Abstract
Objective
There have been few studies of the pharmacologic modulation of facial emotion recognition. The present study aimed to replicate and extend the finding that recognition of facial anger was selectively impaired by diazepam. The hypothesis was that, in comparison with placebo, diazepam would impair the recognition of facial anger in healthy volunteers, but not the recognition of 5 other basic emotions: happiness, surprise, fear, sadness and disgust.
Design
A randomized, counterbalanced, double-blind, placebo-controlled, within-subjects comparison of diazepam with placebo.
Setting
A university psychopharmacology research unit.
Participants
Healthy male (n = 6) and female (n = 22) volunteers, aged 18–45 years.
Procedures
Subjects were tested on 2 tasks following the administration of diazepam, 15 mg, and placebo on separate occasions. In the first “multimorph” task, images of facial expressions were morphed to produce continua between the neutral and full expressions of 6 basic emotions. Accuracy and identification thresholds were assessed for stimuli in which the intensity of expression gradually increased. In the second “emotional hexagon” task, facial expressions were morphed between pairs of emotions. Single images were presented, and accuracy and speed of response were assessed.
Results
Diazepam produced broad impairments in response accuracy, recognition thresholds and response speed on the facial emotion tasks that were not limited to angry expressions.
Conclusions
The present study found that diazepam, 15 mg, impaired facial emotion recognition, but not selectively. In the emotional hexagon task, a reaction-time analysis suggested that the identification of facial anger might be differentially sensitive to variations in stimulus duration, complicating the interpretation of this paradigm.
Medical subject headings: diazepam, emotions, facial expression, gamma-aminobutyric acid, perception
Abstract
Objectif
Les études sur la modulation pharmacologique de la reconnaissance des émotions sur le visage sont peu nombreuses. La présente étude visait à reproduire et à étendre la constatation selon laquelle le diazépam nuit de façon sélective à la reconnaissance de l'expression faciale de la colère. L'hypothèse posée était la suivante : chez des volontaires en bonne santé, le diazépam, comparativement à un placebo, nuirait à la reconnaissance de l'expression faciale de la colère mais non à la reconnaissance de cinq autres émotions de base : bonheur, surprise, peur, tristesse et dégoût.
Conception
Comparaison randomisée, compensée, à double insu et contrôlée par placebo, chez les sujets mêmes, entre le diazépam et un placebo.
Contexte
Service de recherche universitaire en psychopharmacologie.
Participants
Hommes (n = 6) et femmes (n = 22) volontaires en bonne santé âgés de 18 à 45 ans.
Interventions
On a soumis les sujets à deux tâches après leur avoir administré 15 mg de diazépam et un placebo à des occasions distinctes. Au cours de la première tâche de «multimorphage», on a transformé graduellement des images d'expressions faciales pour produire des continuums entre l'expression neutre et complète de six émotions de base. On a évalué des seuils d'exactitude et d'identification reliés aux stimuli où l'intensité de l'expression a augmenté graduellement. Dans la deuxième tâche dite de «l'hexagone affectif», on a transformé des expressions du visage entre des paires d'émotions. On a présenté des images individuelles et évalué l'exactitude et la vitesse de la réponse.
Résultats
Le diazépam a produit des déficits généraux aux niveaux de l'exactitude de la réponse, des seuils de reconnaissance et de la vitesse de réaction dans les tâches reliées aux émotions faciales non limitées à l'expression de colère.
Conclusion
Cette étude a révélé que 15 mg de diazépam nuisaient à la reconnaissance des émotions sur le visage, mais non de façon sélective. Dans le contexte de la tâche dite de l'hexagone affectif, une analyse du temps de réaction a indiqué que l'identification de la colère sur le visage pourrait être d'une sensibilité différentielle à des variations de la durée du stimulus, ce qui complique l'interprétation de ce paradigme.
