Abstract
We systematically reviewed the localization of focal brain lesions that cause isolated hallucination in a single sensory modality. Case reports of post-lesion nonparoxysmal hallucination in 1 (and only 1) of 3 sensory modalities (i.e., visual, auditory, somatic) were reviewed, and the content of the qualitative descriptions was analyzed for each modality. The lesion is practically always located in the brain pathway of the sensory modality of the hallucination. There seem to exist localized sensory brain circuits that in healthy people diminish the intensity of internal sensory representation. After a lesion, hallucinosis seems to be caused also by compensatory overactivation of tissue in the nearby brain sensory pathway. This type of hallucination may indeed be termed a “release” form, whereby patients are aware of the hallucinatory nature of their experience, but not usually of “dream centres” as proposed by Lhermitte. Instead, we propose that it is dreaming that should be considered a special case of neural “release.”
Nous avons passé en revue systématiquement l'emplacement des lésions cérébrales focales qui causent des hallucinations isolées dans un seul mode sensoriel. On a analysé des rapports de cas portant sur l'hallucination non paroxystique postlésionnelle dans un mode sensoriel (et un seulement) sur trois (c.-à-d. visuel, auditif, somatique), et on a analysé le contenu des descriptions qualitatives de chaque mode. La lésion est presque toujours située dans la voie cérébrale du mode sensoriel de l'hallucination. Il semble y avoir des circuits cérébraux sensoriels localisés qui, chez les gens en bonne santé, «atténuent» l'intensité de la représentation sensorielle interne. Après une lésion, l'hallucinose semble être causée aussi par une suractivation compensatoire de tissus de la voie sensorielle cérébrale voisine. On peut en fait qualifier ce type d'hallucination de forme de «libération», dans laquelle les patients sont conscients de la nature hallucinatoire de leur expérience, mais non habituellement de «centres oniriques» comme le propose Lhermitte. Nous proposons plutôt de considérer le rêve comme un cas spécial de «neurolibération».
Medical subject headings: auditory pathways, brain, dreams, epilepsy, hallucinations, psychotic disorders, visual pathways
Introduction
The purpose of this article is to systematically review the localization of brain lesions that cause isolated hallucination in a single sensory modality, specifically, vision, audition or somesthesia. Here we define hallucination widely as any false perception.
Everyone has experienced hallucinosis, that is, has recognized a false perception in oneself, such as the black points or “phosphenes” from eyeball rubbing, the tinnitus or “acouphenes” after a rock concert or with a fever, or “somatophenes” such as the illusion of weightlessness caused by the legs being crossed for too long. However, when the false perception is more spectacular, we immediately think of psychosis. The lesion approach, that is, the notion that psychosis can be caused by brain lesions, to the understanding of psychosis is full of pitfalls. Psychosis is a heterogenous phenomenon: patients present with various neurotic traits and affective symptoms, differing modalities of hallucination, paranoid or nonparanoid delusions, negative or positive symptom complexes, dementia, delirium, paramnesia, agitation and adynamia, among other symptoms. Finding patients who have developed such a mixture of disorders after a focal lesion probably would not provide a neat set of lesion loci. Cummings1 and Pollack et al2 have argued that the diverse lesion sites apt to produce psychosis tend to involve dopaminergic circuits. However, dopaminergic circuits are widespread, and this idea must be considered tentative.
We reviewed single case reports of post-lesion psychosis involving hallucination and delusion and found that the temporal lobe was the most common lesion site3,4,5,6,7,8,9,10,11,12 followed by the frontal lobe13,14,15,16,17,18 and, then, tissue around the third ventricle.8,19,20,21,22 We hesitate to believe that this is indicative of a predilection for lesion loci to be located within dopaminergic pathways. Limiting oneself to a review of case reports of pure delusional states also does not yield a neat set of lesion loci. Cases have been described of patients with frontal lesions,23,24 temporal lesions,7,25 parietal lesions18,26 and lesions of the basal ganglia.27,28 It has been suggested that psychotic auditory hallucination is modulated by internal speech, and brain metabolic imaging of hallucinating psychotic patients and measurement of perioral musculature in psychotic patients both support that point of view.29 This possible mechanism has not, as far as we know, been discussed in post-lesion cases, although there are several reports of patients who have reported hearing voices (more commonly singing). Indeed, the “internal speech” mechanism seems unlikely in post-lesion cases for a number of reasons. Namely, the lesion involves the auditory pathway and not the expressive speech areas, the content of the hallucinated speech does not correspond to any apparent emotional or moral obsession of the patient (as seems to occur in psychosis), the speech is often a conversation among several people known to the patient or singing by a person heard previously by the patient, and the speech is often of a person of the opposite sex. This leads us to wonder whether the perioral activity of patients with schizophrenia during hallucinated speech might not consist of a form of subliminal echolalia, a phenomenon that we would not expect in post-lesion hallucinations. Still, measurement of perioral activity during post-lesion hallucination of speech would be worth investigating.
With the ever-increasing number of single-case post-lesion reports, it has now become possible to review meaningfully large sets of patients with focal lesions who subsequently developed highly specific and limited false perceptions. The present literature review, which is based on multiple single case reports, will therefore focus on cases of pure hallucination, in specific sensory modalities, without neurotic, affective or delusional symptoms. Our method for reviewing this literature consisted of searches of the entire MEDLINE database and then detailed follow-up using the bibliographies of the articles thus obtained. This approach allows for a clear hypothesis that has been formulated on several occasions in single case reports and more limited multiple case reviews, namely, that pure isolated syndromes of hallucination tend to result from lesions located in the sensory brain pathways of the modality in question. Although it would be conceivable to review all the senses, post-lesion gustatory or olfactory hallucinations are too rare to support inference testing. Thus, we shall limit this review to the 3 most epicritic of the senses, namely vision, audition and somesthesia, restricting ourselves to nonparoxysmal cases manifesting hallucinations in only 1 of these modalities with the localizing hypothesis in mind. By primary visual pathway of the brain, we mean the main nuclei of that pathway and important cortical radiations: retina; optic nerve; chiasma; diagonal bands; lateral geniculate body; temporal, parietal and occipital lobes; and superior colliculus. By primary auditory pathway, we mean the pons, inferior colliculus, medial geniculate body and temporal lobe. By the somesthetic pathway, we mean the spinal cord, medulla, pons, thalamus and parietal lobe.
Epilepsy that accompanies a lesion may or may not override and even contradict lesion effects by irritating functional tissue and is sufficient cause of psychiatric symptoms including hallucination.30 Depending upon whether a symptom complex is released or destroyed by a lesion, or is activated or inhibited by pathologic irritation of brain tissue, any symptom complex could conceivably result from many brain disorders. Hallucination in epileptic patients is known to abate after resection.31 To the extent that we can clearly distinguish cases with or without irritative conditions based on clinical electroencephalography (EEG), then some order may be brought to the whole issue. In fact, epilepsy is only 1 irritative condition of the brain. Migraine is another, as are toxic conditions, some tumours, and so on. Certain mitotic lesions have proximal electrical and metabolic irritative effects that can override the effect of tissue loss in the tumour area. This is well illustrated by Filley and Kleinschmidt,5 who reported alleviation of psychiatric symptoms in all 5 of their operable neoplastic cases by surgical removal of the tumour. Consequently, in the multiple case review that we are proposing here, careful attention was paid to the origin of the lesion, as well as to clinical EEG parameters and any mention of seizures.
Two types of neurogenic hallucination were distinguished early on (1973) by Cogan:32 the irritative and release forms. The irritative forms are associated with migraine, tumour and, of course, paroxysmal EEG. They are reputed to be stereotyped and simple, as in migraine auras, and to have some localizing value. They may or may not be associated with brain lesions. When they are, the association is with diffuse radiologically unidentifiable lesions. The patient is not typically aware of the hallucinatory nature of his or her perception (the false perception is then termed hallucinosis). The release forms are associated with silent localized lesions, have little or no localizing value, often represent remarkably complex scenes and vary in the same patient. The patient is typically perfectly lucid concerning the “hallucinatory” nature of the perception. The image is often recognized from memory, suggesting that it is in fact more an overly vivid recollection, recognized as such or not, than a false perception.
Lhermitte33 proposed in 1922 a special midbrain syndrome, which he termed “peduncular hallucinosis,” possibly involving release, presumably via destruction of an inhibitory influence, of dream-like activity. Sleep disorders have on a few occasions been associated with this type of hallucination. Lhermitte associated the sleep disorder with the hallucination and likened the hallucinosis to a mechanism similar to narcoleptic hallucinosis. Several cases have since been reported of post-lesion hallucinosis without sleep disorder. In addition, we will demonstrate that the lesion loci for these types of hallucinations can be anywhere from the pons to the thalamus to the cerebral cortex. Thus, Lhermitte's idea that such highly lucid and articulated hallucinations might be a form of midbrain release of dream processes seems doubtful.
