Normal cardiac function relies on the tight coupling of functionally-related ion channels and transporters in the sarcolemma (plasma membrane and transverse-tubule network, TTs) with calcium-release channels (ryanodine receptors, type 2, RyR2) in the sarcoplasmic reticulum (SR), the intracellular Ca2+ storage organelle (reviewed in [1]). Cardiac excitation-contraction (EC) coupling is initiated by membrane depolarization during the action potential (AP) that activates voltage-gated L-type Ca2+ channels (LTCC) in the sarcolemma. The small increase in local [Ca2+]i due to the Ca2+ flux through the plasma membrane Ca2+ channels is detected by nearby (15 nm) clusters of RyR2s in the junctional SR (jSR) to produce Ca2+ sparks. This amplification system (termed “Ca2+-induced Ca2+ release” or CICR) operates at high gain with great stability and is referred to as "local control" because there is a high [Ca2+]i only locally between the LTCC and the jSR (in small space between them called the "subspace") [2–4]. The synchronization of Ca2+ sparks by the AP produces the cell-wide [Ca2+]i transient that activates contraction. Instability in cardiac Ca2+ management may be due to altered RyR sensitivity ("RyR2 tuning" - see [2, 5]), altered spatial organization of local Ca2+ release sites, or mutations and variants of the RyR2 protein (as those found in specific diseases such as catecholaminergic polymorphic ventricular tachycardia). These changes in cardiac Ca2+ signaling may result in defects in myocyte electrical activity and multiple human cardiac disease phenotypes, including arrhythmia, myopathy, and heart failure [6].
While RyR2 Ca2+ release channels have received significant attention by molecular cardiologists, in the past five years the role of a second pathway for internal Ca2+-release has largely been ignored. Specifically, the cellular role(s) for inositol 1,4,5-trisphosphate receptors (IP3R) have remained elusive. However, there is great and growing interest in cardiac IP3 signaling due to the known importance of several IP3-inducing agonists (e.g. angiotensin II, endothelin, and norepinephrine) in hypertrophy and heart failure [7–15].
While agonist-induced IP3-dependent Ca2+ release is readily observed in most tissues, the role of IP3Rs in cardiac tissue is less clear. The subcellular localization of IP3Rs in cardiac myocytes has received increasing attention as the field attempts to define the function of these channels. In ventricular myocytes, immunofluorescence studies show that IP3Rs are found at the Z-lines, in the perinuclear region and in the nuclear membrane [7, 16, 17]. Moreover, IP3Rs are found in similar locations in both atrial [12] and Purkinje myocytes [18–20]). The role(s) played by these IP3Rs have yet to be convincingly demonstrated, but provocative suggestions for their function include modulation of transcription [21], amplification of RyR2 Ca2+ signals [9], and independent activation through diverse pathways that generate IP3 [22, 23]. While expression of IP3Rs (mainly type 2 in atrial and ventricular myocytes and type 1 in Purkinje myocytes [18–20]) is about 50-fold less than RyR2s in ventricular myocytes [24], there are still about 20,000 copies per ventricular myocyte and likely more per cell in both atrial and Purkinje myocytes [18].
In this issue of The Journal of Molecular and Cellular Cardiology, Hirose and colleagues identify a small population of wide long-lasting Ca2+-release events (WLE) in isolated canine cardiac Purkinje cells that are triggered from subsarcolemmal and perinuclear domains [25]. The biophysical basis of these unusual Ca2+ release events is unclear. Do they arise from clusters of RyR2s with some IP3Rs nearby? Furthermore, what is the stoichiometry and organization of the RyR2s and the IP3Rs? How is Ca2+ release terminated? What role is played by the SR/ER/nuclear envelope Ca2+ content? How important are the various lumenal Ca2+ buffers such as calsequestrin and calreticulin? What is the biophysical basis for Ca2+ wave generation and propagation? How do IP3Rs contribute to the origin and propagation of Ca2+ waves? The answers to these questions are paramount for understanding Purkinje fiber Ca2+ signaling and also for understanding the contributions of IP3Rs to all myocyte Ca2+ signaling.
