Abstract
Two new phenylacetylene derivatives, 5-((4-(2-(2-(2-fluoroethoxy)ethoxy)ethoxy)phenyl)ethynyl)indoline 8 and 5-((4-(2-(2-(2-fluoroethoxy)ethoxy)ethoxy)phenyl)ethynyl)-1H-indole 14, targeting β-amyloid (Aβ) plaques have been prepared. In vitro binding carried out in tissue homogenates prepared from postmortem AD brains with [125I]IMPY (6-iodo-2-(4’-dimethylamino-)phenylimidazo[1,2-a]pyridine) as the radioligand indicated good binding affinities (Ki = 4.0 and 1.5 nM for 8 and 14, respectively). Brain penetration of the corresponding radiofluorinated ligands, evaluated in the normal mice, showed good initial brain penetration (4.50 and 2.43% ID/g (injected dose/gram) for [18F]8 and [18F]14 at 2 min after injection) with moderate to low washout rates from the brain (1.71% ID/g at 2 h and 2.10% ID/g at 3 h, respectively). Autoradiography and homogenate binding studies demonstrated the high specific binding of [18F]14 to the Aβ plaques; however, [18F]8 showed low specific binding. These preliminary results identified that indolylphenylacetylene, 14, may be a good lead for further structural modification to develop a useful Aβ plaque imaging agent.
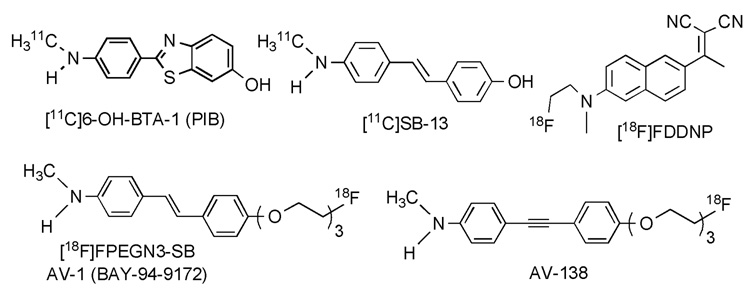
Alzheimer’s disease (AD) is a neurodegenerative disease which currently affects millions of elderly people worldwide. The key pathological features of AD are the formation of senile plaques aggregated by β-amyloid peptide and neurofibrillary tangles piled from phosphorylated tau protein in the brain. Based upon the amyloid cascade hypothesis, formation of Aβ plaques in the brain are the primary influence driving AD pathogenesis.1–3 Imaging techniques such as positron emission tomography (PET) and single photon emission tomography (SPECT) are potentially useful for diagnosis of AD through imaging Aβ plaques in the brain. Various radiolabeled ligands (including the most well-known agent, [11C]-PIB, N-methyl-[11C]2-(4′-methylaminophenyl)-6-hydroxybenzothiazole) had been tested clinically and demonstrated potential usage (Figure 1).4–10
Figure 1.
Reported five reported agents for imaging Aβ plaques in the brain.
