Abstract
Repeated treatment with the dopamine D2/D3 receptor agonist quinpirole produces a sensitized behavioral response in rats manifested as an increase in locomotor activity. Pre-treatment with certain monoamine oxidase inhibitors, such as Ro 41-1049 [N-(2-aminomethyl)-5-(3-fluorophenyl)-4-thiazolecarboxamide HCl], changes the sensitized response from locomotion to stationary, self-directed mouthing. In this study, the effects of quinpirole sensitization, with and without pre-treatment with Ro 41-1049, were determined on dopamine D2-like receptors in the nucleus accumbens and the striatum. Long-Evans rats were pre-treated with Ro 41-1049 (1 mg/kg) 90 min prior to administration of quinpirole (0.5 mg/kg, 8 injections, every 3–4 days). Dopamine D2-like receptor binding was determined 3 days after the last injection by ex vivo radioligand assays using [3H]spiperone and [3H]quinpirole. Densities of [3H]spiperone- and [3H]quinpirole-labeled sites were both increased 32% in the nucleus accumbens of rats with demonstrated locomotor sensitization to quinpirole. In contrast, the density of dopamine D2-like receptors in quinpirole-sensitized rats pre-treated with Ro 41-1049 was not different from saline controls. These findings support the involvement of alterations in dopamine D2-like receptors in the development of locomotor sensitization to quinpirole and suggest that modification of these alterations in dopamine D2-like receptors contributes to the change from sensitized locomotion to mouthing observed when rats are pre-treated with Ro 41-1049.
Keywords: Dopamine D2 receptor, Sensitization, Quinpirole, Striatum, Nucleus accumbens
1. Introduction
Dopaminergic psychostimulants produce sensitized behavioral responses with repeated, intermittent administration in which the response is greater than after a single, acute administration of the same dose (Robinson and Becker, 1986). This long-lasting phenomenon is thought to contribute to the etiology of addiction, with enhanced activation of the mesolimbic dopamine system contributing to the development of drug craving (Di Chiara, 1995; Robinson, 1993). Similarly, sensitization to other stimuli, such as stressors or chemicals, is hypothesized to contribute to the development of neuropsychiatric disorders including mania, psychosis, obsessive-compulsive disorder, post-traumatic stress disorder, and multiple chemical sensitivity (Ellison, 1994; Post and Contel, 1981; Schmidt and Beninger, 2006; Szechtman et al., 1998). Functional changes in the “motive circuit”, which includes the mesolimbic dopamine system, and changes in glutamatergic neurotransmission, appear to contribute to the augmented behavioral response; however, the mechanisms underlying sensitization are not yet fully understood (for review see: Pierce and Kalivas, 1997; Steketee, 2003; Vanderschuren and Kalivas, 2000; Vezina, 2004; Wolf, 2002).
Quinpirole, a selective dopamine D2/D3 receptor agonist (Levant et al., 1992; Tsuruta et al., 1981), produces a sensitized locomotor response in rats. The quinpirole-sensitized rat is of particular interest as these animals compulsively revisit the same location in the open field, akin to compulsive “checking” behavior of humans with obsessive-compulsive disorder; thus, the quinpirole preparation may represent a putative animal model (Szechtman et al., 1998; 1999; 2001). Altered cortico-striatal-thalamic-cortical activity, and dysregulation of certain serotonergic and dopaminergic systems, are believed to contribute to the pathogenesis of obsessive-compulsive disorder (Stein, 2002); however, the exact neurobiological basis is not fully understood. Serotonin reuptake inhibitors are used in the treatment of obsessive-compulsive disorder, but are not effective in many patients (Piccinelli et al., 1995). Furthermore, it is not understood why the particular obsessions or compulsions of obsessive-compulsive disorder patients vary (McKay et al., 2004) or why the obsession and/or compulsion changes over time in almost two-thirds of patients (Skoog and Skoog, 1999).
Based on findings that certain monoamine oxidase inhibitors (MAO inhibitors) and related compounds inhibited the in vitro binding of [3H]quinpirole, apparently uniquely among dopamine D2-like receptor radioligands (Levant et al., 1993; 1996; 2001), Culver and colleagues found that pre-treatment of rats with the MAOA–selective MAO inhibitors clorgyline or Ro 41-1049 [N-(2-aminomethyl)-5-(3-fluorophenyl)-4-thiazolecarboxamide HCl] changed the sensitized behavior produced by quinpirole from locomotion to self-directed “mouthing” activity that included nibbling of paws or tail, and licking of hindquarters or body fur (Culver et al., 2000; Culver and Szechtman, 1997; 2003; Dvorkin et al., 2006). The mechanism underlying the effects of these drugs on quinpirole-induced behaviors remains to be fully determined; however, it appears to be unrelated to inhibition of MAOA because moclobemide, an MAOA inhibitor with low affinity in competition with [3H]quinpirole (Levant et al., 1996), produced similar inhibition of MAOA to clorgyline, but did not alter the sensitized response to quinpirole (Culver et al., 2000). Consistent with in vitro findings (Levant et al., 1993; 1996; 2001), modulation of dopamine uptake and interactions with the sigma and imidazoline sites have also been eliminated as potential mediators of clorgyline’s or Ro 41-1049’s effects on quinpirole-sensitized behaviors (Culver et al., 2000; 2002; Culver and Szechtman, 2003). Furthermore, clorgyline altered quinpirole-sensitized behavior when administered intermittently by injection, continuously by osmotic pump during the sensitizing treatment, or by injection after sensitization had occurred, indicating that the mode of administration and pharmacokinetics were not critical in producing the modification of the sensitized response (Culver et al., 2000; Culver and Szechtman, 2003). Thus, we hypothesize that these drugs modulate [3H]quinpirole binding to the dopamine D2 receptor, and in turn, quinpirole-induced behavior, perhaps by an allosteric mechanism (Levant, 2000; 2002).