In their new manuscript, Hirose and colleagues demonstrate that wide long-lasting Ca2+-release events are augmented by the IP3-generating alpha-adrenergic agonist phenylephrine but not in the presence of a phospholipase C inhibitor U73122 (or the putative IP3R blocker 2APB). Consistent with their previous findings [20], Hirose and colleagues describe type 1 IP3Rs in the subsarcolemma. However, in their new manuscript, with a new antibody, Hirose et al. identify a second population of perinuclear IP3Rs not previously observed [20]. Specifically, they show IP3Rs localize with RyR2s near the nucleus. This proximity of IP3Rs to RyR2s near the nucleus may underlie the amplification of perinuclear IP3R Ca2+ signals and support a regional Ca2+ wave or mini-wave at those sites. Hirose and colleagues also show that on rare occasions wide long-lasting Ca2+-release events may generate cell-wide waves.
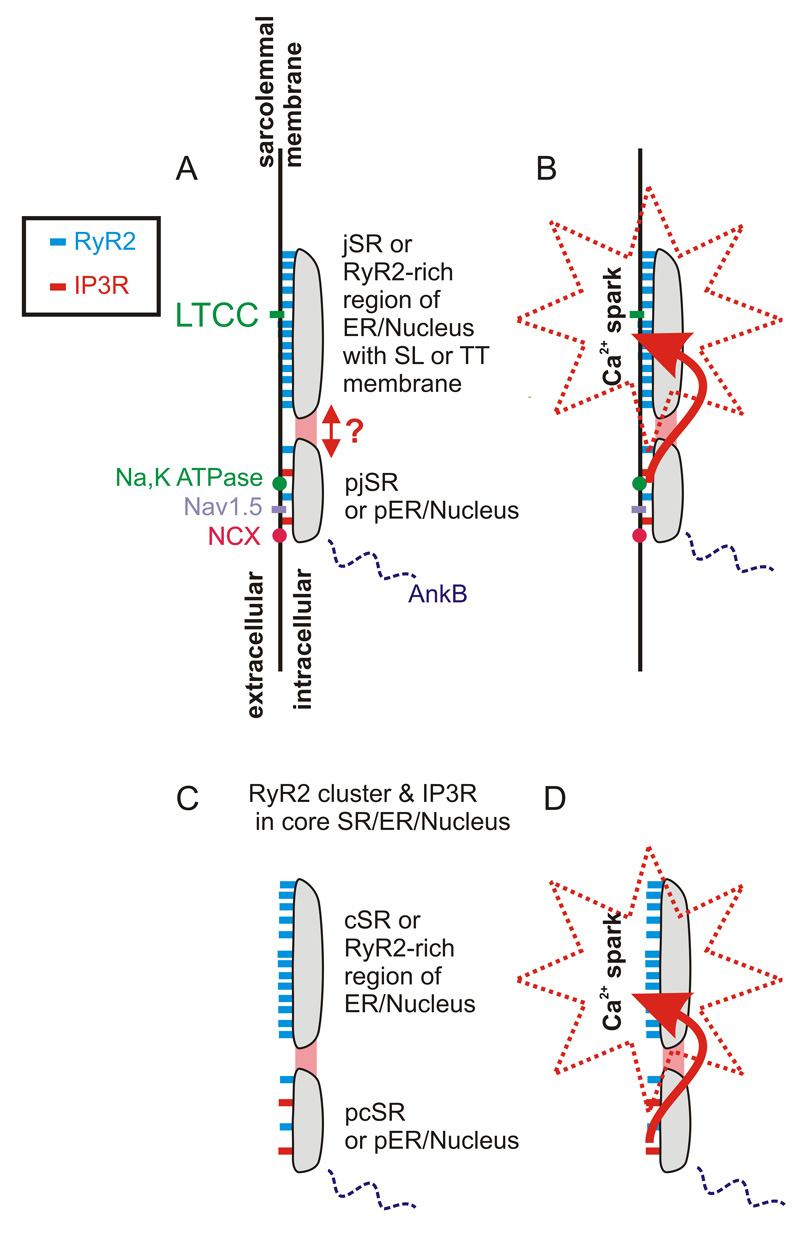
Mounting evidence suggests there may be two classes of organized SR Ca2+ release sites in myocytes (See Fig. 1). To date, however, high resolution electron micrographs have not specifically revealed such an organization - nor have they denied it. Nevertheless, RyR2s are organized tightly with LTCCs as shown by the Moore group [26], a requirement for local control of EC coupling [27–29], while our group and others have demonstrated that IP3Rs, ankyrin-B, Na+/K+ ATPase, Na+/Ca2+ exchanger and Na+ channels (Nav1.5) are co-localized nearby at the Z-line [16, 17, 30, 31]. These findings suggest a spatial organization shown in Fig. 1 which, while based on information in the literature, remains somewhat speculative. In this paradigm, the parajunctional SR (pjSR), a region of the SR near the jSR, contains IP3Rs, and only a few RyR2s. The pjSR is positioned near Na+ channels, the Na+/Ca2+ exchanger, the Na+/K+ ATPase and stabilized by ankyrin-B. In cells with few TT, such as atrial and Purkinje fiber cells, there may be a "corbular" SR (cSR) structure and a "para-corbular" structure (pcSR) in the cell core and in and around the nucleus and ER.