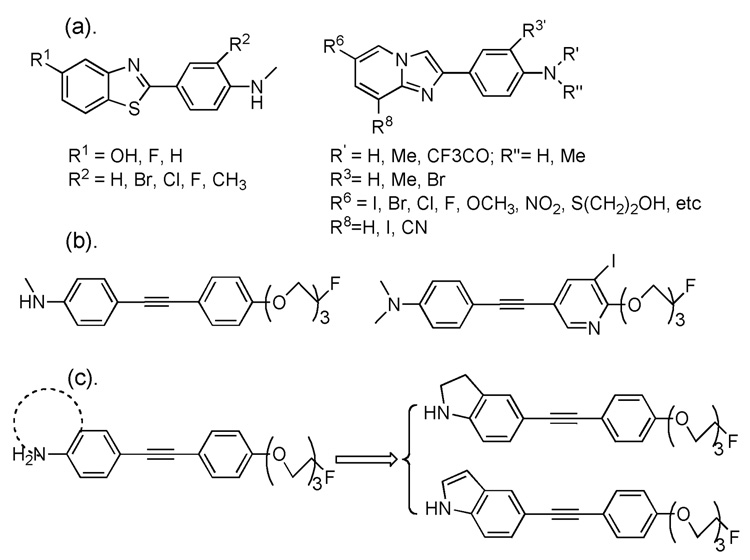
Most of the reported ligands own a common structural feature, they contain a terminal p-N-methyl- or p-N,N-dimethylaminophenyl- group, and these groups are critical for successful binding affinity.6, 8 In many situations, however, it is speculated that the relatively low metabolic stability of these radioligands was mainly due to a rapid N-demethylation in vivo. Until now, efforts have been made to overcome this obstacle by adding an extra neighboring substituent, such as methyl, bromide, chloride or fluoride group, ortho- to the N-methylamino- group on the phenyl ring to reduce the in vivo N-demethylation while maintaining the desired Aβ plaque binding (Figure 2. (a)).11, 12 N-[11C]-labeled aminophenylbenzothiazoles substituted with fluorine in different positions have been synthesized and evaluated. It was suggested that the substitution pattern of the phenyl ring and the benzothiazole moiety has an influence on slowing down the in vivo metabolism, which in turn has an effect on the brain uptake kinetics.11 The data suggest that a strategy of reducing in vivo N-demethylation may improve the brain kinetics for agents targeting Aβ plaques in the brain.
Figure 2.
Examples of potential Aβ plaque imaging agents: (a) N-methyl group ortho-substituted benzothiazole and imidazo[1,2-a]pyridine derivatives. (b) Fluoro-pegylated diphenylacetylene and iodinated azadiphenylacetylene derivatives. (c) Designed ring-fusing targets based on N-methylamino phenylacetylene: indolinyl- and indolylphenylacetylenes.
Recently, the pegylation methodology has been adapted in the design and synthesis of PET Aβ plaque imaging agents in order to adjust the ligands’ pharmacokinetic properties.13, 14 We have since reported a series of fluoro-pegylated diphenylacetylene and iodinated azadiphenylacetylene derivatives as potential PET and SPECT Aβ plaque imaging agents (Figure 2. (b)).15, 16 Following these successful results, we decided to further extend our search for PET imaging agents using this diphenylacetylene core structure. By fusing the N-methylamino group with the phenyl ring, we hope to prevent the probability of in vivo N-demethylation. In order to do so, indolinyl and indolyl groups were chosen to replace the p-N-methyl- or p-N,N-dimethylamino phenyl- group. As such by locking the N-methyl group into a heterocyclic indole or indoline system, we hope to effectively prevent the in vivo N-demethylation. (Figure 2. (c)) We reported herein the synthesis and initial biological evaluation of two phenylacetylene derivatives as improved probes for imaging Aβ plaques in the brain.