Sensory deprivation due to peripheral damage, as in Charles Bonnet syndrome, is believed to remove competition with endogenous representation (imagination) and thus to lower the threshold for hallucination.34 Indeed, most reports of post-lesion hallucinosis in the visual modality, for example, consist of representations located in the hemianopic field,35 and they commonly occur at twilight or at night.36 Hearing loss is common in post-lesion auditory hallucinosis,37 and somesthetic deficits are common in hallucinations of the body image.38 It is interesting to note that Lhermitte's 1922 patient also presented ophthalmologic problems, retrospectively making that case a reasonable candidate for Charles Bonnet syndrome.
At present, it is not known whether children may also manifest pure, namely, isolated hallucinations subsequent to lesions, as is the case for adults. Consequently, we will pay special attention to that issue as well.
Visual hallucinosis
Where are visual hallucinations generated in the brain?
Evidence from stimulation and lesion studies
Simple visual hallucinations from electrocortical stimulation are most typically evoked by occipital probing, whereas complex ones involving people, animals and scenes are more readily evoked by temporo-occipital or parieto-occipital stimulation, according to Penfield and Perot.31 In addition, the same authors found that occipital stimulation tended to produce hallucinations in the contralateral field, whereas temporal stimulation produced hallucinations in both fields. Although the stimulation site that is likely to produce visual hallucinations is typically in the visual pathway, a few sites outside the visual pathway have also been reported. For example, stimulation of the subthalamic nucleus produced visual hallucinosis in a parkinsonian patient that was promptly relieved by clozapine.39
Visual hallucinations in the adult have been stated to result from lesions anywhere in the visual pathway, including the retina, brainstem pathways and occipital lobe.40 Lesions in the suprasellar area diencephalon have been reported to produce visual hallucinosis in 2 patients,41 presumably because of the lesions' proximity to the diagonal bands or chiasma. Smith et al42 described a patient with visual hallucinations with several lesions, but only 1 of which involved the visual pathway, namely, the optic chiasm. In his study of 120 patients with hemianopia or quadrantanopia, Kölmel35 found that for the 16 with hallucinations, the associated lesions located by computed tomography were especially in the occipital lobe but extended sometimes toward the parietal lobe or the temporal lobe, or both. Parkinson et al43 studied patients with occipital lobe lesions and found that this lesion locus was associated with hallucinations in 12 cases. In a vast study of patients with visual loss and spontaneous visual phenomena, Lepore44 showed that visual hallucinations were associated with lesions of the visual pathway, no matter the exact location, including the brain stem. Galasko et al45 also found, in a review of the literature (MEDLINE, 1966–1987) on the psychiatric disorders associated with mass lesions, an association between visual hallucinations and lesions to various parts of the visual pathway.
Evidence from metabolic imaging studies
Ffytche et al46 carried out functional magnetic resonance imaging (fMRI) of visual hallucination in 5 cases of Charles Bonnet syndrome. They found selective activation in the parts of the visual cortex that are known to process the types of images that appeared in each subject's hallucinations. In addition, the patients as a group had tonic activation in the visual cortex even when not hallucinating. Howard et al47 published similar findings and interpretation. Adachi et al48 reported single photon emission computed tomography (SPECT) using iodine 123 iodoamphetamin (IMP) and MRI studies of 5 patients with Charles Bonnet syndrome while they were having visual hallucinations. All patients developed complex visual hallucinations after suffering from eye disease. SPECT disclosed hyperperfusion areas with some asymmetrical appearances in the lateral temporal cortex, striatum and thalamus. This suggested to the authors that when elderly people suffer from eye disease, subsequent excessive cortical compensation in the lateral temporal cortex, striatum and thalamus may precipitate the development of visual hallucinations.
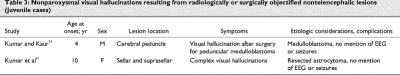
Case reports of pure visual hallucination subsequent to radiologically or surgically objectified focal lesions of the brain are listed in Table 1,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65 Table 266,67,68,69,70,71,72,73 and Table 3.74
Table 1
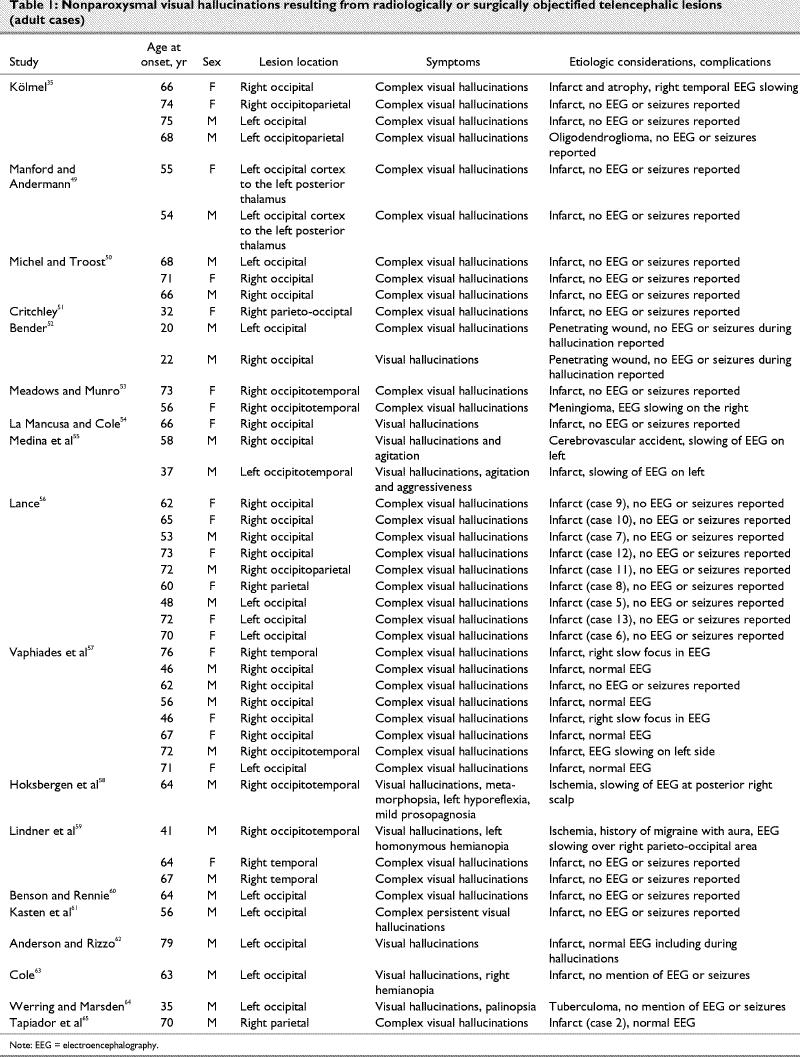
Table 2
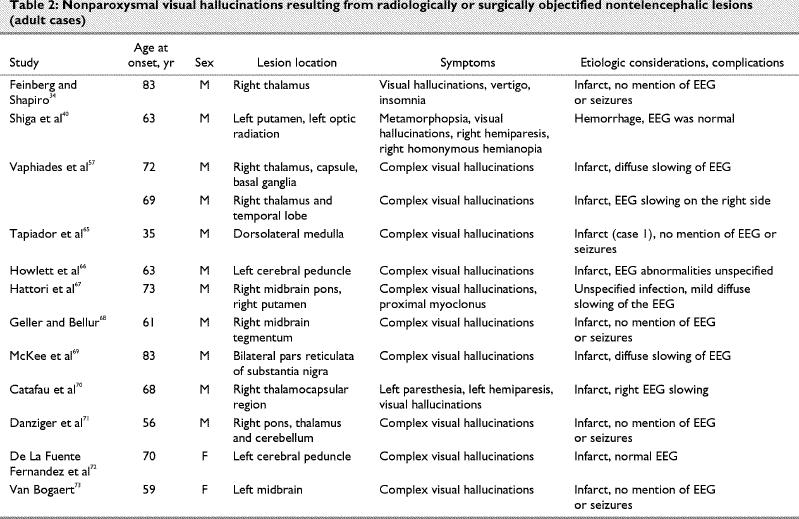
Table 3
What is the content of nonparoxysmal post-lesion visual hallucinations?
Charles Bonnet syndrome is usually characterized by the presence of vivid and complex visual hallucinations, which are recognized as unreal (i.e., pseudohallucinations, parahallucinations or hallucinosis) and occur in the absence of any other psychiatric symptoms or cerebral lesion. Some researchers suggest that isolated visual hallucinations in older adults, often with visual impairment, and frequently sedentary and sensorially deprived, may be an indication of early stages of dementia. Contrary to what was considered for a long time, the syndrome seems to occur frequently. Santhouse et al75 reported a detailed content analysis of the hallucinations of 39 patients with Charles Bonnet syndrome. They found that the most common hallucination involved landscapes with small figures in costumes with hats. The next most common hallucination comprised grotesque faces with prominent eyes and teeth. Finally, the third content category consisted of visual perseveration or palinopsia, or both.
