Abstract
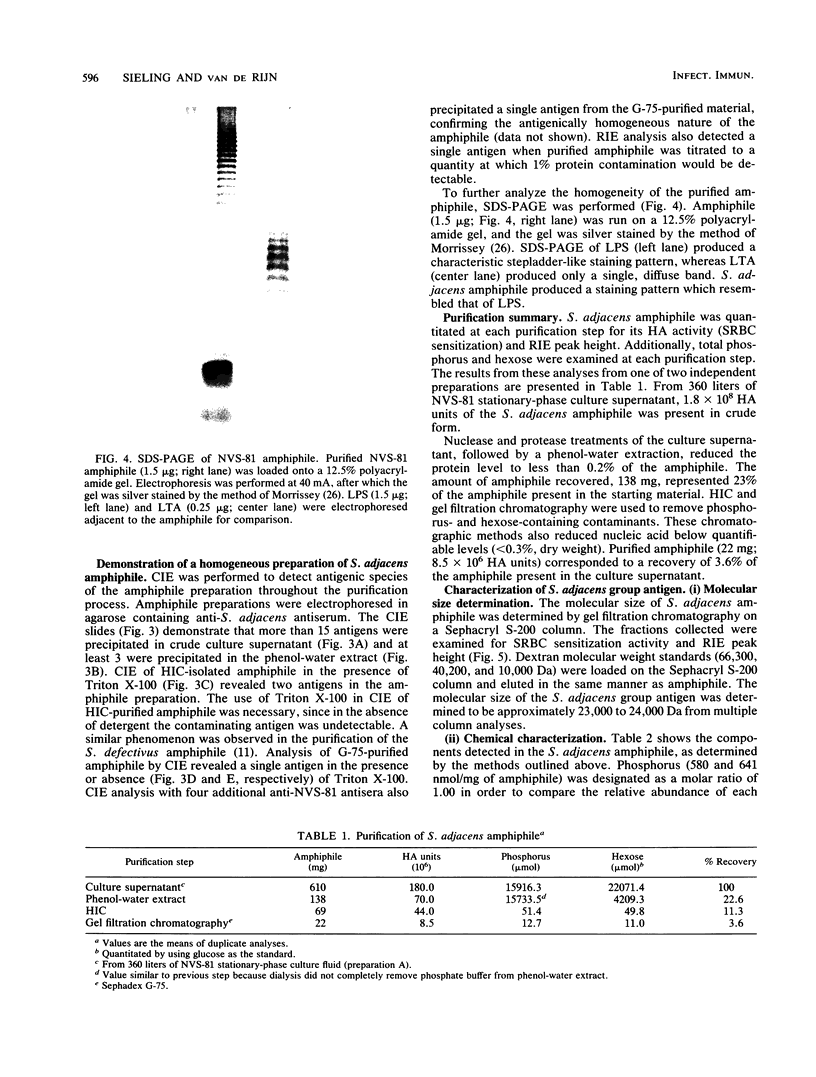
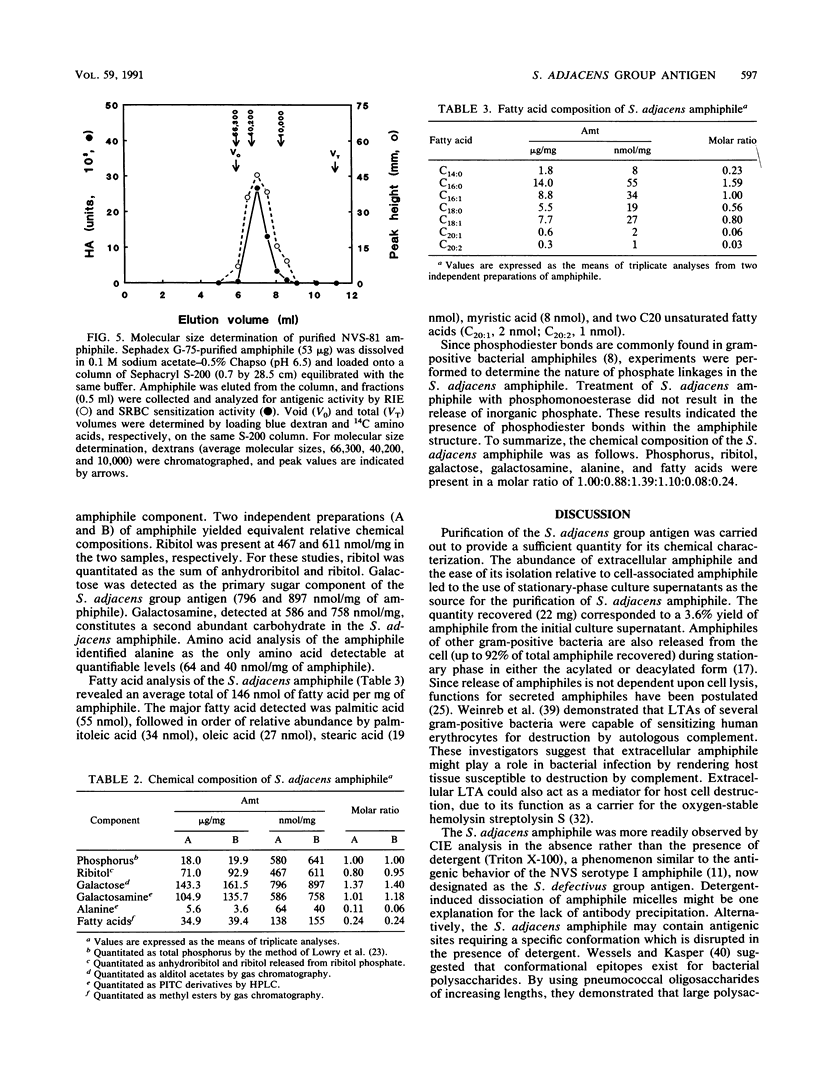
Nutritionally variant streptococci (NVS) possess amphiphiles which are serologically distinct from lipoteichoic acid and which serve as group-specific antigens for NVS. The objective of this study was to purify and characterize the NVS serotype II (Streptococcus adjacens) amphiphile. Amphiphile was isolated from stationary-phase culture supernatants of NVS strain 81 (NVS serotype II). Phenol-water extracts of culture supernatants were subjected to hydrophobic interaction chromatography and gel filtration chromatography. A homogeneous preparation of amphiphile (22 mg; 8.5 x 10(6) hemagglutination units) was recovered, and its approximate molecular size (23,000 to 24,000 Da) and chemical composition were determined. Purified S. adjacens amphiphile contained phosphorus, ribitol, galactose, galactosamine, alanine, and fatty acids in molar ratios of 1.00:0.88:1.39:1.10:0.08:0.24. Since ribitol, galactose, and galactosamine were the primary carbohydrate components, the amphiphile may exist as a polyribitol phosphate with galactose and galactosamine substituents. Preliminary structural analysis demonstrated the presence of phosphodiester bonds within the amphiphile structure. Finally, the amphiphile serves as the S. adjacens group antigen.
Full text
PDF

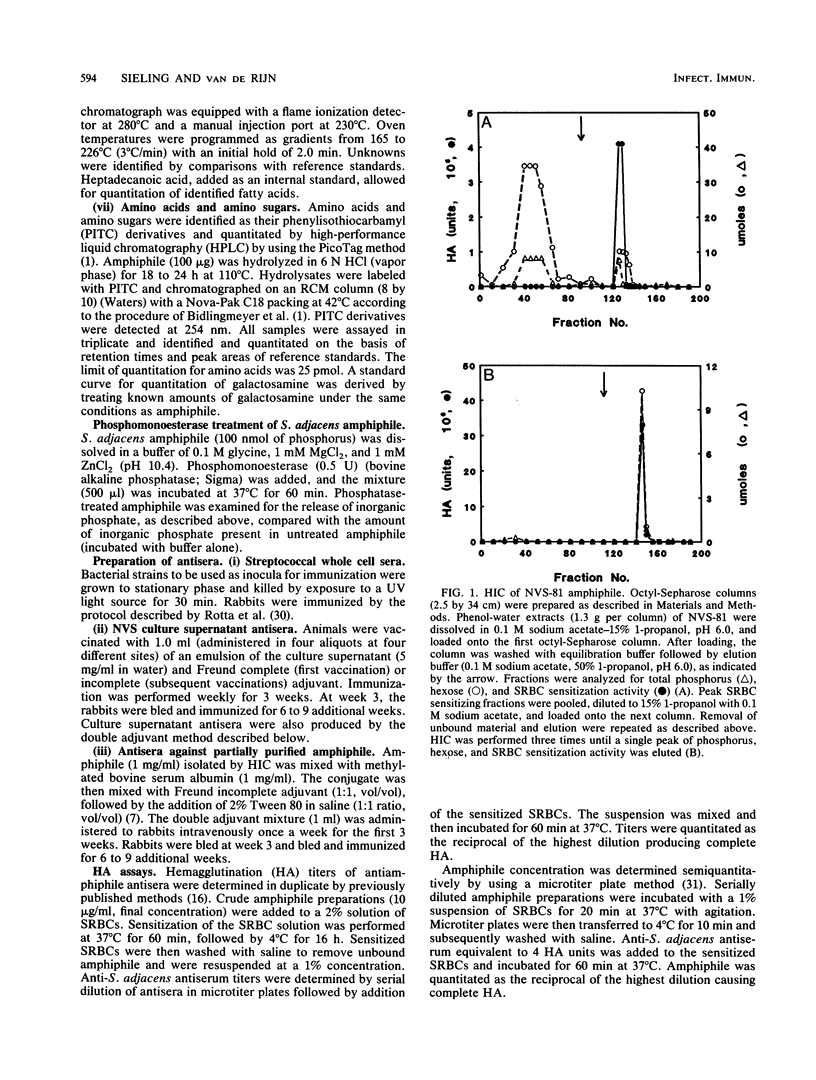
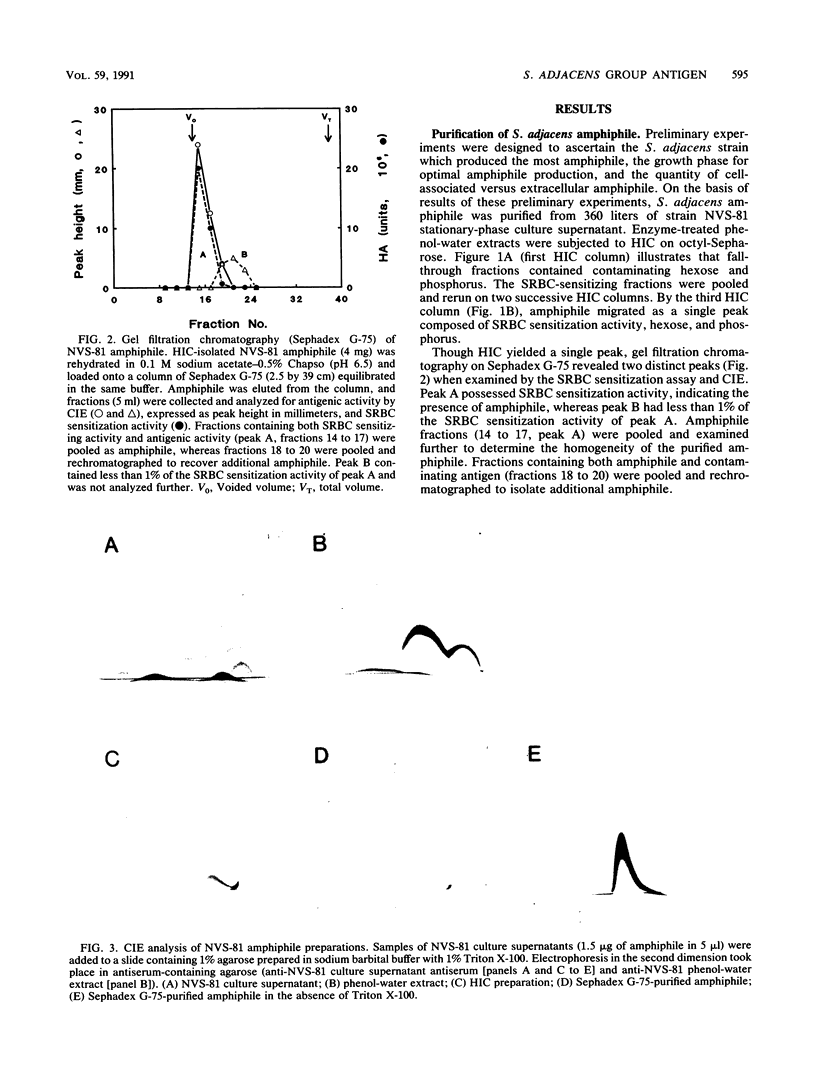


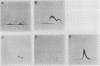
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bidlingmeyer B. A., Cohen S. A., Tarvin T. L. Rapid analysis of amino acids using pre-column derivatization. J Chromatogr. 1984 Dec 7;336(1):93–104. doi: 10.1016/s0378-4347(00)85133-6. [DOI] [PubMed] [Google Scholar]