To further assess mechanisms underlying behavioral sensitization, this study sought to assess changes in dopamine D2-like receptor binding in rats sensitized to quinpirole. The effects of pre-treatment with the MAOA inhibitor Ro 41-1049 on behavior and dopamine D2-like receptor binding were also examined to determine whether the shift in sensitized responding from locomotion to mouthing is related to modulation of D2-like receptors. Ro 41-1049 was used because, unlike the preponderance of other MAO inhibitors, it has no potentially confounding activities at the sigma or imidazoline sites (Levant et al., 1993). In addition, the effects of these treatments on the ability of Ro 41-1049 to modulate [3H]quinpirole binding ex vivo were determined.
2. Materials and Methods
All animal treatments and testing were performed at McMaster University and were conducted in compliance with the guidelines described in the Guide to the Care and Use of Experimental Animals (Canadian Council on Animal Research, 1993).
2.1. Subjects
Experimentally naïve male Long-Evans rats (Charles-River, Canada) weighing 200–230 g at the start of treatment were used. Rats were individually housed in a temperature-controlled colony room (22° C) under a 12-h light-dark cycle (8:00 am to 8:00 pm), with free access to food and water. Rats were allowed to acclimatize to the colony room for 1 week following arrival, and were handled 2 min daily for 7 days before starting experiments. All treatments and testing were conducted during light hours.
2.2. Procedures
Rats (n = 8–10 per group) were pre-treated with Ro 41-1049 (N-(2-aminomethyl)-5-(3-fluorophenyl)-4-thiazolecarboxamide HCl; 1 mg/kg, s.c.; RBI, Natick, MA) or saline 90 min prior to administration of quinpirole (0.5 mg/kg, s.c., RBI, Natick, MA) or saline, twice weekly (3–4 days apart) for a total of eight pairs of injections as previously described (Culver and Szechtman, 2003). An acute quinpirole group was also included, which received eight pairs of saline injections followed by a single saline-quinpirole treatment. All drugs were administered in the testing environment. Rats were returned to the colony room between the pre-treatment and administration of quinpirole. Immediately following each quinpirole or saline injection, rats were placed in Plexiglas locomotor activity chambers (40 × 40 × 35 cm; Accuscan Instruments, Columbus, OH) and their activity recorded for 90 min. Since Ro-1049 sensitizes self-directed mouthing behavior in quinpirole-treated rats, mouthing activity was also measured during the first and last 15 min of the 90-min testing period according to the protocol described in Culver et al. (Culver et al., 2000). Mouthing activity was defined as any contact between the rat’s mouth and/or tongue with either an external object (e.g. licking or gnawing Plexiglas chamber) or parts of its own body (e.g., nibbling of paws or tail, licking of hindquarters or body fur) and is reported as total mouthing although externally and self-directed mouthing activity were scored separately. Three days after the last drug treatment, rats were euthanized by decapitation and brains rapidly removed, frozen on dry ice, and stored at −80° C.
2.3. Radioligand binding assays
Brains were shipped on dry ice to the University of Kansas Medical Center for assessment of dopamine D2-like receptor binding.
Binding of the dopamine D2-like receptor agonist [3H]quinpirole and antagonist [3H]spiperone was performed as previously described in detail (Levant et al., 1992). The nucleus accumbens and striatum were dissected on ice from each brain and homogenized in 20 volumes of assay buffer (50 mM Tris, 5 mM KCl, 2 mM MgCl2, 2 mM CaCl2; pH 7.4 at 23° C) and washed twice. The resulting pellets were reconstituted in assay buffer. In order to facilitate the assessment of multiple endpoints in each individual animal, initial binding analysis was performed using a single concentration of each radioligand. Those endpoints exhibiting significant differences between groups were further examined by Scatchard analysis using pooled tissues from all rats in each group.
[3H]Quinpirole (55.5 Ci/mmol; New England Nuclear, Boston, MA) was used at a final concentration of 2 nM for single-point binding studies or five concentrations (0.38 – 10 nM) for Scatchard analysis. The membrane protein concentration was ~200 μg/tube. Non-specific binding was defined in the presence of spiperone (1 μM). MAO inhibitor-inhibitable [3H]quinpirole binding was determined in the presence of Ro 41-1049 (10 μM). Assay tubes were incubated at 23° C for 4 hrs. Binding reactions were terminated by rapid vacuum filtration and bound radioactivity determined using a Beckman 6500 liquid scintillation counter. Membrane homogenate protein concentrations were determined by the BCA method (Pierce, Rockford, IL). Specific binding is expressed as fmol bound/mg protein.
[3H]Spiperone binding was performed as described for [3H]quinpirole except the membrane protein concentration was ~35 μg/tube; [3H]spiperone (24 Ci/mmol; Amersham, Arlington Heights, IL) was used at a final concentration of 0.2 nM for single-point studies or six concentrations (0.02–1.0 nM) for Scatchard analysis; non-specific binding was defined in the presence of (+)-butaclamol (1 μM); and assay tubes were incubated at 23° C for 90 min.
2.4. Analysis
Data are expressed as the mean ± S.E.M. Scatchard binding experiments were analyzed for KD and Bmax values using SigmaPlot v.8.0.2. The percentage of receptors in the high affinity state was calculated as the ratio of sites labeled by the agonist [3H]quinpirole to sites labeled by the antagonist [3H]spiperone. The percentage of MAO inhibitor-inhibitable [3H]quinpirole binding (%MQB) was calculated as the percentage of binding inhibited by Ro 41-1049. Binding data was analyzed for statistically significant differences by analysis of variance followed by the Student-Newman-Keuls multiple comparisons tests (GraphPad Instat 3). Statistical significance was set at P<0.05.