We were intrigued by a statement by Kölmel35 to the effect that whenever a directional movement of the hallucinated representation was well identified (6 patients), it was from the periphery to the macular area. We found 12 reports that specified the direction of the movement of a hallucinated object: indeed, the movement was from the periphery to the macular portion of the field in 8 cases.51,57,58,76,77 However, 4 patients have been described40,57,62 whose hallucinated object always moved from the meridian to the periphery. In short, the evidence refutes any suggestion of a systematic direction of moving hallucinated objects.
We were also intrigued by Kölmel's statement to the effect that “identical replication,” namely, synchronous systematic repetition of a visual image (variably termed polyopia, tesselopsia and palinopsia) may be interpreted as an expression of the functional architecture of the primary visual cortex.35 Various such recurring forms are frequent in the cases reported by other authors (see Table 4 for details of our content analysis) and are obtained by electrical stimulation of the occipital cortex.78
Table 4

Finally, content analysis of hallucinosis in eye disease led Ffytche and Howard79 to report an intriguing hallucination consisting of “tree” shapes, which they termed “dendropsia” (14% frequency). The authors entertained the fascinating idea that this perception could result from intrusion of retinal vasculature but opted, rather, for long-range release of inhibition as a potential cause. Dendropsia was not observed in our extra-ocular cases (Table 4), suggesting that there might be more grounds for the former than the latter speculation.
Auditory hallucinosis
Where are auditory hallucinations generated in the brain?
Evidence from stimulation and lesion studies
Electrical brain stimulation that is apt to produce reports of auditory experiences is typically located in the temporal cortex, in either hemisphere. Spontaneous ictal auditory hallucinations are most common in temporal lobe epilepsy but also occur, less frequently, in cases with foci well localized in other lobes. Complex hallucinations such as voices or music are nearly universally associated with temporal cortex stimulation or irritation, whereas simple ones such as hissing, buzzing, and so on, can result from subcortical or insular stimulation or irritation.31
Post-lesion auditory hallucination, though less frequent than visual, follows the same logic as the visual forms: the typical lesion is situated anywhere along the auditory pathway, including the brain stem.71 It is quite common in post-lesion auditory hallucination for the patient to manifest deficits of audition such as distortion, poor localization, hypoacusis80 or even deafness,37 suggesting that deafferentation can mediate the effect.
Evidence from metabolic imaging studies
Dierks et al81 used an fMRI protocol to study brain activation during auditory hallucinations in 3 patients with schizophrenia. They found maximal activation in the transverse temporal gyrus of the dominant hemisphere and other activation in the posterior superior temporal gyrus, middle temporal gyrus, frontoparietal operculum, hippocampus, amygdala and sensorimotor cortex. The patients were asked to press a button when their auditory hallucinations started, and to release it when they stopped. Unfortunately, the authors did not use a button-pressing task as a control condition. Activation reported therefore contains mixed signal produced by the auditory hallucination and the motor task. A recent fMRI study82 of 6 patients with schizophrenia found maximal activation in the right inferior colliculus, right and left insula, left parahippocampal gyrus, right temporal gyrus, right thalamus, middle frontal and anterior cingulate gyri, and right inferior and superior temporal lobe during auditory hallucinations. The authors conclude that, because the areas involved in auditory verbal hallucinations are also involved in inner speech, auditory hallucinations result from defective monitoring of inner thoughts. In another study, continuous whole-brain fMRI with a 3-T magnet was used to map the cerebral activation associated with auditory hallucinations in 4 subjects with schizophrenia.83 The subjects experienced episodes of hallucination while in the scanner, so periods of hallucination could be compared with periods of rest in the same individuals. Group analysis demonstrated shared areas of activation in the right and left superior temporal gyri, left inferior parietal cortex and left middle frontal gyrus. When the data were examined on an individual basis, the temporal cortex and prefrontal cortex areas were activated during episodes of hallucination in all 4 subjects. The authors concluded that these findings support the theory that auditory hallucination reflects abnormal activation of normal auditory pathways. As is to be expected from the functional imaging method, activated areas tend to be numerous and several of these are probably epiphenomenal. Positron emission tomography (PET) was used to study the brain state associated with the occurrence of hallucinations in 5 patients with schizophrenia with classic auditory verbal hallucinations despite medication.84 During the hallucinations, there was activation in the subcortical nuclei thalamic, striatal, limbic structures (especially hippocampus and paralimbic regions, parahippocampal and cingulate gyri, as well as the orbitofrontal cortex). The authors propose that activity in deep brain structures, identified with group analysis, may generate or modulate the hallucinations, and the particular neocortical regions involved in individual patients may affect the specific content of their hallucinations. Our reading of this literature suggests to us that in psychotic auditory hallucination, the most likely mechanism is release of inhibition of the auditory cortex by other cortical auditory neural assemblies, including those in the contralateral hemisphere.85 This appears to be the same mechanism as in post-lesion musical hallucinosis: activation in one such case was in the areas (posterior temporal lobes) that would have been expected in normal hearing of such sounds in healthy individuals.86 Case reports of post-lesion auditory hallucinosis are presented in Table 587,88,89,90,91 and Table 6.92,93,94
Table 5
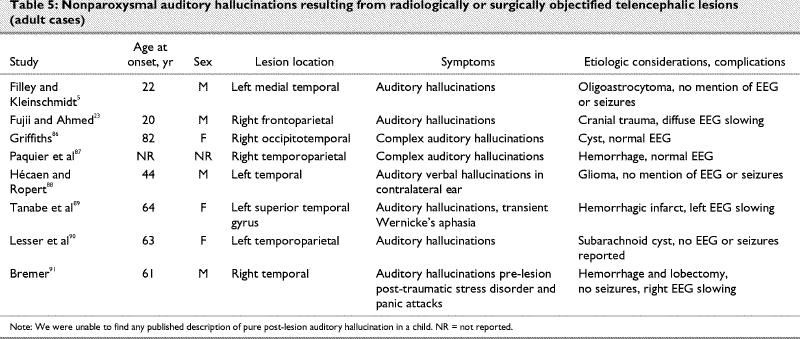
Table 6
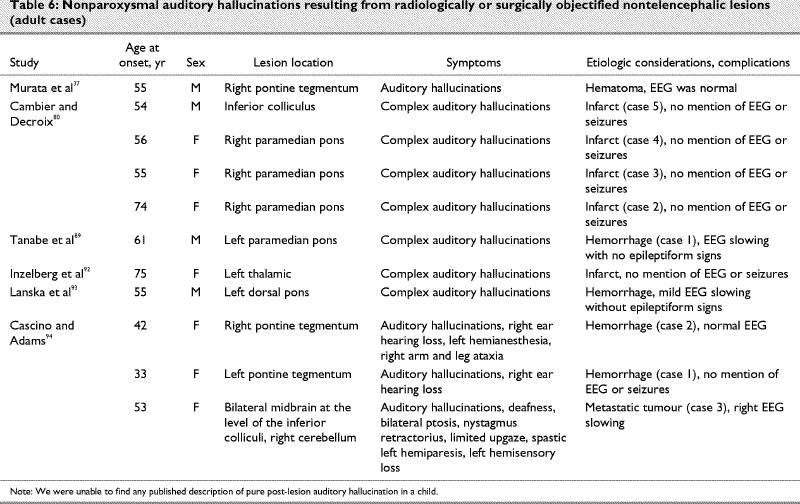
What is the content of nonparoxysmal post-lesion auditory hallucinations?
Parallel to the visual modality, a repetitive simple sound is probably the most common form of post- lesion hallucination, and it will usually be diagnosed as an acouphene (tinnitus). The cause is often obscure, but it can be the result of a vascular abnormality of the cervical region, skull base or cranium, or a tumour. Interestingly, more complex auditory hallucinations, such as music and human voices, are not so unusual subsequent to brainstem lesions, evoking the surprisingly complex representations described by patients with visual post-lesion hallucinosis (Table 1, Table 2 and Table 3). In Table 7, we classify the contents of auditory hallucinations of cases reviewed in Table 5 and Table 6.
Table 7

Somatic hallucinosis
Where are somatic hallucinations generated in the brain?