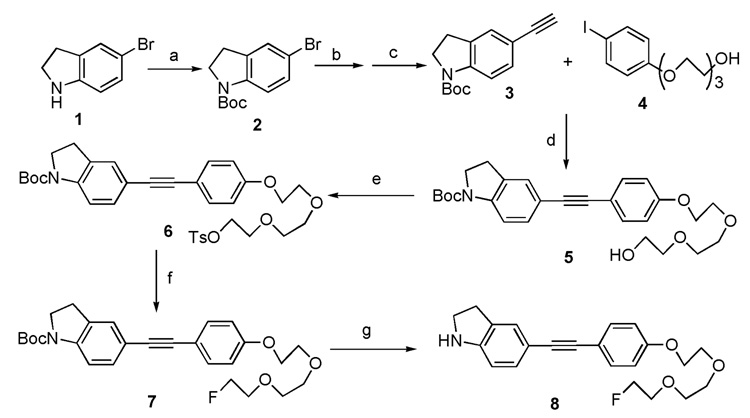
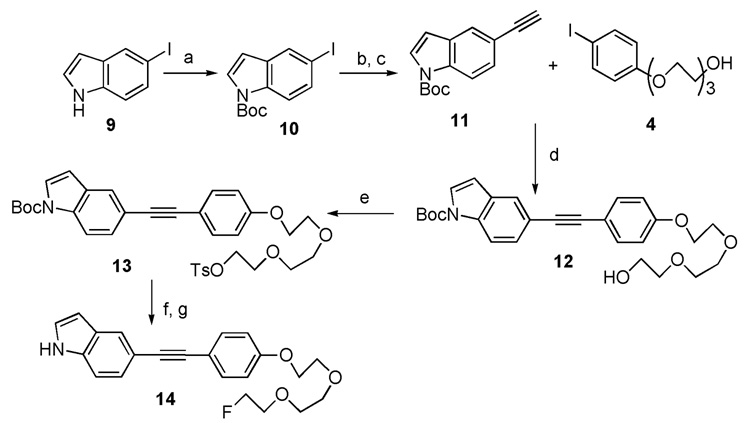
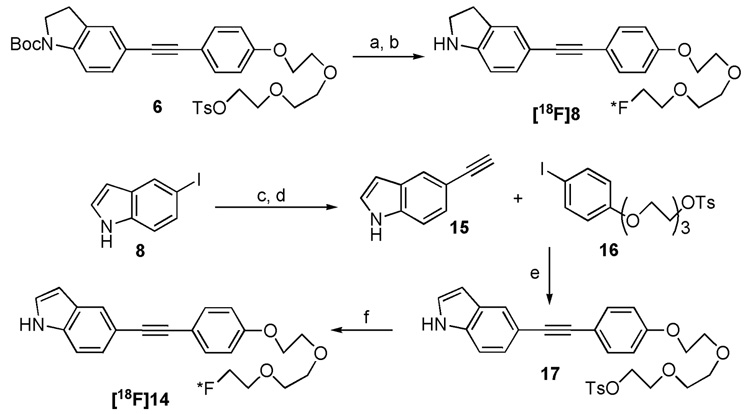
The synthetic procedures for these two target molecules are illustrated in Scheme 1 and Scheme 2. Copper and palladium co-catalyzed Sonogashira coupling reaction plays a vital role in the assembly of target molecules 8 and 14. Starting from 5-bromoindoline 1, the t-butoxycarbonyl (Boc) group protection, microwave heating promoted Sonogashira coupling and following basic deprotection of trimethylsilyl (TMS) group, afforded the intermediate 3. The Sonogashira reaction was employed a second time to couple intermediate 3 and 4. This was followed by tosylation, fluorination and finally N-Boc deprotection. Only four steps were needed to achieve the desired product 8 from 3. For the synthesis of indolylphenylacetylene 14, a similar synthetic route was adopted with two exceptions: (1) the synthesis of intermediate 11 only requires very mild reaction condition (room temperature, 0.7 h); (2) a relatively harsh condition (microwave heating to 140 °C) was utilized for the final N-Boc deprotection step.
Scheme 1.
Reagents and conditions: (a) (Boc)2O, THF, rt, 24 h; (b) Pd(PPh3)4, CuI, trimethylsilylacetylene, diethylamine, DMF, 130 °C, microwave heating, 0.5 h; (c) KOH, MeOH/THF, rt, 0.5 h; (d) Pd(PPh3)4, CuI, Et3N, CH3CN, 0 °C to rt, 1.5 h; (e) TsCl, Et3N, DMAP, DCM, 0 °C to rt, 2 h; (f) TBAF, THF, 75 °C, 1 h; (g) TMSOTf, 2,6-lutidine, DCM, 0 °C to rt, 1.8 h.
Scheme 2.
