P, Crumbliss AL, Fridovich I. Inorg Chem. 1999;38:4011–4022. [Google Scholar]
- 2.Rebouças JS, Spasojevi I, Tjahjono DH, Richaud A, Mendez F, Benov L, Batini-Haberle I. Dalton Trans. 2008:1233–1242. doi: 10.1039/b716517j. [DOI] [PubMed] [Google Scholar]
- 3.Spasojevi I, Batini-Haberle I, Rebouças JS, Idemori YM, Fridovich I. J Biol Chem. 2003;278:6831–6837. doi: 10.1074/jbc.M211346200. [DOI] [PubMed] [Google Scholar]
- 4.Rebouças JS, DeFreitas-Silva G, Spasojevi I, Idemori YM, Benov L, Batini-Haberle I. Free Radical Biol Med. 2008 doi: 10.1016/j.freeradbiomed.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reboucas JS, Spasojevi I, Batini-Haberle I. J Biol Inorg Chem. 2008;13:289–302. doi: 10.1007/s00775-007-0324-9. [DOI] [PubMed] [Google Scholar]
- 6.Ferrer-Sueta G, Vitturi D, Batini-Haberle I, Fridovich I, Goldstein S, Czapski G, Radi R. J Biol Chem. 2003;278:27432–27438. doi: 10.1074/jbc.M213302200. [DOI] [PubMed] [Google Scholar]
- 7.Coulter AD, Emerson JP, Kurtz DM, Cabelli DE. J Am Chem Soc. 2006;122:11555–11556. [Google Scholar]
- 8.Batini-Haberle I, Spasojevi I, Fridovich I. Free Radical Biol Med. 2004;37:367–374. doi: 10.1016/j.freeradbiomed.2004.04.041. [DOI] [PubMed] [Google Scholar]
- 9.Trostchansky A, Ferrer-Sueta G, Batthyany C, Botti H, Batini-Haberle I, Radi R, Rubbo H. Free Radical Biol Med. 2003;35:1293–1300. doi: 10.1016/j.freeradbiomed.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 10.Ferrer-Sueta G, Quijano C, Alvarez B, Radi R. Methods Enzymol. 2002;349:23–37. doi: 10.1016/s0076-6879(02)49318-4. [DOI] [PubMed] [Google Scholar]
- 11.Batini-Haberle I, Spasojevi I, Stevens RD, Hambright P, Fridovich I. J Chem Soc, Dalton Trans. 2002:2689–2696. [Google Scholar]
- 12.Batini-Haberle I, Liochev SI, Spasojevi I, Fridovich I. Arch Biochem Biophys. 1997;343:225–233. doi: 10.1006/abbi.1997.0157. [DOI] [PubMed] [Google Scholar]
- 13.Zhao Y, Chaiswing L, Oberley TD, Batini-Haberle I, St Clair W, Epstein CJ, St Clair D. Cancer Res. 2005;65:1401–1405. doi: 10.1158/0008-5472.CAN-04-3334. [DOI] [PubMed] [Google Scholar]
- 14.Moeller BJ, Batini-Haberle I, Spasojevi I, Rabbani ZN, Anscher MS, Vujaskovi ž, Dewhirst MW. Int J Rad Oncol Biol Phys. 2005;63:545–552. doi: 10.1016/j.ijrobp.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 15.Benov L, Batini-Haberle I. Free Radical Res. 2005;38:81–88. doi: 10.1080/10715760400022368. [DOI] [PubMed] [Google Scholar]
- 16.Sheng H, Spasojevi I, Warner DS, Batini-Haberle I. Neurosci Lett. 2004;366:220–225. doi: 10.1016/j.neulet.2004.05.050. [DOI] [PubMed] [Google Scholar]
- 17.Sheng H, Enghild J, Bowler R, Patel M, Calvi CL, Batini-Haberle I, Day BJ, Pearlstein RD, Crapo JD, Warner DS. Free Radic Biol Med. 2002;33:947–961. doi: 10.1016/s0891-5849(02)00979-6. [DOI] [PubMed] [Google Scholar]
- 18.Vujaskovi I, Batini-Haberle ZN, Rabbani Q-F, Feng SK, Kang I, Spasojevi TV, Samulski I, Fridovich Dewhirst MW, Anscher MS. Free Radic Biol Med. 2002;33:857–863. doi: 10.1016/s0891-5849(02)00980-2. [DOI] [PubMed] [Google Scholar]
- 19.Piganelli JD, Flores SC, Cruz C, Koepp J, Young R, Bradley B, Kachadourian R, Batini-Haberle I, Haskins K. Diabetes. 2002;51:347–355. doi: 10.2337/diabetes.51.2.347. [DOI] [PubMed] [Google Scholar]
- 20.Mackensen GB, Patel M, Sheng H, Calvi CL, Batini-Haberle I, Day BJ, Liang LP, Fridovich I, Crapo JD, Pearlstein RD, Warner DS. J Neurosci. 2001;21:4582–4592. doi: 10.1523/JNEUROSCI.21-13-04582.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aslan M, Ryan TM, Adler B, Townes TM, Parks DA, Thompson JA, Tousson A, Gladwin MT, Tarpey MM, Patel RP, Batini-Haberle I, White CR, Freeman BA. Proc Natl Acad Sci USA. 2001;98:15215–15220. doi: 10.1073/pnas.221292098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bottino R, Balamurugan AN, Bertera S, Pietropaolo M, Trucco M, Piganelli JD. Diabetes. 2002;51:2561–2567. doi: 10.2337/diabetes.51.8.2561. [DOI] [PubMed] [Google Scholar]