- Bouvet A., Villeroy F., Cheng F., Lamesch C., Williamson R., Gutmann L. Characterization of nutritionally variant streptococci by biochemical tests and penicillin-binding proteins. J Clin Microbiol. 1985 Dec;22(6):1030–1034. doi: 10.1128/jcm.22.6.1030-1034.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvet A., van de Rijn I., McCarty M. Nutritionally variant streptococci from patients with endocarditis: growth parameters in a semisynthetic medium and demonstration of a chromophore. J Bacteriol. 1981 Jun;146(3):1075–1082. doi: 10.1128/jb.146.3.1075-1082.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLIOTT S. D. TEICHOIC ACID AND THE GROUP ANTIGEN OF LACTIC STREPTOCOCCI (GROUP N). Nature. 1963 Dec 21;200:1184–1185. doi: 10.1038/2001184a0. [DOI] [PubMed] [Google Scholar]
- Fiedel B. A., Jackson R. W. Immunogenicity of a purified and carrier-complexed streptococcal lipoteichoic acid. Infect Immun. 1976 Jun;13(6):1585–1590. doi: 10.1128/iai.13.6.1585-1590.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer W., Koch H. U., Rösel P., Fiedler F. Alanine ester-containing native lipoteichoic acids do not act as lipoteichoic acid carrier. Isolation, structural and functional characterization. J Biol Chem. 1980 May 25;255(10):4557–4562. [PubMed] [Google Scholar]
- Fischer W. Physiology of lipoteichoic acids in bacteria. Adv Microb Physiol. 1988;29:233–302. doi: 10.1016/s0065-2911(08)60349-5. [DOI] [PubMed] [Google Scholar]
- George M., van de Rijn I. Nutritionally variant streptococcal serotype I antigen. Characterization as a lipid-substituted poly(ribitol phosphate). J Immunol. 1988 Mar 15;140(6):2008–2015. [PubMed] [Google Scholar]
- George M., van de Rijn I. Purification of serotype I antigen from nutritionally variant streptococci. Infect Immun. 1988 May;56(5):1222–1231. doi: 10.1128/iai.56.5.1222-1231.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gephart J. F., Washington J. A., 2nd Antimicrobial susceptibilities of nutritionally variant streptococci. J Infect Dis. 1982 Oct;146(4):536–539. doi: 10.1093/infdis/146.4.536. [DOI] [PubMed] [Google Scholar]
- Grimes W. J., Greegor S. Carbohydrate compositions of normal, spontaneously transformed, and virally transformed cells derived from BALB/c mice. Cancer Res. 1976 Nov;36(11 Pt 1):3905–3910. [PubMed] [Google Scholar]