Evidence from stimulation and lesion studies
Somatic hallucination is crudely classifiable into 3 basic forms: pain, paresthesia and complex somatoparaphrenia. In a review of 16 cases of post-lesion pain, Gonzales et al95 concluded that “central pain” results from lesions anywhere in the somesthetic projection system. Bowsher et al96 came to the same conclusion on the basis of their investigation of 73 cases of post-lesion pain. These cases include focal unilateral parietal lesions,97 but thalamic lesions are most likely to produce pain resembling a psychiatric syndrome: “pain in the thalamic syndrome”98 is of the burning sort, is generalized and may be provoked by benign stimuli (allodynia). Even spinal cord injuries can induce pain below the lesion. Finnerup et al99 studied 436 spinal cord injury cases and found that 77% of individuals reported pain or unpleasant sensations (“dysesthesias” in the authors' terminology). Complex somatic hallucinations involve feelings of supernumerary or abnormally sized or deformed body parts. This condition was termed somatoparaphrenia by Gerstmann in 1942. The lesion that produces this syndrome is nearly always left parietal, although a few cases with lesions localized elsewhere have been reported.
Evidence from metabolic imaging studies
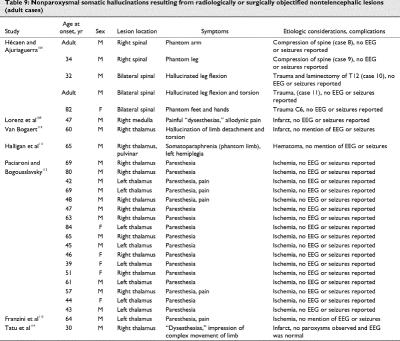
Using fMRI, Lotze et al100 investigated 14 upper-limb amputees and 7 healthy controls during the execution of hand and lip movements and imagined movements of the phantom limb or left hand. Only patients with phantom limb pain showed a shift of the lip activation into the deafferented primary motor and somatosensory hand areas during lip movements. Displacement of the lip representation in the primary motor and somesthetic cortex was positively correlated with the amount of phantom limb pain. Thalamic activation was only present during executed movements in the healthy controls. The cerebellum showed no evidence of reorganizational changes. In amputees, movement of the intact hand showed a level of activation similar to movement of the right dominant hand in the healthy controls. During imagination of moving the phantom hand, all patients showed significantly higher activation in the contralateral primary motor and somatosensory cortices compared with imagination of hand movements in the controls. In the patients with phantom limb pain but not the pain-free amputees, imagined movement of the phantom hand activated the neighbouring facial area. These data suggest selective coactivation of the cortical hand and mouth areas in patients with phantom limb pain. This reorganizational change may be the neural correlate of phantom limb pain. With PET imaging, phantom pain has been found to be significantly related to activity in the thalamus, anterior cingulate and lateral prefrontal cortex.101 Shergill et al102 examined a patient with schizophrenia with fMRI. They compared the distribution of brain activity during somatic and auditory (verbal) hallucinations, occurring at different times. Somatic hallucinations were associated with activation in the primary somatosensory and posterior parietal cortex, areas that normally mediate tactile perception. Auditory hallucinations were associated with activation in the middle and superior temporal cortex, areas involved in processing external speech. They proposed that hallucinations in a given modality seem to involve areas that normally process sensory information in that modality. Case reports of somatic hallucination after brain lesions are reviewed in Table 8103,104,105,106,107,108 and Table 9.109,110,111,112,113,114
Table 8

Table 9
What is the content of nonparoxysmal post-lesion somatic hallucinations?
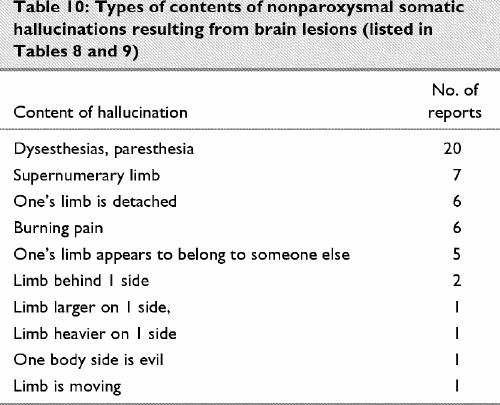
There is a large neurologic and neuropsychologic literature on negative forms of complex disorders of body image, for example, neglect, anosognosia. These forms are not the object of the present review. It is well recognized that somatic hallucinations can derive from organic conditions, such as the paresthesias of delirium tremens (e.g., insects creeping on the skin or vermin under it). The most common forms of post-lesion somatic hallucination are simple, namely, paresthesias such as burning, picking, tingling. These can readily result from thalamic lesions (Table 9). As for positive expressions of complex disorders of body image, these can include supernumerary limbs,115 hatred of the affected limb (misoplegia),116 overestimation of the strength of the affected limb,117 over- or underestimation of the size of the affected limb or mislocation of the affected limb.118 The content of hallucinations in cases reviewed in Table 8 and Table 9 is classified in Table 10.
Table 10
Discussion
The localization of lesions that produce an isolated nonparoxysmal syndrome of hallucination in 1 sensory modality is strikingly coherent, more so than indicated by functional imaging studies. It is nearly always in the primary sensory pathway of that modality in the brain. Take the visual versus auditory modalities as a natural test of this idea. The primary visual pathway does not extend below the midbrain, and the primary auditory pathway contains a major part of its circuitry in the pons itself. If low-level lesions in the pathway can easily produce hallucinations in the relevant modality, then visual lesions should result from peduncular lesions, and auditory hallucinations should result from pontine lesions. This is exactly what occurs. Somesthesia offers an even clearer test of this basic principle: Table 9 illustrates that spinal lesions can cause somatic hallucinations, but such lesions are never reported to cause visual or auditory hallucinations.
Post-lesion hallucination in children?
Why are there so few cases of children manifesting isolated syndromes of unimodal hallucination after a focal lesion? One reason could simply be that few children have focal lesions and that case reports will accrue with time. However, there are numerous cases of children with brain lesions, though admittedly fewer than in adults. There is no penury of juvenile cases manifesting florid psychosis after a focal lesion with hallucinations and delusions.3,4,8,9,11,17,21,22 We also suspect that there are few pediatric cases of pure nonparoxysmal post- lesion hallucinosis because the various comorbidities that facilitate hallucination are usually not present in children (incipient dementia, modal peripheral sensory loss, confusional state, and so on). At any rate, we were not able to find any case report of post-lesion unimodal hallucination in the auditory or somesthetic modalities. We found only 4 cases, limited to the visual modality, and 2 of them (not presented here) had paroxysms on EEG and seizures.
Toward a neurophysiologic model
We speculate that a hallucination, especially a complex one involving full-fledged articulated scenes or events, resulting from a focal lesion, is indeed a release phenomenon — though not necessarily dream-like. More specifically, we propose that the lesioned tissue (supposing that there is no paroxysmal activity and no irritation of brain tissue) must have previously contained a predominance of inhibitory over excitatory neurons for the sensory modality in question. These inhibitory neurons, operating normally, would be capable of “gauging down” brain circuits containing complex sensory representations, which would be presumably cortical (see Fuster119), even though they are sometimes situated in the brain stem. Perhaps such modules are less developed in children simply because juveniles have a less built-up store of such complex representations, or because of lack of experience or lack of re-experiencing (rehearsal) of such complex events, or both. Considering that there are frequent comorbidities of sensory impairment or deprivation in post-lesion hallucinosis and Charles Bonnet syndrome, it seems likely that compensatory overactivation of remaining nearby tissue after a lesion contributes greatly to the risk of hallucinosis. Again, children would be less at risk for these comorbidities (e.g., cataracts, hearing loss, prolonged immobilization, fatigue, incipient dementia) and thus for post-lesion hallucinosis. Indeed, sensory deprivation alone, in healthy adults, leads to hallucinosis.120 Immediately after a retinal lesion, field-adjacent cortical cells increase their receptive fields.121
This interpretation suggests that Lhermitte's fascinating proposal concerning visual hallucinosis may have put things upside down: visual representation might not be generated during dreaming by a brainstem “dream centre,” as Lhermitte speculated,33 but by the visual pathway itself. In other words, we propose that in the visual pathway, neurons and neuronal assemblies specialized for waking vision and for voluntary visual imagination are responsible for the hallucinosis of people with narcolepsy and epilepsy, and of patients with migraine and patients with tumours, as well as the visual evocations of patients receiving exploratory electrical stimulation and, of course, hallucinosis of patients with Charles Bonnet syndrome, hallucination of patients with psychosis and of post-lesion hallucinosis, as well as visual aspects of normal dreams. After all, it is well established that cortical cell assemblies (occipital, inferotemporal and parietal) are repositories of fully formed dynamic images and are extraordinarily specialized for this function. There is also no evidence whatsoever to the effect that such fully formed conscious images could be generated in any subcortical tissue. Within this interpretation, all hallucinations need not derive from conscious memory: they may derive from unconscious memory (forgotten material), or they may even be constructions resulting from the architectonic structure of the neural assemblies stimulated and the specific local effect of any pathology of the neural assemblies. Each pathologic condition creates a particular context for activation of these assemblies, providing a specific coloration.