Figure 1. Hypothesized organization of the RyR2 and IP3Rs in cardiac myocytes.
Recent studies suggest the network of intracellular Ca2+ storage organelles consists of two components, one containing a large cluster of only RyR2s (labeled here as the junctional SR or corbular SR) and another containing RyR2s mixed with IP3Rs (labeled the para-junctional SR or para-corbular SR). Because the SR, ER and nuclear envelope Ca2+ storage organelles are interconnected, they are represented as a single element in each of the drawings. The interconnecting "network SR" or "free SR" is not shown in these drawings but serves to connect all jSR and cSR and pjSR and pcSR to the ER and nuclear components. A. The jSR and pjSR are located along the SL and TT (when present) membranes that contain diverse channels and transporters. B. Close proximity of the pjSR to the jSR and the distinct kinetics of pjSR Ca2+ release channels enable the pjSR to influence Ca2+ signaling and Ca2+ sparks in the jSR. C. RyR2 clusters not associated with an extracellular membrane are here loosely termed the corbular SR (cSR) and its nearby para-corbular SR (pcSR). D. Close proximity of the pcSR to the cSR and the distinct kinetics of pcSR Ca2+ release channels enable the pcSR to influence Ca2+ signaling and Ca2+ sparks in the cSR.
Under normal conditions in cardiac Purkinje fiber cells, depolarization triggers an increase in subsarcolemmal (SSL) Ca2+ followed by passive diffusion of Ca2+ into the non-SSL region (cellular core) [32] without much CICR amplification. This unique Ca2+ signal characterized by restriction of AP-triggered Ca2+ release to the SSL region and absence of propagated CICR is attributable to the lack of TTs and the relatively small amount of Ca2+ released in the SSL region combined with the insensitivity of core RyR2s in cSR/ER/nuclear regions. Activation of other Ca2+ release channels (e.g. IP3Rs) may have a large effect in myocytes with few TTs such as atrial and Purkinje fiber cells. Under these conditions, we envision five scenarios governing the cellular impact of Ca2+ release from IP3R-rich sites: 1) It may increase local [Ca2+] at RyR2 clusters in jSR/cSR/ER/nuclear regions, thereby increasing the probability of triggering a Ca2+ spark; 2) It may increase the Ca2+ spark duration due to the fact that IP3Rs have unique channel kinetics; 3) It may increase the spatial extent of a single Ca2+ spark because Ca2+ release occurs away from the Ca2+ spark center; (4) It may enhance instability because a Ca2+ spark site will be more distant from the central site of elevated [Ca2+]i (spatial disarray); (5) Additional Ca2+ release triggers may be possible due to the sensitivity of the pjSR or pcSR collection of RyR2s and IP3Rs. The overall actions of IP3R-dependent altered Ca2+ spark rate, and size or likelihood of Ca2+ wave propagation, will be constrained by the requirements of pump-leak balance of the SR/ER/nuclear Ca2+ stores (see [9]). The provocative studies of Hirose et al [25] in Purkinje fiber cells appear to be consistent with the model described in Fig. 1 and may also be relevant to atrial and ventricular myocytes.
Purkinje fibers constitute a specialized conduction system in the heart allowing for the rapid and coordinated transfer of the propagating depolarization wave through the large ventricular mass. This special role played by Purkinje fibers in heart combined with their spatially intermittent isolation from the ventricular mass make them potential arrhythmogenic sources as illustrated by mapping studies in idiopathic ventricular fibrillation, long-QT and Brugada syndromes, and following myocardial infarction [33–35]. Several groups have explored the link between Ca2+ waves and afterdepolarizations in Purkinje fiber cells as a possible mechanism for triggered arrhythmias [36–38]. The findings by Hirose and colleagues raise the possibility that activation of IP3Rs promotes the development of Ca2+ sparks and waves in Purkinje fiber cells and suggests that IP3R activation may be pro-arrhythmic. The role of IP3R-dependent enhanced Ca2+ release in the ER and nuclear and peri-nuclear regions may affect EC coupling and may also contribute to Ca2+-dependent transcription modulation. Despite provocative and suggestive work to date [21, 39], details of targeted Ca2+-dependent transcriptional regulation are missing in heart and represent an important experimental and conceptual challenge. Perhaps the greatest outstanding questions about IP3Rs in cardiac myocytes remain the most practical: What do they do? Why are they placed where they are? How do they influence Ca2+ signaling?