Reagents and conditions: (a) (Boc)2O, DMAP, DCM, rt, 1.25 h; (b) Pd(PPh3)4, CuI, trimethylsilylacetylene, diethylamine, DMF, rt, 0.7 h; (c) KOH, MeOH/THF, rt, 2.5 h; (d) Pd(PPh3)4, CuI, Et3N, CH3CN, 0 °C to rt, 1.0 h; (e) TsCl, Et3N, DMAP, DCM, 0 °C to rt, 3 h; (f) TBAF,
The binding affinities of two non-radioactive ligands 8 and 14 were tested by a competitive binding assay (with [125I]IMPY in postmortem AD brain homogenates).17 Both new ligands displayed excellent binding affinities; the Ki values are 4.0 ± 0.8 and 1.5 ± 0.3 nM (three determinations were made for each Ki value), respectively.
Encouraged by the binding data observed for these two ligands, we carried out further biological evaluations with the [18F] labeled probes. Standard nucleophilic substitution reactions of [18F]fluoride with the corresponding tosylate precursors 6 and 17, in the presence of Kryptofix 222 (K222) as phase transfer catalyst (PTC), were successfully performed.18 For the product [18F]8, however, one extra microwave heating step had been executed for the N-Boc deprotection (Scheme 3). The subsequent HPLC purified radioligands, [18F]8 and [18F]14, showed greater than 97% radiochemical purities with 16% and 11% overall yields (decay corrected) and ~670 Ci/mmol and ~1900 Ci/mmol specific activities, respectively. The partition coefficient (PC, commonly measured as log P) of two radiofluorinated ligands at PH 7.4 were measured (log P = 2.95 and 2.56).19 The data illustrate both ligands own suitability as a brain imaging agent.
Scheme 3.
Reagents and conditions: (a) [18F]KF, K222, K2CO3, DMSO, 120 °C, 4.0 min; (b) Microwave heating, 150 °C, 2.5 min; (c) Pd(PPh3)4, CuI, trimethylsilylacetylene, diethylamine, DMF, rt, 0.7 h; (d) KOH, MeOH/THF, rt, 2.5 h; (e) Pd(PPh3)4, CuI, Et3N, CH3CN, 0 °C to rt, 1.0 h; (f) [18F]KF, K222, K2CO3, DMSO, 120 °C, 4.0 min.
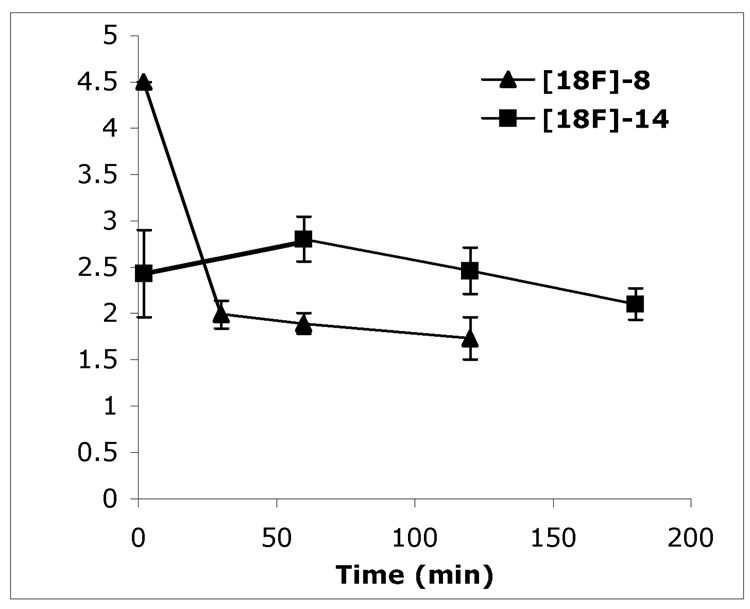
Two radiofluorinated ligands, [18F]8 and [18F]14, displayed good initial penetrations of the blood-brain barrier with excellent initial brain uptakes (4.50 and 2.43 % ID/g at 2 min after tracer injection) in normal mice. However, these two radioligands only displayed moderate to slow washouts with 1.71% ID/g remaining in the brain at two hours after the tracer injection and 2.10% ID/g at three hours, respectively (Figure 3). These results show promise; but the rate of brain washout is not optimal for an Aβ plaque-targeting imaging agent since a fast washout rate may helps generate the better signal-to-noise ratios and therefore may be better for Aβ plaque detection.20
Figure 3.