In their attempt to articulate a pathophysiologic model of hallucinosis, Manford and Andermann49 proposed a neurotransmitter-based explanation. We do not dispute the plausibility of serotonin dysfunction as an important modulating factor of hallucination in narcolepsy, dementing processes, drug consumption and, importantly, a few cases of peduncular hallucinosis. However, the cases presented here are best explained by a neurotransmitter-independent, modality-specific neuronal loss, resulting in connection-based release of inhibition in the sensory cortex. The disconnection is often corticocortical (see Dierks et al81 for fMRI evidence), but it can also be remote. Manford and Andermann49 propose that in the latter case, a thalamocortical gate is usually unbridled. We find this quite plausible, because the thalamus is known to gauge sensory arousal and because thalamic lesions commonly suffice to produce hallucinosis. Not mentioned by Manford and Andermann is the strong piece of evidence to the effect that thalamic bursting is well known to occur during post-lesion pain.122 We remain somewhat perplexed, however, by the paucity of published cases of auditory hallucinosis after a thalamic lesion. Noda et al123 reported 2 patients with cases of thalamic infarction, 1 right and 1 left, who both had visual and auditory hallucinations consisting of clear recollections of events dating as far back as 50 years.
Finally, it has been proposed that lesions of the right hemisphere are more likely than lesions of the left to produce visual hallucinations.50,52 In the current analysis, visual hallucinations resulted from right hemisphere lesions in 35 cases and from left hemisphere lesions in 20 (χ2 = 4.1, p < 0.05), suggesting some truth to that assertion. The other modalities yielded nonsignificant prevalence differences by hemisphere, but there were not enough cases to support meaningful inference tests. The laterality of the expected lesion in visual hallucinosis suggests (but does not prove) a corticocortical mediation related to hemispheric specialization, again leading us to argue that neither a neurotransmitter-based nor a thalamic-gating mechanism can suffice to explain entirely the neural basis of post-lesion hallucination.
What remains to be done with regard to the neural bases of hallucination?
The study of hallucinosis and hallucination could contribute to the advancement of psychophysics and sensory neuroscience, as well as to better health care. We have presented a modest test of a statement by Kölmel35 claiming peripheral-to-macular directionality of hallucinated movement in the damaged field. We have provided another modest test of one of Ffytche and collaborator's models of dendropsia.46 The same authors propose a relation between “near projection” and “far projection” and micropsia and macropsia, respectively. This remains to be investigated systematically. There are many other questions about the relation between sensation, perception, memory and the brain that only studies of hallucination can answer.
It would be interesting to determine, in detail, whether the contents of paroxysmal hallucinations in cases without identifiable lesions (migraine, drug induced, epileptic) are similar to those of lesioned cases without paroxysms. Post-lesion hallucinations seem akin to hallucinations evoked in epileptic patients by the neurosurgeon's stimulating probe exploring the cortex for epileptic foci: they are often complex, nonmorbid, lucidly identified as unreal, and they are evoked by lesions or stimulation in the same general areas of the cortex (see Stephane et al124 for a comprehensive review), though the effects of subcortical lesions are another matter.
It would be even more informative to compare, in detail, quantitatively, large corpuses of contents of hallucinations as a function of each cause of hallucination, including focal lesions, psychosis, narcolepsy, idiopathic Parkinson's disease, migraine coma, Charles Bonnet syndrome, drugs, and, of course, epilepsy.49 Let us consider one of these comparisons more closely. Similar to the hallucinations of patients with psychosis, the post-lesion hallucination is often of a complex scene (moving humans or animals, or both, or music including symphonies or voices, or body image distortion, supernumerary body parts, and so on). However, in contrast to the hallucinations of psychotic patients, the content of the post-lesion hallucinations seems benign rather than morbid (i.e., life-threatening visual scenes, molestation, ominous compulsive dangerous voices, feelings of body decay, worm infestation and so on). The patient with brain lesions seems to be typically more lucid about the hallucinatory nature of the experience than the patient with psychosis. Post-lesion hallucination often involves important components derived from memory. The proportion has been found to be 33% in patients with Charles Bonnet syndrome.125 The contents of psychotic hallucination may appear stranger to the patient. Another difference between hallucinations of psychotic patients and post-lesion cases is that the former are more frequently auditory and the latter more frequently visual. We need to find out why such differences exist.
Let us consider another contrast, between patients with Charles Bonnet syndrome and patients with lesions. Post-lesion hallucination, in the visual modality, is very much akin to that of Charles Bonnet syndrome, except that palinopsia and dendropsia seem to be less frequent in the former. Otherwise, content analysis of the case reports reviewed in the present investigation (Table 1, Table 2 and Table 3) yields a classification of the same types and in the same proportions as the content analysis of 39 patients with Charles Bonnet syndrome recently published by Santhouse et al.75 There are several important implications to this:
· Charles Bonnet syndrome is probably none other than a post-lesion syndrome in forme fruste (ischemia; mild neuronal or glial degeneration, or both; metabolic insufficiency).
· Expression of Charles Bonnet syndrome is simply facilitated by emerging dementia, advanced age, visual pathology, sleep disorders and surrounding darkness (e.g., deafferentation, sensory deprivation).
· Damage to the peripheral components of the neurosensory system may actually add characteristics of hallucinosis in Charles Bonnet syndrome (e.g., dendropsia).
· The logic of Charles Bonnet syndrome applies just as readily to the other sensory-perceptual modalities, audition and somesthesia, as to vision, and more attention should be paid to these other modalities, and the 3 syndromic entities should be recognized and labelled coherently.
Auditory post-lesion hallucinations are also very similar to those described in direct electrical stimulation of the temporal cortex (see Table 4 and Penfield and Perot31). Much less is known about somatic hallucinations, especially the complex ones.126 Parietal epileptic foci are rare in the somesthetic cortex, such that this cortex has not been probed much electrically, and post-lesion positive somesthetic syndromes are rare (Table 9). Post-lesion case reports will thus remain a precious commodity in this modality for a long time to come.
What can be done to help patients with post-lesion hallucinosis?
The first implication of the literature on post-lesion hallucination is that post-lesion hallucination is under- reported, probably because patients do not wish to appear “crazy.”127 Thus, patients with documented or suspected fresh lesions should be questioned about hallucination. A second important implication is that an isolated report of hallucination should be taken as a potential symptom of a brain lesion and should be diagnostically explored as such. Medical treatment of a brain lesion in addition to the more obvious provision of “psychologic” support could be life saving.
According to Pelaez,128 pattern completion in a neural network model of the thalamus and a biologically plausible model of synaptic plasticity are key concepts for analyzing cognitive disorders that involve hallucinations of several kinds: visual hallucinations in Charles Bonnet syndrome and psychedelic drug consumption, somatic hallucination in phantom limbs, and cognitive hallucinations in schizophrenia and even in multiple personality disorders. It has been suggested that all these types of hallucination are the result of pattern completion dynamics in thalamic deafferented areas. Effective treatments of some of these disorders would thus, suggests Pelaez,128 involve peripheral stimulation jointly with central inhibition so that the neural circuits that generate the disorders are depressed according to the proposed model of synaptic plasticity.
Certain antipsychotic drugs such as olanzapine or risperidone129 are known to exert a specific anti-hallucinatory effect. Could such agents be recommended for isolated unimodal hallucinations resulting from a lesion? If one is to take Charles Bonnet syndrome as a benign example of post- lesion hallucinosis, we must concur with Batra et al130 that few successful methods of treatment have been described. Therapies with classic neuroleptics, antidepressants or benzodiazepines have generally been found to be unpromising. Batra et al129 treated the condition successfully in 3 patients using the atypical neuroleptic melperone. Improvement resulting from anticonvulsant treatment has been observed in a few cases,76 of course primarily cases with epileptiform signs on EEG. Burke et al131 reported the successful treatment of a case of probable Charles Bonnet syndrome with the cholinesterase inhibitor donepezil.
Acknowledgments
This research was made possible by grants to C.M.J.B. by the Fonds de la recherche en santé du Québec (FRSQ), the Fonds concerté d'action et de recherche (FCAR) of the government of Quebec and the Natural Sciences and Engineering Research Council of Canada (NSERC).
Footnotes
Competing interests: None declared.