- 23.Sompol P, Ittarat W, Tangpong J, Chen Y, Doubinskaia I, Batini-Haberle I, Mohammad Abdul H, Butterfield A, St Clair DK. Neuroscience. 2007 doi: 10.1016/j.neuroscience.2008.01.044. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okado-Mastsumoto A, Batini-Haberle I, Fridovich I. Free Radic Biol Med. 2004;37:401–410. doi: 10.1016/j.freeradbiomed.2004.04.040. [DOI] [PubMed] [Google Scholar]
- 25.Benov L, Batini-Haberle I, Spasojevi I, Fridovich I. Arch Biochem Biophys. 2002;402:159–165. doi: 10.1016/S0003-9861(02)00062-0. [DOI] [PubMed] [Google Scholar]
- 26.Batini-Haberle I, Benov L, Spasojevi I, Fridovich I. J Biol Chem. 1998;273:24521–24528. doi: 10.1074/jbc.273.38.24521. [DOI] [PubMed] [Google Scholar]
- 27.Batini-Haberle I, Spasojevi I, Stevens RD, Hambright P, Neta P, Okado-Matsumoto A, Fridovich I. J Chem Soc, Dalton Trans. 2004:1696–1702. doi: 10.1039/b400818a. [DOI] [PubMed] [Google Scholar]
- 28.Batini-Haberle I, Benov L, Fridovich I. Anal Biochem. 1999;275:267. doi: 10.1006/abio.1999.4318. [DOI] [PubMed] [Google Scholar]
- 29.Waud WR, Brady FO, Wiley RD, Rajagopalan KV. Arch Biochem Biophys. 1975;169:695–701. doi: 10.1016/0003-9861(75)90214-3. [DOI] [PubMed] [Google Scholar]
- 30.Richards RA, Hammons K, Joe M, Miskelly GM. Inorg Chem. 1996;33:1940–1944. [Google Scholar]
- 31.McCord JM, Fridovich I. J Biol Chem. 1969;244:6049–6055. [PubMed] [Google Scholar]
- 32.Spasojevi I, Batini-Haberle I, Stevens RD, Hambright P, Thorpe AN, Grodkowski J, Neta P, Fridovich I. Inorg Chem. 2001;40:726–739. doi: 10.1021/ic0004986. [DOI] [PubMed] [Google Scholar]
- 33.Imlay JA, Linn S. J Bacteriol. 1987;169:2967–2976. doi: 10.1128/jb.169.7.2967-2976.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Munroe W, Kingsley C, Daruzo A, Gralla EB, Imlay JA, Srinivasan C, Valentine JS. J Inorg Biochem. 2007;101:1875–1882. doi: 10.1016/j.jinorgbio.2007.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vance CK, Miller AF. J Am Chem Soc. 1998;120:461–467. [Google Scholar]
- 36.Michel E, Nauser T, Sutter B, Bounds PL, Koppenol WH. Arch Biochem Biophys. 2005;439:234–240. doi: 10.1016/j.abb.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 37.Goldstein S, Fridovich I, Czapski G. Free Radical Biol Med. 2006;41:937–941. doi: 10.1016/j.freeradbiomed.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 38.Lawrence GD, Sawyer DT. Biochemistry. 1979;18:3045–3050. doi: 10.1021/bi00581a021. [DOI] [PubMed] [Google Scholar]
- 39.Barrette WC, Jr, Sawyer DT, Free JA, Asada K. Biochemistry. 1983;22:624–627. doi: 10.1021/bi00272a015. [DOI] [PubMed] [Google Scholar]
- 40.Faraggi M. In: O2•− Dismutation Catalyzed by Water- soluble Porphyrins: A Pulse Radiolysis Study. Bors W, Saran M, Tait D, editors. Walter de Gruyter; Berlin: 1984. pp. 419–430. [Google Scholar]
- 41.Batini-Haberle I, Spasojevi I, Stevens RD, Bondurant B, Okado-Matsumoto A, Fridovich I, Vujaskovi, Dewhirst MW. Dalton Trans. 2006:617–624. doi: 10.1039/b513761f. [DOI] [PubMed] [Google Scholar]
- 42.Kachadourian R, Batini-Haberle I, Fridovich I. Inorg Chem. 1999;38:391–396. [Google Scholar]
- 43.Ou Z, Shao J, D’Souza F, Tagliatesta P, Kadish KM. J Porphyrins Phthalocyanines. 2004;8:201–214. [Google Scholar]
- 44.Rebouças JS, de Carvalho MEMD, Idemori YM. J Porphyrins Phthalocyanines. 2002;6:50–57. [Google Scholar]
- 45.do Nascimento E, deF Silva G, Caetano FA, Fernandes MAM, da Silva DC, de Carvalho MEMD, Pernaut JM, Rebouças JS, Idemori YM. J Inorg Biochem. 2005;99:1193–1204. doi: 10.1016/j.jinorgbio.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 46.Foster N. J Magn Reson. 1984;56:140–143. [Google Scholar]
- 47.Riley DP, Henke SL, Lennon PJ, Aston K. Inorg Chem. 1999;38:1908–1917. doi: 10.1021/ic981319b. [DOI] [PubMed] [Google Scholar]
- 48.Aston K, Rath N, Naik A, Slomczynska U, Schall OF, Riley DP. Inorg Chem. 2001;40:1779–1789. doi: 10.1021/ic000958v. [DOI] [PubMed] [Google Scholar]
- 49.Riley DP. Adv Supramol Chem. 2000;6:217–244. [Google Scholar]