Brain uptake and washout of [18F]8 and [18F]14 in normal mice. Data are presented as % ID/g of three mice ± standard deviation.
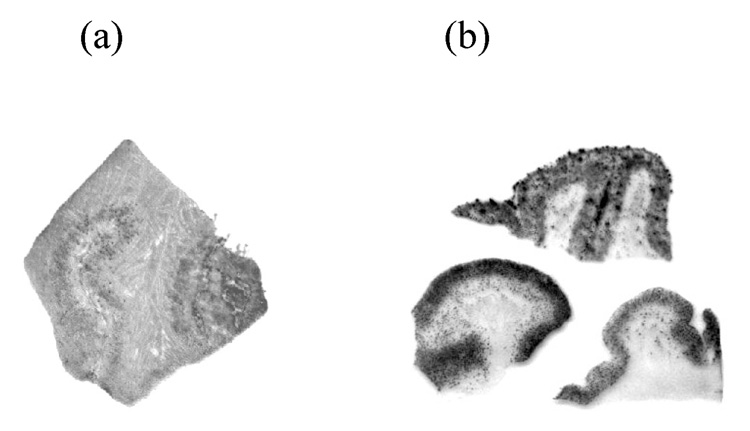
To confirm the specific binding of radiofluorinated ligands 8 and 14 to Aβ plaques, we performed the in vitro film autoradiography. As shown in Figure 4, [18F]14 distinctively labeled plaques on AD brain sections with low background labeling. On the contrary, in addition to plaque labeling, [18F]8 displayed significant white matter labeling.
Figure 4.
In vitro autoradiography of frozen human brain sections of AD patients with (a) [18F]8 and (b) [18F]14. [18F]14 showed excellent binding to the Aβ plaques with very low background labeling.
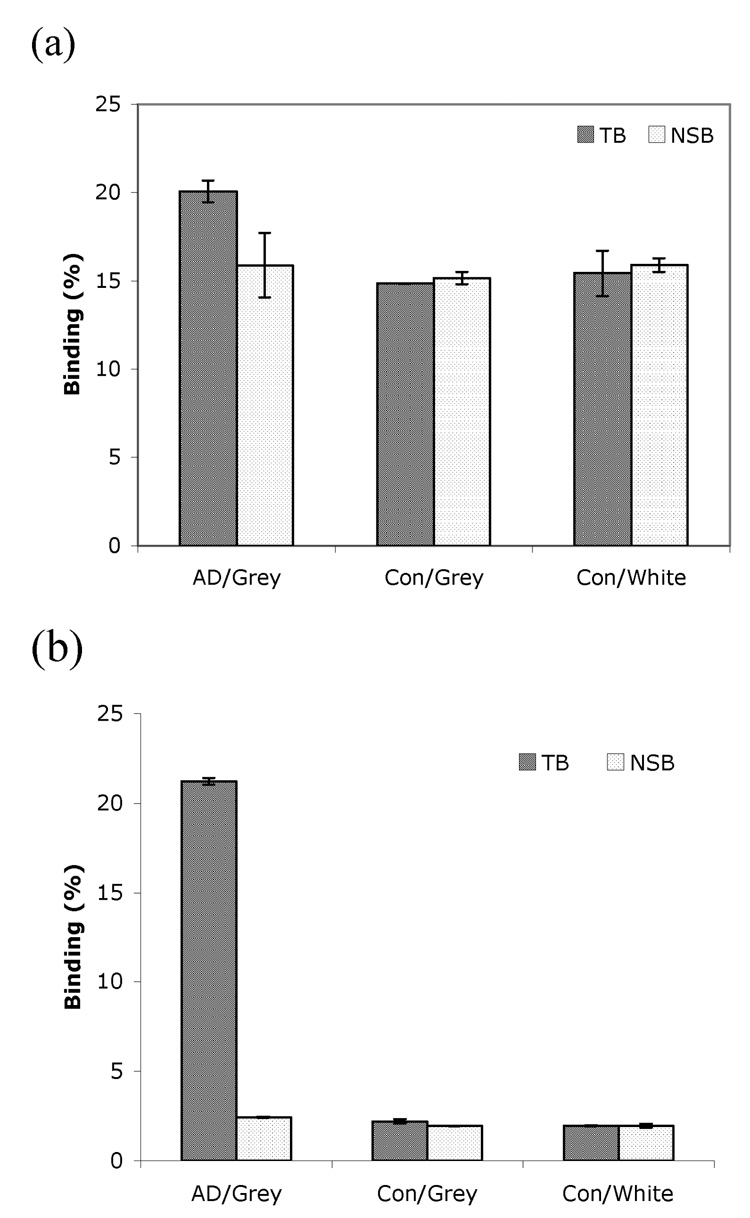
Furthermore, the in vitro binding assay using homogenates prepared from AD and control brain tissues were conducted to evaluate the binding specificity of these two radioligands to Aβ plaques (Figure 5).21 [18F]14 showed a very high specific binding in homogenate prepared from grey matter of an AD patient with low non-specific binding. In contrast, [18F]8 displayed similar binding in homogenates prepared from an AD patient and control with high non-specific binding. These results are consistent with in vitro binding results derived from autoradiography of AD brain sections. Preliminary in vivo and in vitro metabolism data of [18F]14 showed a complex pattern of metabolism in plasma and in liver (data not shown). It is likely that the indole or indoline ring may have removed the chances of N-demethylation; however, other metabolic reaction(s) at different sites of these two molecules may have occurred. Further exploration of the structure-activity relationship of this series of agents will be needed in the future. Nonetheless, the novel core structures showing excellent binding affinities to Aβ aggregates in human brain tissue provide potential insight for developing useful imaging agents.
Figure 5.
Specific binding of (a) [18F]8 and (b) [18F]14 to AD and control (Con) brain tissue homogenate. Grey and white matter was dissected from the cortical regions. [18F]14 showed high specific binding mainly in the gray matter of AD brain. (NSB: non-specific binding; TB: total binding; two determinations were made).