3. Results
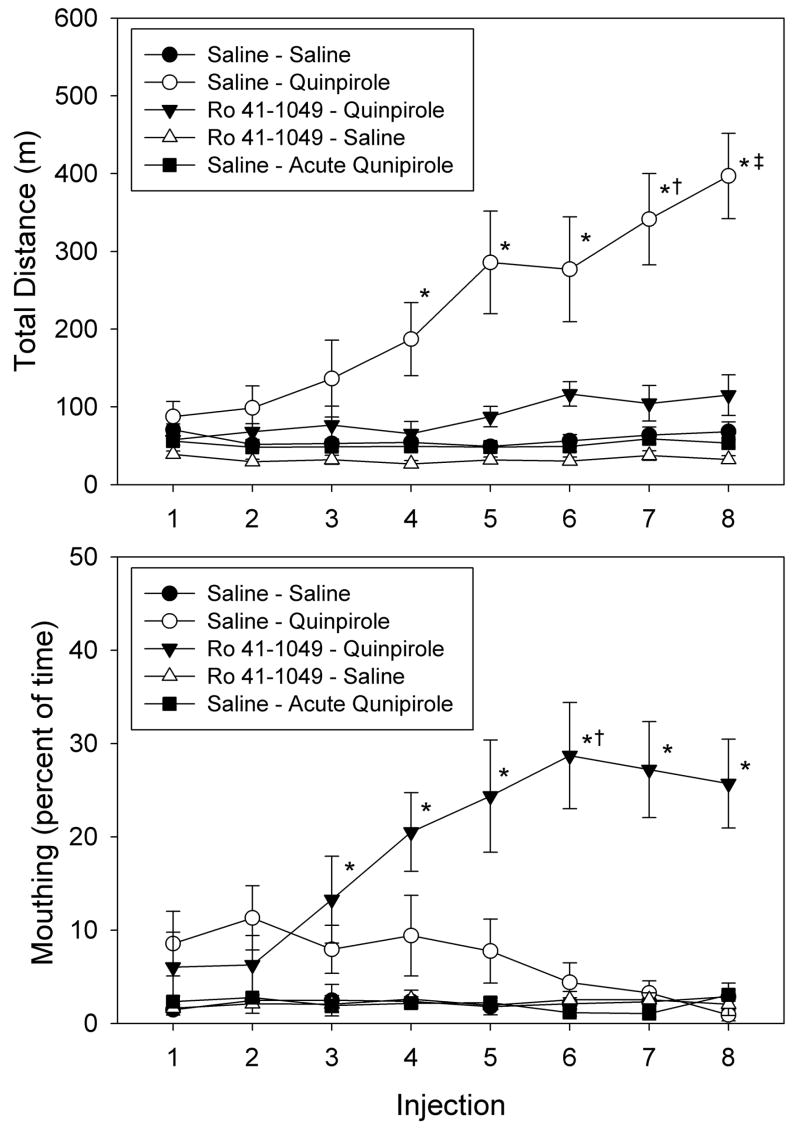
Repeated injections of quinpirole induced behavioral sensitization manifested as an almost 4-fold increase in distance traveled after injection 8 compared to injection 1 (P<0.01) (Figure 1). Pretreatment with Ro 41-1049 changed the sensitized behavior induced by quinpirole from locomotion to mouthing activity. Over 90% of the total mouthing activity was directed primarily at the rat’s own body and included behaviors such as nibbling and licking of paws, tail, or body fur (Culver and Szechtman, 2003). Total mouthing in the Ro 41-1049 pretreated animals after injection 8 was 7-times higher than in rats treated with saline (P<0.01), whereas distance traveled was not different from saline controls. Distance traveled or mouthing by rats treated only with Ro 41-1049 was not different from that of saline controls.
Figure 1. Locomotor and mouthing responses to quinpirole, with and without pretreatment with Ro 41-1049.
Rats were pretreated with with Ro 41-1049 (1 mg/kg, s.c) or saline 90 min prior to administration of quinpirole (0.5 mg/kg, s.c.) or saline twice weekly (3–4 days apart) for a total of 8 pairs of injections. Data are presented as the mean ± SEM (n = 8 for Saline-Saline, n = 10 for all other groups). *P<0.05 vs. Saline-Saline, same injection by ANOVA and Student-Newman-Keuls test. †P<0.05 vs. injections 1 and 2, same treatment. ‡P<0.05 vs. injections 1–4, same treatment. These data were previously reported in Culver and Szechtman (2003).
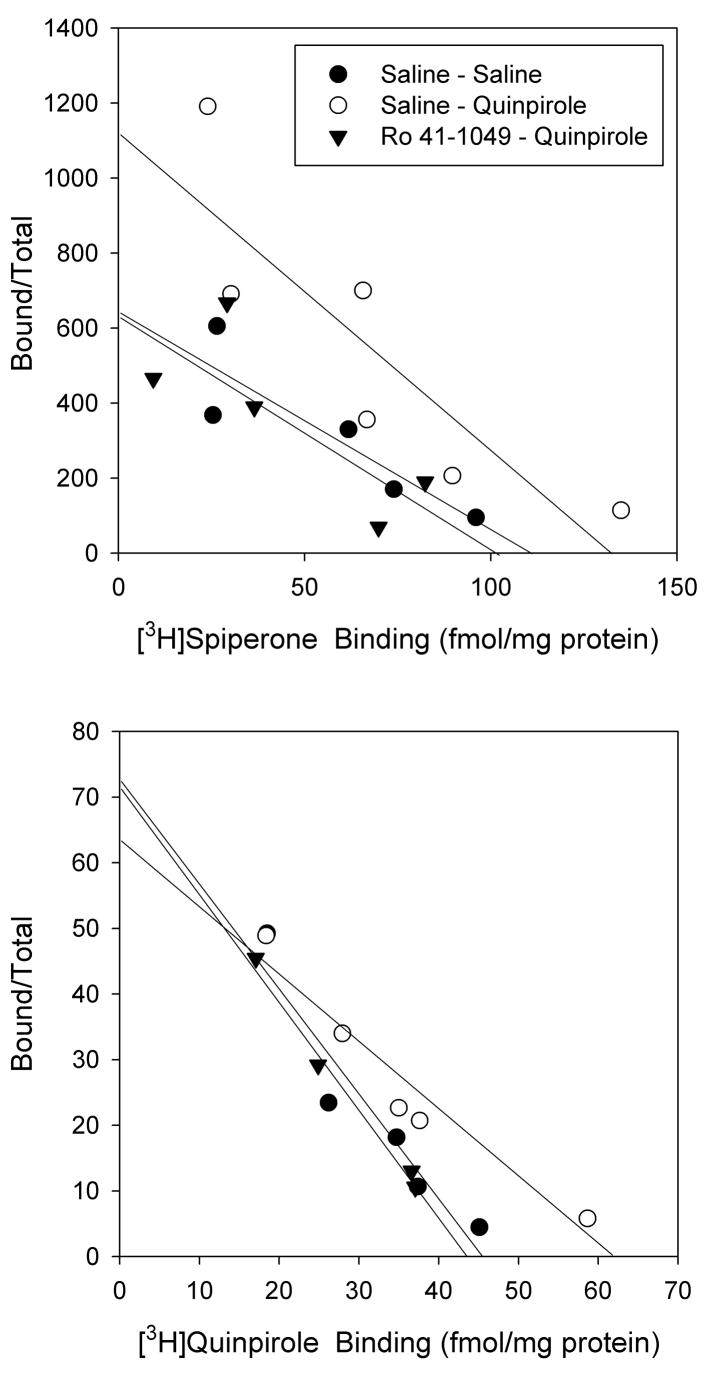
Dopamine D2-like receptor binding was assessed in brains collected three days after the last injection. In the nucleus accumbens, single-point binding assays indicated that [3H]quinpirole and [3H]spiperone binding were both 32% higher in quinpirole-sensitized rats compared to rats treated with saline (P<0.05) (Table 1). [3H]Spiperone binding was 38% higher in quinpirole-sensitized rats than in rats pre-treated with Ro 41-1049 prior to administration of quinpirole (P<0.05). [3H]Quinpirole binding was 14% higher in quinpirole-sensitized rats without Ro 41-1409 pre-treatment than in rats pre-treated with Ro 41-1049, but this difference was not statistically significant. Neither treatment with Ro 41-1049 alone, nor an acute injection of quinpirole, altered [3H]quinpirole or [3H]spiperone binding in the nucleus accumbens. None of the treatments altered the percentage of dopamine D2-like receptors in the high affinity state or the percentage of MAO inhibitor-inhibitable [3H]quinpirole binding (%MQB). Scatchard analysis performed using pooled samples from each treatment group indicated that the increases in [3H]quinpirole and [3H]spiperone binding observed in the quinpirole-sensitized rats resulted from an increase in receptor density (Bmax), which was higher than Saline-Saline for both [3H]spiperone and [3H]quinpirole (Figure 2). Minor alterations in binding site affinity may also have occurred; however, the single replication of the Scatchard analyses, due to the limited amount of tissue available, affords only qualitative assessment of this data.
Table 1.
Effects of sensitization to quinpirole, with and without pre-treatment with Ro 41-1049, on [3H]spiperone and [3H]quinpirole binding in the nucleus accumbens.