Correspondence to: Dr. Claude M.J. Braun, Department of Psychology, Université du Québec à Montréal, CP 8888, Succursale Centre-ville, Montréal QC H3C 3P8; fax 514 987-8952; Braun.Claude@uqam.ca
Submitted Mar. 15, 2002 Revised Dec. 11, 2002 Accepted Feb. 24, 2003
References
- 1.Cummings JL. Organic psychosis. Psychosomatics 1988;29:16-26. [DOI] [PubMed]
- 2.Pollack IF, Polinko P, Albright AL, Towbin R, Fitz C. Mutism and pseudobulbar symptoms after resection of posterior fossa tumors in children: incidence and pathophysiology. Neurosurgery 1995;37:885-93. [DOI] [PubMed]
- 3.Andermann LF, Savard G, Meencke HJ, McLachhan R, Moshé S, Andermann F. Psychosis after resection of ganglioglioma or DNET: evidence for an association. Epilepsia 1999;40:83-7. [DOI] [PubMed]
- 4.Apter A, Kaminer Y, Tyano S, Skurnik N, Weitz R. Irritation temporale et pathologie arachnoide: réaction pseudo-psychotique chez un adolescent et evolution sous traitement. Ann Méd Psychol (Paris) 1982;140:776-8. [PubMed]
- 5.Filley CM, Kleinschmidt BK. Neurobehavioral presentations of brain neoplasms. West J Med 1995;163:19-25. [PMC free article] [PubMed]
- 6.Levine DN, Finklestein S. Delayed psychosis after right temporoparietal stroke or trauma: relation to epilepsy. Neurology 1982; 32:267-73. [DOI] [PubMed]
- 7.Mace CJ, Trimble MR. Psychosis following temporal lobe surgery: a report of six cases. J Neurol Neurosurg Psychiatry 1991; 54:639-44. [DOI] [PMC free article] [PubMed]
- 8.Malamud N. Psychiatric disorder with intracranial tumors of limbic system. Arch Neurol 1967;17:113-23. [DOI] [PubMed]
- 9.McDade G. Symptomatic schizophrenia with Moya Moya disease. Behav Neurol 1991;4:25-8. [DOI] [PubMed]
- 10.Minski L. Mental symptoms associated with 58 cases of cerebral tumors. J Neurol Psychopathol 1933;13:330-43. [DOI] [PMC free article] [PubMed]
- 11.Savard G, Andermann F, Olivier A, Rémillard GM. Postictal psychosis after partial complex seizures: a multiple case study. Epilepsia 1991;32:225-31. [DOI] [PubMed]
- 12.Stevens J. Psychiatric consequences of temporal lobectomy for intractable seizures: a 20-30 year follow-up of 14 cases. Psychol Med 1990;20:529-45. [DOI] [PubMed]
- 13.Andy OJ, Webster JS, Carranza J. Frontal lobe lesions and behavior. South Med J 1981;74:968-72. [DOI] [PubMed]
- 14.Berg RA. Cancer. In: Tarter RE, Van Thiel DH, Edwards KL, editors. Medical neuropsychology: the impact of disease on behavior. New York: Plenum Press; 1988.
- 15.Blustein J, Seeman MV. Brain tumors presenting as functional psychiatric disturbances. Can Psychiatr Assoc J 1972;17:59-63. [DOI] [PubMed]
- 16.Burke JG, Dursun SM, Reveley MA. Refractory schizophrenia resulting from frontal lobe lesion: response to clozapine. J Psychiatry Neurosci 1999;24:456-61. [PMC free article] [PubMed]
- 17.Golden CJ. Clinical implications of neuropsychological assessment of children. In: Bell RW, Elias J, Greene RL, Harvey JH, editors. Developmental psychobiology and clinical neuropsychology. Lubbock: Texas Tech Press; 1984. p. 109-33.
- 18.Price BH, Mesulam M. Psychiatric manifestations of right hemisphere infarctions. J Nerv Ment Dis 1985;173:610-4. [DOI] [PubMed]
- 19.Fumihiko O, Toshimit A, Hiroshi A. Schizophrenic symptoms induced by a tumor of the left basal ganglia with ipsalateral cerebral hemiatrophy. Ann Clin Psychiatry 1992;4:105-9.
- 20.Guard O, Bellis F, Mabille JP, Dumas R, Boisson D, Devic M. Thalamic dementia after a unilateral hemorrhagic lesion of the right pulvinar [in French]. Rev Neurol (Paris) 1986;142:759-65. [PubMed]
- 21.Okada F, Toshimitsu A, Abe H. Schizophrenic symproms induced by a tumor of the left basal ganglia with ipsilateral hemiatrophy. Ann Clin Psychiatry 1992;4:105-9.
- 22.Ponsot G, Diebler C, Plouin P, Nardou M, Dulac O, Chaussain JL, et al. Hypothalamic hamartoma and gelastic crises. A propos of 7 cases [in French]. Arch Fr Pediatr 1983;40:757-61. [PubMed]
- 23.Fujii DEM, Ahmed I. Psychosis secondary to traumatic brain injury. Neuropsychiatry Neuropsychol Behav Neurol 1996;9:133-8.
- 24.Waldfogel S, Field HL, Wu L. Oedipism in a patient with frontal lobe encephalomalacia. Brain Inj 1994;8:377-81. [DOI] [PubMed]
- 25.Selecki BR. Intracranial space-occupying lesions among patients admitted to mental hospitals. Med J Aust 1965;1:383-90. [DOI] [PubMed]
- 26.Mesulam M. Dissociative states with abnormal temporal lobe EEG. Arch Neurol 1981;38:176-81. [DOI] [PubMed]
- 27.Miller BL, Lesser IM, Boone K, Goldberg M, Hill E, Miller MH, et al. Brain white-matter lesions and psychosis. Br J Psychiatry 1989; 155:73-8. [DOI] [PubMed]
- 28.Su K, Hsu C, Hsieh S, Shen WW. Magnetic resonance imaging findings in patients with delusional disorder due to diffuse cerebrovascular disease: a report of seven cases. Psychiatry Clin Neurosci 2001;55:121-7. [DOI] [PubMed]
- 29.Bentaleb LA, Stip E, Beauregard M. Psychopathologie et bases neurobiologiques des hallucinations auditives dans la schizophrénie. Sante Ment Que 2000;25:241-57. [PubMed]
- 30.Young WB, Heros DO, Ehrenberg BL, Hedges TR. Metamorphopsia and palinopsia: association with periodic lateralized eplieptiform discharges in a patient with malignant astrocytoma. Arch Neurol 1989;46:820-2. [DOI] [PubMed]
- 31.Penfield W, Perot P. The brain's record of auditory and visual experiences: a final summary and discussion. Brain 1963; 86:595-696. [DOI] [PubMed]
- 32.Cogan DG. Visual hallucinations as release phenomena. Graefes Arch Clin Exp Ophthalmol 1973;188:139-50. [DOI] [PubMed]
- 33.Lhermitte J. Syndrome de la callote du pédoncule cérébral. Les troubles psycho-sensoriels dans les lésions du mésencéphale. Rev Neurol 1922;38:1359-65.
- 34.Feinberg TE, Shapiro RM. Misidentification-reduplication and the right hemisphere. Neuropsychiatry Neuropsychol Behav Neurol 1989;2:39-48.