In conclusion, we have demonstrated that indolinyl- and indolyl-phenylacetylenes 8 and 14 can be successfully prepared. They showed high binding affinities to β-amyloid plaques by in vitro binding assay. The radiofluorinated derivatives, [18F]8 and [18F]14, displayed good brain penetration with moderate to relatively low rate of washout in normal mice. The combination of in vitro autoradiography of postmortem AD brain sections and brain tissue homogenate binding assay depicted that radioligand [18F]14 showed specific Aβ plaque labeling signal. Taken together, the results suggest that the indolylphenylacetylene ligand, 14, may be a lead for further structural modification in order to improve the in vivo stability and in vivo kinetics desirable for a useful Aβ plaque imaging agent.
Acknowledgements
This work was supported by grants from the National Institutes of Health (AG-022559 to H.F.K.) and Avid Radiopharmaceuticals. The authors thank Pathology Core Laboratories at The Children’s Hospital of Philadelphia for assembling the human macro-array brain sections.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Goedert M, Spillantini MG. Science. 2006;314:777. doi: 10.1126/science.1132814. [DOI] [PubMed] [Google Scholar]
- 2.Hardy J. J Alzheimers Dis. 2006;9:151. doi: 10.3233/jad-2006-9s317. [DOI] [PubMed] [Google Scholar]
- 3.Hardy J, Selkoe DJ. Science. 2002;297:353. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 4.Mathis CA, Wang Y, Holt DP, Huang G-F, Debnath ML, Klunk WE. J. Med. Chem. 2003;46:2740. doi: 10.1021/jm030026b. [DOI] [PubMed] [Google Scholar]
- 5.Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, Bergstrom M, Savitcheva I, Huang G-F, Estrada S, Ausen B, Debnath ML, Barletta J, Price JC, Sandell J, Lopresti BJ, Wall A, Koivisto P, Antoni G, Mathis CA, Långström B. Ann. Neurol. 2004;55:306. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 6.Cai L, Innis RB, Pike VW. Curr. Med. Chem. 2007;14:19. doi: 10.2174/092986707779313471. [DOI] [PubMed] [Google Scholar]
- 7.Rowe CC, Ackerman U, Browne W, Mulligan R, Pike KL, O'Keefe G, Tochon-Danguy H, Chan G, Berlangieri SU, Jones G, Dickinson-Rowe KL, Kung HP, Zhang W, Kung MP, Skovronsky D, Dyrks T, Holl G, Krause S, Friebe M, Lehman L, Lindemann S, Dinkelborg LM, Masters CL, Villemagne VL. Lancet Neurol. 2008;7:129. doi: 10.1016/S1474-4422(08)70001-2. [DOI] [PubMed] [Google Scholar]