Introduction
The recognition of emotional expressions is an important aspect of social communication that has come under increasing research scrutiny, with the identification through lesion and neuroimaging studies1,2,3 of neural structures that contribute to emotion recognition and the identification of alterations in emotion recognition in specific disorders, such as psychopathy4 and Huntington's disease,5 or in association with particular experiences such as child abuse.6 An area of investigation in which research results are starting to appear is the study of pharmacologic manipulation of emotion recognition. An early study showed impairment of anger recognition after the administration of alcohol.7 More recently, studies have begun to report findings using more graduated tasks in which the intensity of emotional expressions is systematically varied using computer morphing techniques.8 These studies have shown that the β-adrenergic antagonist propranolol may subtly delay recognition of facial sadness, without impairing recognition accuracy,9 that the D2 dopamine receptor antagonist sulpiride may selectively impair recognition of facial anger10 and, in the first published study of this type, that the positive allosteric modulator of the γ-aminobutyric acid type A receptor diazepam selectively impairs recognition of facial anger.11 The last finding was replicated recently, except that the impairment was not fully selective for facial anger, because fear recognition was also impaired.12 As a lead-in to more extensive investigation of the pharmacologic manipulation of facial emotion recognition, the present study also aimed to test whether recognition of facial anger was selectively impaired by diazepam, using both a modification of the original paradigm11 and a second task, in order to determine whether impairments were related to specific emotional expressions independently of other task parameters.
Methods
Subjects
The study was approved by the University of Alberta Health Research Ethics Board. Healthy male (n = 6) and female (n = 22) volunteers aged 18–45 (mean 26, standard deviation 7) years were recruited and gave written informed consent to their participation. All subjects were university undergraduates or graduates. Subjects were screened to exclude axis I psychiatric disorders using the Mini-International Neuropsychiatric Interview,13 which was modified to probe for lifetime disorders. Subjects were also excluded for the following reasons: not raised in Canada; not fluent in English; history of significant medical and particularly neurologic disorders; use of medications other than oral contraception or simple analgesics; history of depression, bipolar disorder or schizophrenia in first-degree relatives; consumption of more than 14 (male) or 7 (female) standard drinks of alcohol per week; consumption of alcohol within 48 hours of testing; frequent recreational use of marijuana or other nonprescription drugs; use of marijuana or other nonprescription drugs within 4 weeks of testing; and being pregnant or lactating. A negative urine pregnancy test was required from female participants before each test. The subjects' usual caffeine intake was permitted on test days. The 1 smoking participant was not permitted to smoke after drug ingestion.
Design
A randomized-order, counterbalanced for drug order, double-blind, placebo-controlled, within-subjects design was used. Subjects attended for 2 test sessions, which were at least 7 days apart. At the first session, subjects were randomly assigned the order in which they would receive diazepam and placebo. All subjects were administered both drugs. At baseline and following drug administration, immediately before testing, subjects completed the Profile of Mood States (POMS), of which the composed–anxious, energetic–fatigued and clearheaded– confused scales have been shown to be sensitive to diazepam.14,15 They also completed the Spielberger State Anxiety Inventory (SSAI).16,17 At baseline at the first session, subjects also completed the Spielberger Trait Anxiety Inventory (STAI) to rate their general level of anxiety.16 Immediately after the baseline ratings were completed, subjects were given diazepam, 15 mg, or placebo (in matching opaque gelatin capsules). Equal numbers of subjects received either diazepam or placebo on each occasion. Subjects completed the second set of ratings after 40 minutes. The emotion recognition tasks were then carried out in a fixed order. The timing of peak plasma diazepam levels has varied between 30 and 90 minutes in pharmacokinetic studies, and the dose and timing used in the present study were consistent with other studies of the effects of diazepam on facial emotion, visual perception and memory.11,12,18,19,20
Test procedures (facial emotion tasks)
Stimuli for both tasks were grey-scale images derived from the Pictures of Facial Affect photographic series, for which there are high levels of agreement between raters as to the depicted emotions.21 The intensity of facial emotion was manipulated by computer morphing, using previously described methods to produce continua,8 either between neutral expressions and the full expression of 6 basic emotions (happy, surprised, fearful, sad, disgusted, angry) or between pairs of basic emotions. The advantages of using morphed stimuli are that the images are realistic, a high degree of control can be exerted over the stimulus intensity, and the degree to which an expression is morphed above neutral is proportional to the accuracy of identification, intensity of perceived emotion and the activation of specific brain regions in neuroimaging studies.22,23