- 35.Kölmel HW. Complex visual hallucinations in the hemianopic field. J Neurol Neurosurg Psychiatry 1985;48:29-38. [DOI] [PMC free article] [PubMed]
- 36.Kölmel HW. Peduncular hallucinations. J Neurol 1991;238:457-9. [DOI] [PubMed]
- 37.Murata S, Naritomi H, Sawada T. Musical auditory hallucinations caused by a brainstem lesion. Neurology 1994;44:156-8. [DOI] [PubMed]
- 38.Nightingale S. Somatoparaphrenia: a case report. Cortex 1982; 18:463-7. [DOI] [PubMed]
- 39.Diederich NJ, Alesch F, Goetz CG. Visual hallucinations induced by deep brain stimulation in Parkinson's disease. Clin Neuropharm 2000;23:287-9. [DOI] [PubMed]
- 40.Shiga K, Makino M, Ueda Y, Nakajima K. Metamorphosia and visual hallucinations restricted to the right visual hemifield after a left putaminal haemorrhage. J Neurol Neurosurg Psychiatry 1996;61:420-2. [DOI] [PMC free article] [PubMed]
- 41.Kumar R, Behari S, Wahi J, Banerji D, Sharma K. Peduncular hallucinosis: an unusual sequel to surgical intervention in the suprasellar region. Br J Neurosurg 1999;13:500-3. [PubMed]
- 42.Smith RA, Gelles DB, Vanderhaeghen JJ. Subcortical visual hallucinations. Cortex 1971;7:162-8. [DOI] [PubMed]
- 43.Parkinson D, Rucker CW, McCraig W. Visual hallucinations associated with tumors of the occipital lobe. Arch Neurol Psychiatry (Chicago) 1952;68:66-8. [DOI] [PubMed]
- 44.Lepore FE. Spontaneous visual phenomena with visual loss: 104 patients with lesions of retinal and neural afferent pathways. Neurology 1990;40:444-7. [DOI] [PubMed]
- 45.Galasko DR, Kwo-On-Yuen, PF, Thal LJ. Intracranial mass lesions associated with late-onset psychosis and depression. Psychiatr Clin North Am 1988;11:151-66. [PubMed]
- 46.Ffytche DH, Howard RJ, Brammer MJ, David A, Woodruff P, Williams S. The anatomy of conscious vision: an fMRI study of visual hallucinations. Nat Neurosci 1998;1:738-42. [DOI] [PubMed]
- 47.Howard R, David A, Woodruff P, Mellers I, Wright J, Brammer M, et al. Seeing visual hallucinations with functional magnetic resonance imaging. Dement Geriatr Cogn Disord 1997;8:73-7. [DOI] [PubMed]
- 48.Adachi N, Watanabe T, Matsuda H, Onuma T. Hyperperfusion in the lateral temporal cortex, the striatum and the thalamus during complex visual hallucinations: single photon emission computed tomography findings in patients with Charles Bonnet syndrome. Psychiatry Clin Neurosci 2000; 54: 157-62. [DOI] [PubMed]
- 49.Manford M, Andermann F. Complex visual hallucinations. Clinical and neurobiological insights. Brain 1998;121:1819-40. [DOI] [PubMed]
- 50.Michel EM, Troost BT. Palinopsia: cerebral localization with computed tomography. Neurology 1980;30:887-9. [DOI] [PubMed]
- 51.Critchley M. Types of visual perseveration: palinopsia and illusory visual spread. Brain 1951;74:267-99. [DOI] [PubMed]
- 52.Bender MB. Polyopia and monocular diplopia of cerebral origin. Arch Neurol Psychiatry 1945;54:323-8. [DOI] [PubMed]
- 53.Meadows JC, Munro SSF. Palinopsia. J Neurol Neurosurg Psychiatry 1977;40:5-8. [DOI] [PMC free article] [PubMed]
- 54.La Mancusa JC, Cole AR. Visual manifestations of occipital lobe infarction in three patients on a geriatric psychiatry unit. J Geriatr Psychiatry Neurol 1988;1:231-4. [DOI] [PubMed]
- 55.Medina JL, Chokroverty S, Rubino FA. Syndrome of agitated delirium and visual impairment: a manifestation of medial temporo-occipital infarction. J Neurol Neurosurg Psychiatry 1977; 40:861-4. [DOI] [PMC free article] [PubMed]
- 56.Lance JW. Simple formed hallucinations confined to the area of a specific field defect. Brain 1976;99:19-34. [DOI] [PubMed]
- 57.Vaphiades MS, Celesia GG, Brigell MG. Positive spontaneous visual phenomena limited to the hemianopic field in lesions of central visual pathways. Neurology 1996;47:408-17. [DOI] [PubMed]
- 58.Hoksbergen I, Pickut BA, Mariën P, Slabbynck H, Kunnen J, De Deyn PP. SPECT findings in an unusual case of visual hallucinosis. J Neurol 1996;243:594-8. [DOI] [PubMed]
- 59.Lindner A, Reiners K, Toyka KV. Meningeal hyperperfusion visualized by MRI in a patient with visual hallucinations and migraine. Headache 1996;36:53-7. [DOI] [PubMed]
- 60.Benson MT, Rennie IG. Formed hallucination in the hemianopic field. Postgrad Med J 1989;65:756-7. [DOI] [PMC free article] [PubMed]
- 61.Kasten E, Muller-Oehring E, Poggel D, Sabel BA. Chronic visual hallucinations and illusions following brain lesions. A single study [in German]. Fortschr Neurol Psychiatr 1998;66:49-58. [DOI] [PubMed]
- 62.Anderson SW, Rizzo M. Hallucinations following occipital lobe damage: the pathological activation of visual representations. J Clin Exp Neuropsychol 1994;16:651-63. [DOI] [PubMed]
- 63.Cole M. When the left brain is not right the right brain may be left: report of personal experience of occipital hemianopia. J Neurol Neurosurg Psychiatry 1999;67:169-73. [DOI] [PMC free article] [PubMed]
- 64.Werring DJ, Marsden CD. Visual hallucinations and palinopsia due to an occipital lobe tuberculoma. J Neurol Neurosurg Psychiatry 1999;66:684. [DOI] [PMC free article] [PubMed]
- 65.Tapiador MJ, Lopez-Lopez A, Ayuso T. A review of the pathogenesis of the hallucinatory state. A description of four cases [in Spanish]. Rev Neurol 1996;24:1529-32. [PubMed]
- 66.Howlett DC, Downie AC, Banerjee AK, Tonge KA, Oakeley HF. MRI of an unusual case of peduncular hallucinosis (Lhermitte's syndrome). Neuroradiology 1994;36:121-2. [DOI] [PubMed]
- 67.Hattori T, Hirayama K, Imai T, Yamada T, Kojima S. Pontine lesion in opsoclonus-myoclonus syndrome shown by MRI. J Neurol Neurosurg Psychiatry 1998;51:1572-5. [DOI] [PMC free article] [PubMed]
- 68.Geller TJ, Bellur SN. Peduncular hallucinosis: magnetic resonance imaging confirmation of mesencephalic infarction during life. Ann Neurol 1987;21:602-4. [DOI] [PubMed]
- 69.McKee AC, Levine DN, Kowall NW, Richardson EP Jr. Peduncular hallucinosis associated with isolated infarction of the substantia nigra pars reticulata. Ann Neurol 1990;27:500-4. [DOI] [PubMed]
- 70.Catafau JS, Rubio F, Serra JP. Peduncular hallucinosis associated with posterior thalamic infarction. J Neurol 1992;239:89-90. [DOI] [PubMed]
- 71.Danziger N, Meary E, Mercier B, Samson Y, Rancurel G. Visual hallucinosis and hyperhedonism in pontine and thalamic infarction [in French]. Rev Neurol(Paris) 1997;153:679-83. [PubMed]
- 72.De La Fuente Fernandez R, Lope J, Rey del Corral P, de la Iglesia Martinez F. Peduncular hallucinosis and right hemiparkinsonism caused by left mesencephalic infarction. J Neurol Neurosurg Psychiatry 1994;57:870. [DOI] [PMC free article] [PubMed]
- 73.Van Bogaert L. L'hallucinose pédonculaire. Rev Neurol (Paris) 1927; 43:608-17.
- 74.Kumar R, Kaur A. Peduncular hallucinosis: an unusual sequelae of medulloblastoma surgery. Neurol India 2000;48:183-5. [PubMed]
- 75.Santhouse AM, Howard RJ, Ffytche DH. Visual hallucinatory syndromes and the anatomy of the visual brain. Brain 2000; 123: 2055-4. [DOI] [PubMed]
- 76.Brust JC, Behrens MM. “Release hallucinations” as the major symptom of posterior cerebral artery occlusion: a report of 2 cases. Ann Neurol 1977;2:432-36. [DOI] [PubMed]
- 77.De Morsier G. Pathogénie de l'hallucinose pédonculaire. À propos d'un nouveau cas. Rev Neurol 1935;64:606-24.