- Harvey D. J., Horning M. G. Characterization of the trimethylsilyl derivatives of sugar phosphates and related compounds by gas chromatography and gas chromatography-mass spectrometry. J Chromatogr. 1973 Feb 7;76(1):51–62. doi: 10.1016/s0021-9673(01)97777-5. [DOI] [PubMed] [Google Scholar]
- Hellerqvist C. G., Sweetman B. J. Mass spectrometry of carbohydrates. Methods Biochem Anal. 1990;34:91–143. doi: 10.1002/9780470110553.ch2. [DOI] [PubMed] [Google Scholar]
- Hewett M. J., Knox K. W., Wicken A. J. Studies on the group F antigen of lactobacilli: detection of antibodies by haemagglutination. J Gen Microbiol. 1970 Mar;60(3):315–322. doi: 10.1099/00221287-60-3-315. [DOI] [PubMed] [Google Scholar]
- Joseph R., Shockman G. D. Synthesis and excretion of glycerol teichoic acid during growth of two streptococcal species. Infect Immun. 1975 Aug;12(2):333–338. doi: 10.1128/iai.12.2.333-338.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R. E., Shockman G. D. Precursor-product relationship of intracellular and extracellular lipoteichoic acids of Streptococcus faecium. J Bacteriol. 1979 Feb;137(2):869–877. doi: 10.1128/jb.137.2.869-877.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R. E., van de Rijn I. Quantitative immunoelectrophoretic analysis of Streptococcus pyogenes membrane. Infect Immun. 1979 Dec;26(3):892–902. doi: 10.1128/iai.26.3.892-902.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox K. W., Hewett M. J., Wicken A. J. Studies on the group F antigen of lactobacilli: antigenicity and serological specificity of teichoic acid preparations. J Gen Microbiol. 1970 Mar;60(3):303–313. doi: 10.1099/00221287-60-3-303. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROBERTS N. R., LEINER K. Y., WU M. L., FARR A. L. The quantitative histochemistry of brain. I. Chemical methods. J Biol Chem. 1954 Mar;207(1):1–17. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Larm O., Lindberg B. The pneumococcal polysaccharides: a re-examination. Adv Carbohydr Chem Biochem. 1976;33:295–322. doi: 10.1016/s0065-2318(08)60284-x. [DOI] [PubMed] [Google Scholar]