- 8.Henriksen G, Yousefi BH, Drzezga A, Wester HJ. Eur J Nucl Med Mol Imaging. 2008;35:S75. doi: 10.1007/s00259-007-0705-x. [DOI] [PubMed] [Google Scholar]
- 9.Mathis CA, Lopresti BJ, Klunk WE. Nucl. Med. Biol. 2007;34:809. doi: 10.1016/j.nucmedbio.2007.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ikonomovic MD, Klunk WE, Abrahamson EE, Mathis CA, Price JC, Tsopelas ND, Lopresti BJ, Ziolko S, Bi W, Paljug WR, Debnath ML, Hope CE, Isanski BA, Hamilton RL, Dekosky ST. Brain. 2008 doi: 10.1093/brain/awn016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henriksen G, Hauser AI, Westwell AD, Yousefi BH, Schwaiger M, Drzezga A, Wester HJ. J. Med. Chem. 2007;50:1087. doi: 10.1021/jm061466g. [DOI] [PubMed] [Google Scholar]
- 12.Cai L, Cuevas J, Temme S, Herman MM, Dagostin C, Widdowson DA, Innis RB, Pike VW. J. Med. Chem. 2007;50:4746. doi: 10.1021/jm0702231. [DOI] [PubMed] [Google Scholar]
- 13.Zhang W, Oya S, Kung MP, Hou C, Maier DL, Kung HF. J. Med. Chem. 2005;48:5980. doi: 10.1021/jm050166g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stephenson KA, Chandra R, Zhuang ZP, Hou C, Oya S, Kung MP, Kung HF. Bioconjug. Chem. 2007;18:238. doi: 10.1021/bc060239q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chandra R, Oya S, Kung MP, Hou C, Jin LW, Kung HF. J. Med. Chem. 2007;50:2415. doi: 10.1021/jm070090j. [DOI] [PubMed] [Google Scholar]
- 16.Bacskai BJ, Frosch MP, Freeman SH, Raymond SB, Augustinack JC, Johnson KA, Irizarry MC, Klunk WE, Mathis CA, Dekosky ST, Greenberg SM, Hyman BT, Growdon JH. Arch. Neurol. 2007;64:431. doi: 10.1001/archneur.64.3.431. [DOI] [PubMed] [Google Scholar]
- 17.Kung M-P, Hou C, Zhuang Z-P, Skovronsky D, Kung HF. Brain Res. 2004;1025:89. doi: 10.1016/j.brainres.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 18.Cai L, Lu S, Pike VW. Eur. J. Org. Chem. 2008:2853. [Google Scholar]
- 19.Qu W, Kung MP, Hou C, Benedum TE, Kung HF. J. Med. Chem. 2007;50:2157. doi: 10.1021/jm070025+. [DOI] [PubMed] [Google Scholar]
- 20.Mathis CA, Wang Y, Klunk WE. Curr. Pharm. Des. 2004;10:1469. doi: 10.2174/1381612043384772. [DOI] [PubMed] [Google Scholar]
- 21.Qu W, Kung MP, Hou C, Oya S, Kung HF. J. Med. Chem. 2007;50:3380. doi: 10.1021/jm070467l. [DOI] [PMC free article] [PubMed] [Google Scholar]