Acknowledgments
Much of this work was supported by research and training grants from the National Institute of Heart Lung and Blood (NHLBI). TJH is supported by T32 HL00731. APZ by NHLBI T32 HL072751, and also by NIAMS T32 AR007592 and NIGMS T32 GM 008181. WJL is supported by NHLBI (P01 HL67849, R01 HL 36974), by the Leducq Foundation and by the State of Maryland Stem Cell Fund. PJM is supported by NHLBI (HL084583 and HL62494) and the Pew Scholars Trust.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bers DM. Excitation-Contraction Coupling and Cardiac Contractile Force. Second ed. Dordrecht: Kluwer Academic Publishers; 2001. [Google Scholar]
- 2.Cheng H, Lederer WJ. Calcium Sparks. Physiol Rev. 2008 doi: 10.1152/physrev.00030.2007. In Press. [DOI] [PubMed] [Google Scholar]
- 3.Niggli E, Lederer WJ. Voltage-independent calcium release in heart muscle. Science. 1990;250(4980):565–568. doi: 10.1126/science.2173135. [DOI] [PubMed] [Google Scholar]
- 4.Stern MD. Theory of excitation-contraction coupling in cardiac muscle. Biophys J. 1992 Aug;63(2):497–517. doi: 10.1016/S0006-3495(92)81615-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kass RS, Lindegger N, Hagen B, Lederer WJ. Another calcium paradox in heart failure. J Mol Cell Cardiol. 2008 doi: 10.1016/j.yjmcc.2008.04.001. In Press. [DOI] [PubMed] [Google Scholar]
- 6.Lehnart SE, Ackerman MJ, Benson DW, Jr, Brugada R, Clancy CE, Donahue JK, et al. Inherited Arrhythmias: A National Heart, Lung, and Blood Institute and Office of Rare Diseases Workshop Consensus Report About the Diagnosis, Phenotyping, Molecular Mechanisms, and Therapeutic Approaches for Primary Cardiomyopathies of Gene Mutations Affecting Ion Channel Function. Circulation. 2007 Nov 13;116(20):2325–2345. doi: 10.1161/CIRCULATIONAHA.107.711689. [DOI] [PubMed] [Google Scholar]
- 7.Bare DJ, Kettlun CS, Liang M, Bers DM, Mignery GA. Cardiac type 2 inositol 1,4,5-trisphosphate receptor: interaction and modulation by calcium/calmodulin-dependent protein kinase II. J Biol Chem. 2005 Apr 22;280(16):15912–15920. doi: 10.1074/jbc.M414212200. [DOI] [PubMed] [Google Scholar]
- 8.Bootman MD, Roderick HL. Why, where, and when do cardiac myocytes express inositol 1,4,5-trisphosphate receptors? Am J Physiol Heart Circ Physiol. 2008 Feb;294(2):H579–H581. doi: 10.1152/ajpheart.01378.2007. [DOI] [PubMed] [Google Scholar]
- 9.Domeier TL, Zima AV, Maxwell JT, Huke S, Mignery GA, Blatter LA. IP3 receptor-dependent Ca2+ release modulates excitation-contraction coupling in rabbit ventricular myocytes. Am J Physiol Heart Circ Physiol. 2008 Feb;294(2):H596–H604. doi: 10.1152/ajpheart.01155.2007. [DOI] [PubMed] [Google Scholar]
- 10.Dorn GW, 2nd, Brown JH. Gq signaling in cardiac adaptation and maladaptation. Trends Cardiovasc Med. 1999 Jan–Feb;9(1–2):26–34. doi: 10.1016/s1050-1738(99)00004-3. [DOI] [PubMed] [Google Scholar]
- 11.Kockskamper J, Seidlmayer L, Walther S, Hellenkamp K, Maier LS, Pieske B. Endothelin-1 enhances nuclear Ca2+ transients in atrial myocytes through Ins(1,4,5)P3-dependent Ca2+ release from perinuclear Ca2+ stores. J Cell Sci. 2008 Jan 15;121(Pt 2):186–195. doi: 10.1242/jcs.021386. [DOI] [PubMed] [Google Scholar]