| Pre-treatment/Treatment | [3H]Spiperone (fmol/mg protein) | [3H]Quinpirole (fmol/mg protein) | % High Affinity | %MQB | |
|---|---|---|---|---|---|
| Saline | Saline | 44 ± 4.3 | 19 ± 1.7 | 49 ± 4.7 | 52 ± 3.0 |
| Saline | Quinpirole | 58 ± 4.0a | 25 ± 1.6 a | 48 ± 5.0 | 49 ± 1.9 |
| Ro 41-1049 | Quinpirole | 40 ± 2.4b | 22 ± 1.5 | 55 ± 4.9 | 44 ± 1.6 |
| Ro 41-1049 | Saline | 46 ± 2.0 b | 20 ± 1.4 b | 43 ± 2.6 | 47 ± 1.7 |
| Saline | Acute Quinpirole | 51 ± 5.4 | 19 ± 1.9 b | 45 ± 3.6 | 47 ± 3.1 |
Data are presented as the mean ± S.E.M. (n = 8 for Saline-Saline, n = 10 for all other groups). Receptor binding data is from single-point assays using tissue from each individual animal with the concentration of radioligand at the KD. The percentage of monoamine-displaceable [3H]quinpirole binding (%MQB) was determined in the presence of Ro 41-1049 (10 μM).
P<0.05 v. Saline-Saline;
P<0.05 v. Saline – Quinpirole by analysis of variance and the Student-Newman-Keuls tests.
Figure 2. Rosenthal plots of [3H]spiperone and [3H]quinpirole binding in the nucleus accumbens of quinpirole-sensitized rats, with or without pretreatment with Ro 41-1049.
Data represent a single determination performed using pooled samples from all individual animals in each treatment group that were used for the single-point analyses presented in Table 1. For [3H]spiperone binding, KD values were 0.17, 0.12, and 0.16 nM; Bmax values were 112, 133, and 102 fmol/mg protein for the Saline-Saline, Saline-Quinpirole, and Ro 41-1049-Quinpirole groups, respectively. For [3H]quinpirole binding, KD values were 0.63, 0.94, and 0.60 nM and Bmax values were 46, 59, and 44 fmol/mg protein for the Saline-Saline, Saline-Quinpirole, and Ro 41-1049-Quinpirole groups, respectively. For clarity, data for the Ro 41-1049–Saline and Saline-Acute Quinpirole groups are not shown.
In the striatum, single-point binding analysis indicated no alterations in [3H]quinpirole or [3H]spiperone binding, the percentage of dopamine D2-like receptors in the high affinity state, or the percentage of MAO inhibitor-inhibitable [3H]quinpirole binding (%MQB) (Table 2).
Table 2.
Effects of sensitization to quinpirole, with and without pre-treatment with Ro 41-1049, on [3H]spiperone and [3H]quinpirole binding in the striatum.
| Pre-treatment/Treatment | [3H]Spiperone (fmol/mg protein) | [3H]Quinpirole (fmol/mg protein) | % High Affinity | %MQB | |
|---|---|---|---|---|---|
| Saline | Saline | 78 ± 4.3 | 45 ± 2.4 | 58 ± 3.7 | 63 ± 3.5 |
| Saline | Quinpirole | 76 ± 5.7 | 48 ± 3.3 | 61 ± 3.1 | 67 ± 3.0 |
| Ro 41-1049 | Quinpirole | 68 ± 5.4 | 39 ± 2.9 | 57 ± 2.2 | 60 ± 2.7 |
| Ro 41-1049 | Saline | 64 ± 2.0 | 39 ± 1.9 | 62 ± 2.6 | 56 ± 2.0 |
| Saline | Acute Quinpirole | 75 ± 4.9 | 41 ± 3.2 | 54 ± 9.8 | 62 ± 3.5 |
Data are presented as the mean ± S.E.M. (n = 8 for Saline-Saline, n = 10 for all other groups). Receptor binding data is from single-point assays using tissue from each individual animal with the concentration of radioligand at the KD. The percentage of monoamine-displaceable [3H]quinpirole binding (%MQB) was determined in the presence of Ro 41-1049 (10 μM). No significant differences between groups were detected by analysis of variance.