- Markham J. L., Knox K. W., Wicken A. J., Hewett M. J. Formation of extracellular lipoteichoic acid by oral streptococci and lactobacilli. Infect Immun. 1975 Aug;12(2):378–386. doi: 10.1128/iai.12.2.378-386.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Roberts R. B., Krieger A. G., Schiller N. L., Gross K. C. Viridans streptococcal endocarditis: the role of various species, including pyridoxal-dependent streptococci. Rev Infect Dis. 1979 Nov-Dec;1(6):955–966. doi: 10.1093/clinids/1.6.955. [DOI] [PubMed] [Google Scholar]
- Rosan B. Absence of glycerol teichoic acids in certain oral streptococci. Science. 1978 Sep 8;201(4359):918–920. doi: 10.1126/science.684416. [DOI] [PubMed] [Google Scholar]
- Rotta J., Krause R. M., Lancefield R. C., Everly W., Lackland H. New approaches for the laboratory recognition of M types of group A streptococci. J Exp Med. 1971 Nov 1;134(5):1298–1315. doi: 10.1084/jem.134.5.1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvestri L. J., Craig R. A., Ingram L. O., Hoffmann E. M., Bleiweis A. S. Purification of lipoteichoic acids by using phosphatidyl choline vesicles. Infect Immun. 1978 Oct;22(1):107–118. doi: 10.1128/iai.22.1.107-118.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toon P., Brown P. E., Baddiley J. The lipid-teichoic acid complex in the cytoplasmic membrane of Streptococcus faecalis N.C.I.B. 8191. Biochem J. 1972 Apr;127(2):399–409. doi: 10.1042/bj1270399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Driel D., Wicken A. J., Dickson M. R., Knox K. W. Cellular location of the lipoteichoic acids of Lactobacillus fermenti NCTC 6991 and Lactobacillus casei NCTC 6375. J Ultrastruct Res. 1973 Jun;43(5):483–497. doi: 10.1016/s0022-5320(73)90025-7. [DOI] [PubMed] [Google Scholar]