- 12.Mackenzie L, Bootman MD, Laine M, Berridge MJ, Thuring J, Holmes A, et al. The role of inositol 1,4,5-trisphosphate receptors in Ca(2+) signalling and the generation of arrhythmias in rat atrial myocytes. J Physiol. 2002 Jun 1;541(Pt 2):395–409. doi: 10.1113/jphysiol.2001.013411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Molkentin JD. Dichotomy of Ca2+ in the heart: contraction versus intracellular signaling. J Clin Invest. 2006 Mar;116(3):623–626. doi: 10.1172/JCI27824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roderick HL, Bootman MD. Pacemaking, arrhythmias, inotropy and hypertrophy: the many possible facets of IP3 signalling in cardiac myocytes. J Physiol. 2007 Jun 15;581(Pt 3):883–884. doi: 10.1113/jphysiol.2007.133819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zima AV, Blatter LA. Inositol-1,4,5-trisphosphate-dependent Ca(2+) signalling in cat atrial excitation-contraction coupling and arrhythmias. J Physiol. 2004 Mar 16;555(Pt 3):607–615. doi: 10.1113/jphysiol.2003.058529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mohler PJ, Davis JQ, Bennett V. Ankyrin-B Coordinates the Na/K ATPase, Na/Ca Exchanger, and InsP(3) Receptor in a Cardiac T-Tubule/SR Microdomain. PLoS Biol. 2005 Dec;3(12):e423. doi: 10.1371/journal.pbio.0030423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mohler PJ, Schott JJ, Gramolini AO, Dilly KW, Guatimosim S, duBell WH, et al. Ankyrin-B mutation causes type 4 long-QT cardiac arrhythmia and sudden cardiac death. Nature. 2003 Feb 6;421(6923):634–639. doi: 10.1038/nature01335. [DOI] [PubMed] [Google Scholar]
- 18.Gorza L, Schiaffino S, Volpe P. Inositol 1,4,5-trisphosphate receptor in heart: evidence for its concentration in Purkinje myocytes of the conduction system. J Cell Biol. 1993;121(2):345–353. doi: 10.1083/jcb.121.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lipp P, Laine M, Tovey SC, Burrell KM, Berridge MJ, Li W, et al. Functional InsP3 receptors that may modulate excitation-contraction coupling in the heart. Curr Biol. 2000;10(15):939–942. doi: 10.1016/s0960-9822(00)00624-2. [DOI] [PubMed] [Google Scholar]
- 20.Stuyvers BD, Dun W, Matkovich S, Sorrentino V, Boyden PA, ter Keurs HE. Ca2+ sparks and waves in canine purkinje cells: a triple layered system of Ca2+ activation. Circ Res. 2005 Jul 8;97(1):35–43. doi: 10.1161/01.RES.0000173375.26489.fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu X, Zhang T, Bossuyt J, Li X, McKinsey TA, Dedman JR, et al. Local InsP3-dependent perinuclear Ca2+ signaling in cardiac myocyte excitation-transcription coupling. J Clin Invest. 2006 Mar;116(3):675–682. doi: 10.1172/JCI27374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poggioli J, Sulpice JC, Vassort G. Inositol phosphate production following alpha 1-adrenergic, muscarinic or electrical stimulation in isolated rat heart. FEBS Lett. 1986 Oct 6;206(2):292–298. doi: 10.1016/0014-5793(86)80999-1. [DOI] [PubMed] [Google Scholar]
- 23.Sugden PH. An overview of endothelin signaling in the cardiac myocyte. J Mol Cell Cardiol. 2003 Aug;35(8):871–886. doi: 10.1016/s0022-2828(03)00153-6. [DOI] [PubMed] [Google Scholar]
- 24.Moschella MC, Marks AR. Inositol 1,4,5-trisphosphate receptor expression in cardiac myocytes. The Journal of cell biology. 1993 Mar;120(5):1137–1146. doi: 10.1083/jcb.120.5.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hirose M, Stuyvers BD, Dun W, Ter Keurs HE, Boyden PA. Wide long lasting perinuclear calcium release events generated by an interaction between ryanodine and IP3 receptors in canine Purkinje cell. J Mol Cell Cardiol. 2008;XXXXX(XXXXX):XXXXX. doi: 10.1016/j.yjmcc.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scriven DR, Dan P, Moore ED. Distribution of proteins implicated in excitation-contraction coupling in rat ventricular myocytes. BiophysJ. 2000;79(5):2682–2691. doi: 10.1016/S0006-3495(00)76506-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Niggli E, Lederer WJ. Voltage-independent calcium release in heart muscle. Science. 1990;250(4980):565–568. doi: 10.1126/science.2173135. [DOI] [PubMed] [Google Scholar]