4. Discussion
The effects of sensitization to quinpirole, with and without pretreatment with Ro 41-1049, on the binding properties of dopamine D2-like receptors were assessed in rats with verified behavioral sensitization.
As evidenced by parallel increases in both [3H]spiperone and [3H]quinpirole binding, sensitizing treatment with quinpirole increased the density of dopamine D2-like receptors in the nucleus accumbens, but not in striatum. Small changes in dopamine D2-like receptor affinity in the nucleus accumbens may also contribute to the sensitized behavior, but the presence of such a change in affinity could not be evaluated in the present study due to the limited quantity of tissue available. The increase in the density of dopamine D2-like sites in the nucleus accumbens after sensitizing treatment with quinpirole was not observed after a single acute injection of the drug, indicating that the increase in receptor density requires multiple injections. The increase in the density of dopamine D2-like receptors in the nucleus accumbens is consistent with previous studies of the effects of sensitization to quinpirole on quinpirole-stimulated local cerebral glucose utilization in which a decrease in glucose utilization in the nucleus accumbens was observed only in sensitized animals and not those treated acutely (Carpenter et al., 2003). Furthermore a decrease in glucose utilization would be consistent with increased numbers of the inhibitory dopamine D2-like receptors. This change in dopamine D2-like receptor density could reflect the increased sensitivity of dopamine D2-like autoreceptors reported by Dwoskin et al. (1988) in amphetamine- or cocaine-sensitized rats. However, other studies of dopamine receptor changes after amphetamine- or cocaine-induced sensitization report decreased sensitivity (Yi and Johnson, 1990) or no change (Bonhomme et al., 1995; Claye et al., 1995; Farfel et al., 1992; Fitzgerald and Reid, 1991; Gifford and Johnson, 1992; King et al., 1994; Mayfield et al., 1992; Peris et al., 1990; Unterwald et al., 1994), perhaps due to differences in the spectra of activity of these drugs.
The increase in the density of dopamine D2-like receptors in the nucleus accumbens produced by sensitizing treatment with quinpirole was not observed in rat pre-treated with the MAO inhibitor Ro 41-1049. This supports a role for the increase in dopamine D2-like receptor density in the nucleus accumbens in the augmented locomotor response and suggests that the attenuation of this effect, at least in part, underlies the change in sensitized behavior from locomotion to mouthing. Since dopamine D2-like receptor binding in the nucleus accumbens was at control levels in these animals, neurobiological changes beyond altered accumbal dopamine D2 receptor density must contribute to the expression of sensitized mouthing. Indeed, quinpirole-sensitized rats, with and without pretreatment with an MAO inhibitor, exhibited differences in quinpirole-stimulated glucose utilization in the locus coeruleus, raphe magnus nucleus, piriform cortex, and septum, whereas glucose utilization in the nucleus accumbens was not different between these groups (Richards et al., 2007). This suggests that altered activity in the neuronal circuits involving one or more of these regions could be involved in mediating the change in sensitized behavior and perhaps also affect the density of dopamine D2-like receptors in the nucleus accumbens. However, the present findings cannot rule out the possibility that discretely localized changes in dopamine D2-like receptor expression in sub-regions of the nucleus accumbens or striatum, which would not be detectable using radioligand binding assays in homogenized grossly-dissected brain regions, may contribute to the particular sensitized behavioral response.
In this study, the ratio of sites labeled by the agonist [3H]quinpirole to those labeled by the antagonist [3H]spiperone was interpreted as representing the proportion of dopamine D2-like receptors in the high affinity state. However, [3H]quinpirole labels both dopamine D2 and D3 sites with roughly equal affinity in our in vitro assay (Levant et al., 1992). Thus, some of the [3H]quinpirole binding in the nucleus accumbens represents the dopamine D3 receptor, though the dopamine D3 receptor comprises only about 10% of dopamine D2-like sites in that region (Levant, 1997). Accordingly, dopamine D3 receptors in the nucleus accumbens certainly contribute to the [3H]quinpirole binding detected in that brain region in this study, and thus the percentage of dopamine D2-like receptors in the high affinity state. The dopamine D3 receptor has been proposed as a mediator of the sensitized behavioral response to psychostimulants, with desensitization of the receptor, which inhibits locomotion, contributing to the augmented locomotor response (Richtand, 2006). This study did not assess the specific contribution of the dopamine D3 receptor due to the limited amount of tissue available. The density of [3H]PD 128907-labeled dopamine D3 receptor in ventral striatum (nucleus accumbens and olfactory tubercle) was not altered in rats with amphetamine sensitization (Richtand et al., 2003); however, when the specific contribution of dopamine D3 receptors was measured in quinpirole-sensitized rats, an increase in percent of dopamine D2 and D3 receptors in the high affinity state was found in the nucleus accumbens and the striatum (Perreault et al., 2007; Seeman et al., 2006), Thus, while the dopamine D3 receptor represents only a small fraction of the [3H]quinpirole binding observed in this study, changes in expression level or regulation of that receptor subtype may be functionally consequential.
The literature on dopamine receptor changes after psychostimulant sensitization is conflicting, though most typically negative (see above). One factor contributing to these variable findings is the use of the widely abused psychostimulants amphetamine and cocaine. Although clinically-relevant, the pharmacology of these drugs is quite complex, involving both pre-synaptic actions resulting in post-synaptic effects at a multitude of dopaminergic, noradrenergic, serotonergic receptors (Jaffe, 1993). This spectrum of activity may obscure the detection of key mediators of sensitization. In this context, quinpirole, which produces sufficient stimulation of dopamine D2/D3 receptors to induce a sensitized behavioral response, represents a tool that may facilitate elucidation of mechanisms of sensitization that may also occur, perhaps transiently, with other psychostimulants. Alternatively, the effects observed in this study may be specific to quinpirole.