- 78.Brindley GS, Lewin WS. The sensations produced by electrical stimulation of the visual cortex. J Physiol 1968;196:479-93. [DOI] [PMC free article] [PubMed]
- 79.Ffytche DH, Howard RJ. The perceptual consequences of visual loss: ‘positive’ pathologies of vision. Brain 1999;122:1247-60. [DOI] [PubMed]
- 80.Cambier J, Decroix JP. Auditory hallucinations in lesions of the brain stem [in French]. Rev Neurol (Paris) 1987;143:255-62. [PubMed]
- 81.Dierks T, Linden DEJ, Jandl M, Formisano E, Goebel R, Lanfermann H, et al. Activation of Heschl's gyrus during auditory hallucinations. Neuron 1999;22:615-21. [DOI] [PubMed]
- 82.Shergill SS, Bramer MJ, Williams SCR, Murray RM, McGuire PK. Mapping auditory hallucinations in schizophrenia using functional magnetic resonance imaging. Arch Gen Psychiatry 2000; 57:1033-8. [DOI] [PubMed]
- 83.Lennox BR, Park SB, Medley I, Morris PG, Jones PB. The functional anatomy of auditory hallucinations in schizophrenia. Psychiatr Res 2000;100:13-20. [DOI] [PubMed]
- 84.Silbersweig DA, Stern E, Frith C, Cahill C, Holmes A, Grootoonk S, et al. A functional neuroanatomy of hallucinations in schizophrenia. Nature 1995;378:176-9. [DOI] [PubMed]
- 85.Bentaleb LA, Beauregard M, Liddle P, Stip E. Cerebral activity associated with auditory verbal hallucinations: a functional magnetic resonance imaging case study. J Psychiatry Neurosci 2002; 27:110-5. [PMC free article] [PubMed]
- 86.Griffiths TD. Musical hallucinosis in acquired deafness. Phenomenology and brain substrate. Brain 2000;123:2065-76. [DOI] [PubMed]
- 87.Paquier P, Van Vugt P, Bal P, Cras P, Parizel PM, Van Haesendonck J, et al. Transient musical hallucinosis of central origin: a review and clinical study. J Neurol Neurosurg Psychiatry 1992;55:1069-73. [DOI] [PMC free article] [PubMed]
- 88.Hécaen H, Ropert R. Hallucinations auditives au cours de syndromes neurologiques. Ann Med Psychol (Paris) 1959;117:257-306. [PubMed]
- 89.Tanabe, H, Sawada T, Asai H, Okuda J, Shiraishi J. Lateralisation phenomenon of complex auditory hallucinations. Acta Psychiatr Scand 1986;74:178-82. [DOI] [PubMed]
- 90.Lesser IM, Jeste DV, Boone KB, Harris MJ, Miller BL, Heaton RK, et al. Late-onset psychotic disorder, not otherwise specified: clinical and neuroimaging findings. Biol Psychiatry 1992; 31: 419-23. [DOI] [PubMed]
- 91.Bremer J. New-onset auditory hallucinations after right temporal lobectomy. Am J Psychiatry 1996;153:442-3. [DOI] [PubMed]
- 92.Inzelberg R, Visnievskaya S, Korczyn AD. Transient musical hallucinosis. J Neurol Neurosurg Psychiatry 1993;56:833. [DOI] [PMC free article] [PubMed]
- 93.Lanska DJ, Lanska MJ, Mendez MF. Brainstem auditory hallucinosis. Neurology 1987;37:1685. [DOI] [PubMed]
- 94.Cascino GD, Adams RD. Brainstem auditory hallucinosis. Neurology 1986;36:1042-7. [DOI] [PubMed]
- 95.Gonzales GR, Lewis SA, Weaver AL. Tactile illusion perception in patients with central pain. Mayo Clin Proc 2001;76:267-74. [DOI] [PubMed]
- 96.Bowsher D, Leijon G, Thuomas KA. Central poststroke pain: correlation of MRI with clinical pain characteristics and sensory abnormalities. Neurology 1998;51:1352-8. [DOI] [PubMed]
- 97.Potagas C, Avdelidis D, Singounas E, Missir O, Aessopos A. Episodic pain associated with a tumor in the parietal operculum: a case report and literature review. Pain 1997;72:201-8. [DOI] [PubMed]
- 98.Déjerine J, Roussy G. Le syndrome thalamique. Rev Neurol 1906; 14:521-32.
- 99.Finnerup NB, Johannesen IL, Sindrup SH, Bach FW, Jensen TS. Pain and dysesthesia in patients with spinal cord injury: a postal survey. Spinal Cord 2001;39:256-62. [DOI] [PubMed]
- 100.Lotze M, Flor H, Grodd W, Larbig W, Birbaumer N. Phantom movements and pain. An fMRI study in upper limb amputees. Brain 2001;124:2268-77. [DOI] [PubMed]
- 101.Willoch F, Rosen G, Tolle TR, Oye I, Wester HJ, Berner N, et al. Phantom limb pain in the human brain: unraveling neural circuitries of phantom limb sensations using positron emission tomography. Ann Neurol 2000;48:842-9. [PubMed]
- 102.Shergill SS, Cameron LA, Brammer MJ, Williams SC, Murray RM, McGuire PK. Modality specific neural correlates of auditory and somatic hallucinations. J Neurol Neurosurg Psychiatry 2001; 71:688-90. [DOI] [PMC free article] [PubMed]
- 103.Halligan PW, Marshall JC, Wade DT. Unilateral somatoparaphrenia after right hemisphere stroke: a case description. Cortex 1995;31:173-82. [DOI] [PubMed]
- 104.Hécaen H, Ajuriaguerra J. Méconnaissances et hallucinations corporelles. Paris: Masson; 1952.
- 105.Rode G, Charles N, Perenin MT, Vighetto A, Trillet M, Aimard G. Partial remission of hemiplegia and somatoparaphrenia through vestibular stimulation in a case of unilateral neglect. Cortex 1992;28:203-8. [DOI] [PubMed]
- 106.Assal G. No, I am not paralyzed, it's my husband's hand [in French]. Schweiz Arch Neurol Neurochir Psychiatr 1983;133:151-7. [PubMed]
- 107.Miura N, Takeda A, Terao S, Tanaka H, Ishikawa S, Mitsuma T. Somatoparaphrenia caused by the lesion in the dominant cerebral hemisphere—a case report [in Japanese]. No To Shinkei 1996; 48: 275-9. [PubMed]
- 108.Fujii Y, Konishi Y, Kuriyama M, Hori C, Sudo M. Lipoma on surface of centroparietal lobes. Pediatr Neurol 1993;9:144-6. [DOI] [PubMed]
- 109.Lorenz J, Kohlhoff H, Hansen HC, Kunze K, Bromm B. A-beta fiber mediated activation of cingulate cortex as correlate of post-stroke pain. Neuroreport 1998;9:659-63. [DOI] [PubMed]
- 110.Van Bogaert L. Sur la pathologie de l'image de soi. Ann Med Psychol (Paris) 1934;92:419-555.
- 111.Halligan PW, Marshall JC, Wade, DT. Three arms: a case study of supernumerary phantom limb after right hemisphere stroke. J Neurol Neurosurg Psychiatry 1993;56:159-66. [DOI] [PMC free article] [PubMed]
- 112.Paciaroni M, Bogousslavsky J. Pure sensory syndromes in thalamic stroke. Eur Neurol 1998;39:211-7. [DOI] [PubMed]
- 113.Franzini A, Ferroli P, Servello D, Broggi G. Reversal of thalamic hand syndrome by long-term motor cortex stimulation. J Neurosurg 2000;93:873-5. [DOI] [PubMed]
- 114.Tatu L, Moulin T, Chavot D, Berges S, Chopard JL, Rumbach L. Hallucinations and thalamic infarction [in French]. Rev Neurol (Paris) 1996; 152:557-9. [PubMed]
- 115.Ehrenwald H. Vernandertes erleben des korperbildes mit konsekutiver wahnbildung bei linksseitiger hemiplegie. Monatsschr Psychiatr Neurol 1930;75:89-97.
- 116.Critchley M. Clinical investigations of disease of the parietal lobes of the brain. Med Clin North Am 1962;46:837-57. [DOI] [PubMed]
- 117.Anastopoulos GK. Die nosoagnostiche uberschatzung. Psychiatr Neurol (Basel) 1961;141:214-28.13683103
- 118.Frederiks JAM. Disorders of the body schema. In: Vinken PJ, Bruyn GW, editors. Handbook of clinical neurology. Vol. 4. Amsterdam: North Holland; 1969.
- 119.Fuster JM. Distributed memory for both short and long term. Neurobiol Learn Mem 1998;70:268-74. [DOI] [PubMed]
- 120.Heron W, Doane BK, Scott TH. Visual disturbance after prolonged perceptual isolation. Can J Psychol 1956;10:13-8. [DOI] [PubMed]
- 121.Gilbert CD, Wiesel TN. Receptive field dynamics in adult primary cortex. Nature 1992;356:150-2. [DOI] [PubMed]
- 122.Boivie J, Leijon G. Clinical findings in patients with central poststroke pain. In: Casey KL. Pain and central nervous system disease: the central pain syndromes. New York: Raven Press; 1991. p. 65-75.
- 123.Noda S, Mizoguchi M, Yamamoto A. Thalamic experiential hallucinosis. J Neurol Neurosurg Psychiatry 1993;56:1224-6. [DOI] [PMC free article] [PubMed]
- 124.Stephane M, Barton S, Boutros NN. Auditory verbal hallucinations and dysfunction of the neural substrates of speech. SchizophrRes 2001;50:61-78. [DOI] [PubMed]
- 125.Teunisse RJ, Cruysberg JR, Hoefnagels WH, Verbeek AL, Zitman FG. Visual hallucinations in psychologically normal people: Charles Bonnet's syndrome. Lancet 1996;347:794-7. [DOI] [PubMed]
- 126.Berrios GE. Tactile hallucinations: conceptual and historical aspects. J Neurol Neurosurg Psychiatry 1982;45:285-93. [DOI] [PMC free article] [PubMed]
- 127.Crohn R. Phantom vision. Arch Neurol 1971;25:468-71. [DOI] [PubMed]
- 128.Pelaez JR. Towards a neural network based therapy for hallucinatory disorders. Neural Netw 2000;13:1047-61. [DOI] [PubMed]
- 129.Edell WS, Tunis SL. Antipsychotic treatment of behavioral and psychological symptoms of dementia in geropsychiatric inpatients. Am J Geriatr Psychiatry 2001;9:289-97. [PubMed]
- 130.Batra A, Bartels M, Wormstall H. Therapeutic options in Charles Bonnet syndrome. Acta Psychiatr Scand 1997;96:129-33. [DOI] [PubMed]
- 131.Burke WJ, Roccaforte WH, Wengel SP. Treating visual hallucinations with donepezil. Am J Psychiatry 1999;156:1117-8. [DOI] [PubMed]