- 28.Cannell MB, Cheng H, Lederer WJ. The control of calcium release in heart muscle. Science. 1995;268(5213):1045–1049. doi: 10.1126/science.7754384. [DOI] [PubMed] [Google Scholar]
- 29.Stern MD. Theory of excitation-contraction coupling in cardiac muscle. Biophysical Journal. 1992;63:497–517. doi: 10.1016/S0006-3495(92)81615-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mohler PJ, Rivolta I, Napolitano C, Lemaillet G, Lambert S, Priori SG, et al. Nav1.5 E1053K mutation causing Brugada syndrome blocks binding to ankyrin-G and expression of Nav1.5 on the surface of cardiomyocytes. Proc Natl Acad Sci U S A. 2004 Dec 14;101(50):17533–17538. doi: 10.1073/pnas.0403711101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scriven DR, Dan P, Moore ED. Distribution of proteins implicated in excitation-contraction coupling in rat ventricular myocytes. Biophys J. 2000;79(5):2682–2691. doi: 10.1016/S0006-3495(00)76506-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cordeiro JM, Spitzer KW, Giles WR, Ershler PE, Cannell MB, Bridge JH. Location of the initiation site of calcium transients and sparks in rabbit heart Purkinje cells. J Physiol. 2001 Mar 1;531(Pt 2):301–314. doi: 10.1111/j.1469-7793.2001.0301i.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haissaguerre M, Extramiana F, Hocini M, Cauchemez B, Jais P, Cabrera JA, et al. Mapping and ablation of ventricular fibrillation associated with long-QT and Brugada syndromes. Circulation. 2003 Aug 26;108(8):925–928. doi: 10.1161/01.CIR.0000088781.99943.95. [DOI] [PubMed] [Google Scholar]
- 34.Haissaguerre M, Shoda M, Jais P, Nogami A, Shah DC, Kautzner J, et al. Mapping and ablation of idiopathic ventricular fibrillation. Circulation. 2002 Aug 20;106(8):962–967. doi: 10.1161/01.cir.0000027564.55739.b1. [DOI] [PubMed] [Google Scholar]
- 35.Szumowski L, Sanders P, Walczak F, Hocini M, Jais P, Kepski R, et al. Mapping and ablation of polymorphic ventricular tachycardia after myocardial infarction. J Am Coll Cardiol. 2004 Oct 19;44(8):1700–1706. doi: 10.1016/j.jacc.2004.08.034. [DOI] [PubMed] [Google Scholar]
- 36.Boyden PA, Barbhaiya C, Lee T, ter Keurs HE. Nonuniform Ca2+ transients in arrhythmogenic Purkinje cells that survive in the infarcted canine heart. Cardiovasc Res. 2003 Mar;57(3):681–693. doi: 10.1016/s0008-6363(02)00725-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boyden PA, Pu J, Pinto J, Keurs HE. Ca(2+) transients and Ca(2+) waves in purkinje cells : role in action potential initiation. Circ Res. 2000 Mar 3;86(4):448–455. doi: 10.1161/01.res.86.4.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cordeiro JM, Bridge JH, Spitzer KW. Early and delayed afterdepolarizations in rabbit heart Purkinje cells viewed by confocal microscopy. Cell Calcium. 2001 May;29(5):289–297. doi: 10.1054/ceca.2000.0192. [DOI] [PubMed] [Google Scholar]
- 39.Guatimosim S, Amaya MJ, Guerra MT, Aguiar CJ, Goes AM, Gomez-Viquez NL, et al. Nuclear Ca(2+) regulates cardiomyocyte function. Cell Calcium. 2008 Jan 15; doi: 10.1016/j.ceca.2007.11.016. [DOI] [PubMed] [Google Scholar]