The shift from locomotion to self-directing mouthing produced in quinpirole sensitized rats by some MAO inhibitors may provide a model of neurobiological mechanisms underlying specific symptom changes observed during chronic drug intake or in some psychiatric disorders. As described by Ellinwood (1967), amphetamine users pass through several behavioral stages during the course of chronic drug intake. The stages are characterized by different psychological pre-occupations: At onset of drug-taking there is an expansion of the scope of attention as users become hyper-responsive to distal stimuli and are interested in the wide environment and broad issues. With chronic intake, the scope of attention narrows progressively as users focus on proximal stimuli and detailed examinations of the immediate environment; ultimately they may become pre-occupied with every minutiae of their own body and self. A parallel shift in the focus of attention from distal to proximal stimuli had been described for the behavior of apomorphine-treated rats (Szechtman et al., 1985) and the observed shift in the present study from locomotor behavior to a focus on the rat’s own body may be considered as a behavioral manifestation of a similar change in the rat’s focus of attention and pre-occupation. Along the same lines, considering that quinpirole-sensitized rats may constitute an animal model of obsessive-compulsive disorder (Szechtman et al 1998), the shift in behavior produced by Ro 41-1049 may prove revealing as to the neurobiological mechanisms underlying a change in obsessive-compulsive disorder symptoms from compulsive checking to compulsive washing observed in some patients (Besiroglu et al., 2007).
In summary, the present data demonstrate an increase in the density of dopamine D2-like receptors in the nucleus accumbens of rats with locomotor sensitization to quinpirole. The increase in dopamine D2-like receptors was not present in quinpirole-sensitized rats that were pre-treated with the MAO inhibitor Ro 41-1049, a treatment regimen which changes the sensitized behavioral response from locomotion to self-directed mouthing. These findings support the involvement of alterations in dopamine D2-like receptors in the nucleus accumbens in the development of locomotor sensitization to quinpirole and suggest that modification of these alterations contributes to the change from sensitized locomotion to mouthing observed when rats are pre-treated with Ro 41-1049. Further studies must elucidate how these effects on dopamine D2-like receptors interact with other neurobiological sequelae of intermittent psychostimulant treatment to produce a particular sensitized behavioral response.
Acknowledgments
Supported by the Canadian Institutes of Health Research Grant MOP-64424 (HS), NIH P30 HD02528 (BL), and NIH P20 RR016475 from the INBRE Program of the National Center for Research Resources (BL).
Footnotes
Supported by the Canadian Institutes of Health Research Grant MOP-64424 (HS), NIH P30 HD02528 (BL), and NIH P20 RR016475 from the INBRE Program of the National Center for Research Resources (BL).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Besiroglu L, Uguz F, Ozbebit O, Guler O, Cilli AS, Askin R. Longitudinal assessment of symptom and subtype categories in obsessive-compulsive disorder. Depression Anxiety. 2007;24:461–466. doi: 10.1002/da.20240. [DOI] [PubMed] [Google Scholar]
- Bonhomme N, Cador M, Stinus L, Le Moal M, Spampinato U. Short and long-term changes in dopamine and serotonin receptor binding sites in amphetamine-sensitized rats: a quantitative autoradiographic study. Brain Res. 1995;675:215–223. doi: 10.1016/0006-8993(95)00067-z. [DOI] [PubMed] [Google Scholar]
- Canadian Council on Animal Research. [accessed 1/2/08];Guide to the Care and Use of Experimental Animals. 1993 on-line: http://www.ccac.ca/en/CCAC_Programs/Guidelines_Policies/GUIDES/ENGLISH/toc_v1.htm.
- Carpenter TL, Pazdernik TL, Levant B. Differences in quinpirole-induced local cerebral glucose utilization between naive and sensitized rats. Brain Res. 2003;964:295–301. doi: 10.1016/s0006-8993(02)04115-x. [DOI] [PubMed] [Google Scholar]
- Claye LH, Akunne HC, Davis MD, DeMattos S, Soliman KF. Behavioral and neurochemical changes in the dopaminergic system after repeated cocaine administration. Molec Neurobiol. 1995;11:55–66. doi: 10.1007/BF02740684. [DOI] [PubMed] [Google Scholar]
- Culver KE, Rosenfeld JM, Szechtman H. A switch mechanism between locomotion and mouthing implicated in sensitization to quinpirole in rats. Psychopharmacology. 2000;151:202–210. doi: 10.1007/s002139900346. [DOI] [PubMed] [Google Scholar]
- Culver KE, Rosenfeld JM, Szechtman H. Monoamine oxidase inhibitor-induced blockade of locomotor sensitization to quinpirole: role of striatal dopamine uptake inhibition. Neuropharmacology. 2002;43:385–393. doi: 10.1016/s0028-3908(02)00128-4. [DOI] [PubMed] [Google Scholar]
- Culver KE, Szechtman H. Monoamine oxidase inhibitor sensitive site implicated in sensitization to quinpirole. Eur J Pharmacol. 1997;339:109–111. doi: 10.1016/s0014-2999(97)01386-1. [DOI] [PubMed] [Google Scholar]
- Culver KE, Szechtman H. Clorgyline-induced switch from locomotion to mouthing in sensitization to the dopamine D2/D3 agonist quinpirole in rats: role of sigma and imidazoline I2 receptors. Psychopharmacology (Berl) 2003;167:211–218. doi: 10.1007/s00213-003-1408-z. [DOI] [PubMed] [Google Scholar]