- WICKEN A. J., ELLIOTT S. D., BADDILEY J. The identity of streptococcal group D antigen with teichoic acid. J Gen Microbiol. 1963 May;31:231–239. doi: 10.1099/00221287-31-2-231. [DOI] [PubMed] [Google Scholar]
- Weinreb B. D., Shockman G. D., Beachey E. H., Swift A. J., Winkelstein J. A. The ability to sensitize host cells for destruction by autologous complement is a general property of lipoteichoic acid. Infect Immun. 1986 Nov;54(2):494–499. doi: 10.1128/iai.54.2.494-499.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessels M. R., Kasper D. L. Antibody recognition of the type 14 pneumococcal capsule. Evidence for a conformational epitope in a neutral polysaccharide. J Exp Med. 1989 Jun 1;169(6):2121–2131. doi: 10.1084/jem.169.6.2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicken A. J., Knox K. W. Bacterial cell surface amphiphiles. Biochim Biophys Acta. 1980 May 27;604(1):1–26. doi: 10.1016/0005-2736(80)90583-0. [DOI] [PubMed] [Google Scholar]
- Wicken A. J., Knox K. W. Lipoteichoic acids: a new class of bacterial antigen. Science. 1975 Mar 28;187(4182):1161–1167. doi: 10.1126/science.46620. [DOI] [PubMed] [Google Scholar]
- van de Rijn I. Analysis of cross-protection between serotypes and passively transferred immune globulin in experimental nutritionally variant streptococcal endocarditis. Infect Immun. 1988 Jan;56(1):117–121. doi: 10.1128/iai.56.1.117-121.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Rijn I., Bouvet A. Characterization of a pH-dependent chromophore from nutritionally variant streptococci. Infect Immun. 1984 Jan;43(1):28–31. doi: 10.1128/iai.43.1.28-31.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Rijn I., George M. Immunochemical study of nutritionally variant streptococci. J Immunol. 1984 Oct;133(4):2220–2225. [PubMed] [Google Scholar]
- van de Rijn I. Role of culture conditions and immunization in experimental nutritionally variant streptococcal endocarditis. Infect Immun. 1985 Dec;50(3):641–646. doi: 10.1128/iai.50.3.641-646.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]