- Di Chiara G. The role of dopamine in drug abuse viewed for the perspective of its role in motivation. Drug Alc Depend. 1995;38:95–137. doi: 10.1016/0376-8716(95)01118-i. [DOI] [PubMed] [Google Scholar]
- Dvorkin A, Culver KE, Szechtman H. Differential effects of clorgyline on sensitization to quinpirole in rats tested in small and large environments. Psychopharmacology (Berl) 2006;186:534–543. doi: 10.1007/s00213-006-0377-4. [DOI] [PubMed] [Google Scholar]
- Dwoskin LP, Peris J, Yasuda RP, Philpott K, Zahniser NR. Repeated cocaine administration results in supersensitivity of striatal D-2 dopamine autoreceptors to pergolide. Life Sci. 1988;42:255–262. doi: 10.1016/0024-3205(88)90634-0. [DOI] [PubMed] [Google Scholar]
- Ellinwood EH. Amphetamine psychosis. I. Description of individuals and process. J Ment Nerv Dis. 1967;144:273–283. [Google Scholar]
- Ellison G. Stimulant-induced psychosis, the dopamine theory of schizophrenia, and the habenula. Brain Res Rev. 1994;19:223–239. doi: 10.1016/0165-0173(94)90012-4. [DOI] [PubMed] [Google Scholar]
- Farfel GM, Kleven MS, Woolverton WL, Seiden LS, Perry BD. Effects of repeated injections of cocaine on catecholamine receptor binding sites, dopamine transporter binding sites and behavior in rhesus monkey. Brain Res. 1992;578:235–243. doi: 10.1016/0006-8993(92)90252-5. [DOI] [PubMed] [Google Scholar]
- Fitzgerald JL, Reid JJ. Chronic cocaine treatment does not alter rat striatal D2 autoreceptor sensitivity to pergolide. Brain Res. 1991;541:327–333. doi: 10.1016/0006-8993(91)91033-w. [DOI] [PubMed] [Google Scholar]
- Gifford AN, Johnson KM. Effect of chronic cocaine treatment on D2 receptors regulating the release of dopamine and acetylcholine in the nucleus accumbens and striatum. Pharmacol Biochem Behav. 1992;41:841–846. doi: 10.1016/0091-3057(92)90236-9. [DOI] [PubMed] [Google Scholar]
- Jaffe JH. Drug addiction and drug abuse. In: Gilman AG, Rall TW, Nies AS, Taylor P, editors. Goodman and Gilman’s The Pharmacological Basis of Therapeutics. McGraw-Hill; New York: 1993. pp. 522–573. [Google Scholar]
- King GR, Ellinwood EH, Jr, Silvia C, Joyner CM, Xue Z, Caron MG, Lee TH. Withdrawal from continuous or intermittent cocaine administration: changes in D2 receptor function. J Pharmacol Exp Ther. 1994;269:743–749. [PubMed] [Google Scholar]
- Levant B. The D3 dopamine receptor: neurobiology and potential clinical relevance. Pharmacol Rev. 1997;49:231–252. [PubMed] [Google Scholar]
- Levant B. Novel drug interactions at D2 dopamine receptors: modulation of [3H]quinpirole binding by monoamine oxidase inhibitors. Life Sci. 2002;71:2691–2700. doi: 10.1016/s0024-3205(02)02109-4. [DOI] [PubMed] [Google Scholar]
- Levant B, Gilliland SL, Culver KE, Szechtman H. Novel drug interactions at CNS dopamine receptors. Potential role in psychopathology and toxicity. In: Williams G, Aruoma OI, editors. Molecular Drug Metabolism and Toxicity. OICA International; St. Lucia: 2000. pp. 201–216. [Google Scholar]
- Levant B, Grigoriadis DE, DeSouza EB. Characterization of [3H]quinpirole binding to D2-like dopamine receptors in rat brain. J Pharmacol Exp Ther. 1992;262:929–935. [PubMed] [Google Scholar]
- Levant B, Grigoriadis DE, DeSouza EB. Monoamine oxidase inhibitors inhibit [3H]quinpirole binding in rat striatal membranes. Eur J Pharmacol. 1993;246:171–178. doi: 10.1016/0922-4106(93)90095-q. [DOI] [PubMed] [Google Scholar]
- Levant B, Moehlenkamp JD, Morgan KA, Leonard NL, Cheng CC. Modulation of [3H]quinpirole binding in brain by monoamine oxidase inhibitors: evidence for a potential novel binding site. J Pharmaco Exp Ther. 1996;278:145–153. [PubMed] [Google Scholar]
- Levant B, Morgan KA, Ahlgren-Beckendorf JA, Grandy DK, Chen K, Shih JE, Seif I. Modulation of [3H]quinpirole binding at striatal D2 dopamine receptors by a monoamine oxidaseA-like site: evidence from radioligand binding studies and D2 receptor- and MAOA-deficient mice. Life Sci. 2001;70:229–241. doi: 10.1016/s0024-3205(01)01400-x. [DOI] [PubMed] [Google Scholar]
- Mayfield RD, Larson G, Zahniser NR. Cocaine-induced behavioral sensitization and D1 dopamine receptor function in rat nucleus accumbens and striatum. Brain Res. 1992;573:331–335. doi: 10.1016/0006-8993(92)90783-6. [DOI] [PubMed] [Google Scholar]
- McKay D, Abramowitz JS, Calamari JE, Kyrios M, Radomsky A, Sookman D, Taylor S, Wilhelm S. A critical evaluation of obsessive-compulsive disorder subtypes: symptoms versus mechanisms. Clin Psychol Rev. 2004;24:283–313. doi: 10.1016/j.cpr.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Peris J, Boyson SJ, Cass WA, Curella P, Dwoskin LP, Larson G, Lin LH, Yasuda RP, Zahniser NR. Persistence of neurochemical changes in dopamine systems after repeated cocaine administration. J Pharmacol Exp Ther. 1990;253:38–44. [PubMed] [Google Scholar]
- Perreault ML, Seeman P, Szechtman H. Kappa-opioid receptor stimulation quickens pathogenesis of compulsive checking in the quinpirole sensitization model of obsessive-compulsive disorder (OCD) Behav Neurosci. 2007;121:976–991. doi: 10.1037/0735-7044.121.5.976. [DOI] [PubMed] [Google Scholar]
- Piccinelli M, Pini S, Bellantuono C, Wilkinson G. Efficacy of drug treatment in obsessive-compulsive disorder. A meta-analytic review. Br J Psychiatry. 1995;166:424–443. doi: 10.1192/bjp.166.4.424. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Kalivas PW. A circuitry model of the expression of behavioral sensitization to amphetamine-like psychostimulants. Brain Res Rev. 1997;25:192–216. doi: 10.1016/s0165-0173(97)00021-0. [DOI] [PubMed] [Google Scholar]
- Post RM, Contel NR. Cocaine-induced behavioral sensitization: a model for recurrent manic illness. In: Perris C, Struwe G, Jansson B, editors. Biological Psychiatry. Elsevier; Amsterdam: 1981. pp. 746–749. [Google Scholar]
- Richards TL, Pazdernik TL, Levant B. Clorgyline-induced modification of behavioral sensitization to quinpirole: effects on local cerebral glucose utilization. Brain Res. 2007;1160:124–133. doi: 10.1016/j.brainres.2007.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richtand NM. Behavioral sensitization, alternative splicing, and D3 dopamine receptor-mediated inhibitory function. Neuropsychopharmacology. 2006;31:2368–2375. doi: 10.1038/sj.npp.1301163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richtand NM, Welge JA, Levant B, Logue AD, Hayes S, Pritchard LM, Geracioti TD, Coolen LM, Berger SP. Altered behavioral response to dopamine D3 receptor agonists 7-OH-DPAT and PD 128907 following repetitive amphetamine administration. Neuropsychopharmacology. 2003;28:1422–1432. doi: 10.1038/sj.npp.1300182. [DOI] [PubMed] [Google Scholar]
- Robinson TE. The neural basis of drug craving: and incentive-sensitization theory of addiction. Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Becker JB. Enduring changes in brain and behavior produced by chronic amphetamine administration: a review and evaluation of animal models of amphetamine psychosis. Brain Res Rev. 1986;11:157–198. doi: 10.1016/s0006-8993(86)80193-7. [DOI] [PubMed] [Google Scholar]
- Schmidt WJ, Beninger RJ. Behavioural sensitization in addiction, schizophrenia, Parkinson’s disease and dyskinesia. Neurotox Res. 2006;10:161–166. doi: 10.1007/BF03033244. [DOI] [PubMed] [Google Scholar]
- Seeman P, Schwarz J, Chen JF, Szechtman H, Perreault M, McKnight GS, Roder JC, Quirion R, Boksa P, Srivastava LK, Yanai K, Weinshenker D, Sumiyoshi T. Psychosis pathways converge via D2high dopamine receptors. Synapse. 2006;60:319–346. doi: 10.1002/syn.20303. [DOI] [PubMed] [Google Scholar]
- Skoog G, Skoog I. A 40-year follow-up of patients with obsessive-compulsive disorder. Arch Gen Psychiatry. 1999;56:121–127. doi: 10.1001/archpsyc.56.2.121. [DOI] [PubMed] [Google Scholar]
- Stein DJ. Obsessive-compulsive disorder. Lancet. 2002;360:397–405. doi: 10.1016/S0140-6736(02)09620-4. [DOI] [PubMed] [Google Scholar]
- Steketee JD. Neurotransmitter systems of the medial prefrontal cortex: potential role in sensitization to psychostimulants. Brain Res. 2003;41:203–228. doi: 10.1016/s0165-0173(02)00233-3. [DOI] [PubMed] [Google Scholar]
- Szechtman H, Culver K, Eilam D. Role of dopamine systems in obsessive-compulsive disorder (OCD): implications from a novel psychostimulant-induced animal model. Polish J Pharmacol. 1999;51:55–61. [PubMed] [Google Scholar]
- Szechtman H, Eckert MJ, Tse WS, Boersma JT, Bonura CA, McClelland JZ, Culver KE, Eilam D. Compulsive checking behavior of quinpirole-sensitized rats as an animal model of Obsessive-Compulsive Disorder(OCD): form and control. BMC Neurosci. 2001;2:4. doi: 10.1186/1471-2202-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szechtman H, Ornstein K, Teitelbaum P, Golani I. The morphogenesis of stereotyped behavior induced by the dopamine receptor agonist apomorphine in the laboratory rat. Neuroscience. 1985;14:783–798. doi: 10.1016/0306-4522(85)90143-5. [DOI] [PubMed] [Google Scholar]
- Szechtman H, Sulis W, Eilam D. Quinpirole induces compulsive checking behavior in rats: a potential animal model of obsessive-compulsive disorder (OCD) Behav Neurosci. 1998;112:1475–1485. doi: 10.1037//0735-7044.112.6.1475. [DOI] [PubMed] [Google Scholar]
- Tsuruta K, Frey EA, Grewe CW, Cote TE, Eskay RL, Kebabian JW. Evidence that LY 141865 specifically stimulates the D-2 dopamine receptor. Nature. 1981;292:463–466. doi: 10.1038/292463a0. [DOI] [PubMed] [Google Scholar]
- Unterwald EM, Ho A, Rubenfeld JM, Kreek MJ. Time course of the development of behavioral sensitization and dopamine receptor up-regulation during binge cocaine administration. J Pharmacol Exp Ther. 1994;270:1387–1396. [PubMed] [Google Scholar]
- Vanderschuren LJMJ, Kalivas PW. Alterations in dopaminergic and glutaminergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology. 2000;151:99–120. doi: 10.1007/s002130000493. [DOI] [PubMed] [Google Scholar]
- Vezina P. Sensitization of midbrain dopamine neuron reactivity and the self-administration of psychomotor stimulant drugs. Neurosci Biobehav Rev. 2004;27:827–839. doi: 10.1016/j.neubiorev.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Wolf ME. Addiction: making the connection between behavioral changes and neuronal plasticity in specific pathways. Mol Interv. 2002;2:146–157. doi: 10.1124/mi.2.3.146. [DOI] [PubMed] [Google Scholar]
- Yi SJ, Johnson KM. Effects of acute and chronic administration of cocaine on striatal uptake, compartmentalization and release of [3H]dopamine. Neuropharmacology. 1990;29:475–486. doi: 10.1016/0028-3908(90)90170-v. [DOI] [PubMed] [Google Scholar]