Task 1: “Multimorph” task
The paradigm from which the test was developed has been described previously.4 The current test version used images of 9 models, each portraying 6 emotions, thus providing 54 stimuli. For each stimulus, the expression was morphed between neutral and the full expression in 40 increments of 2.5%. Stimuli were presented on a computer monitor as continuous sequences, in which the intensity of expression gradually transformed from 2.5% to 100% at a rate of 5% per second, giving a total duration of 20 s. The stimulus order was randomized. Six response buttons with emotion labels were presented onscreen to the right of the stimulus and the order of the response buttons was also randomized between sessions. Subjects responded with a mouse click on the appropriate label as soon as they could identify the emotion. They could change their response by clicking again. At the end of the stimulus, the 100% image remained onscreen until subjects confirmed their final choice. The lowest intensity at which each emotion stimulus was recognized correctly without subsequent alteration was recorded. An incorrect final choice was assigned an intensity of 102.5%. A mean threshold for each emotion was calculated based on the 9 models. Before each test, a practice version was run with 12 stimuli derived from a separate model. In a group of subjects who were tested twice on this task, intraclass correlations for identification thresholds were > 0.70 for all emotions (Dr. Nick J. Coupland, Mr. Ryan A. Sustrik, Ms. Patricia Ting, Dr. R. James Blair: unpublished data, 2002). The paradigm was sensitive to predicted deficits in the recognition of facial sadness and disgust in children with psychopathic tendencies.4 In healthy subjects, lower positive affect predicted a higher threshold for recognizing happy faces, whereas higher negative affect predicted a lower threshold for recognizing disgust, suggesting that the task engages affective systems, rather than purely cognitive processing.24
Task 2: “Emotional hexagon” task
The test stimuli have been described previously.11 In brief, the 30 images were produced by morphing between pairs of emotions for a single model (J.J.) from the Pictures of Facial Affect series.21 The pairs were selected in a sequence (happy–surprised, surprised–fearful, fearful–sad, sad–disgusted, disgusted–angry) on the basis that each expression is more likely to be misidentified as its immediate neighbours than more distant neighbours. An angry–happy pairing was used to complete the sequence. Five morphs were produced for each pair, in which the intensity of the first emotion in the pair increased in 20% increments from 10% (e.g., happy 10%–surprised 90%; happy 30%–surprised 70%; happy 50%–surprised 50%; happy 70%–surprised 30%; happy 90%–surprised 10%). Previous studies have shown that subjects tend to perceive images containing 70% or 90% of an emotion categorically as that emotion and that increasing intensity of emotion is associated with decreased reaction times in recognition tests.8 Each image was presented for 5000 ms. Labels for 6 basic emotions were presented to the right of the image, starting at the onset of the image and remaining onscreen for 7000 ms. A 1000-ms interstimulus interval was used. Subjects were instructed to respond with a left mouse click as soon as they were sure that they had identified the emotion. Five blocks of 30 stimuli were presented. The stimuli and the order of the emotion labels were randomized in each block. A single block of 30 stimuli was used as a practice run before each test. Responses were counted each time an emotion was identified that was present at an intensity of 70% or 90% in the image, so that there was a maximum of 20 correct responses for each expression (5 blocks with each emotion present in 2 pairs). The percentage identification at each intensity was then analyzed. The mean response times for correct identifications were analyzed and also the response times for each intensity level (50%, 70%, 90%, all emotions combined). Studies using this stimulus set have shown reduced disgust recognition associated with obsessive–compulsive disorder,25 Huntington's disease5 or an insular cortex lesion26 and decreased anger recognition following the administration of diazepam or sulpiride.10,11,12 However, reliability statistics have not been reported.
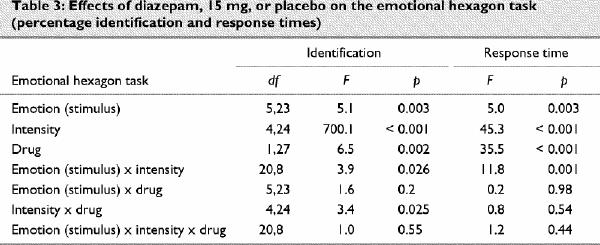
Statistical analysis
Data were analyzed using multivariate analysis of variance (ANOVA). For the multimorph threshold and accuracy data, there were 2 within-subject factors, emotion (6 levels for the 6 emotion categories) and drug (2 levels for diazepam and placebo), plus an interaction factor. In the response-time data for the hexagon task, reaction times were for specific intensity of stimuli, rather than for identification of particular emotions: the factors were, therefore, stimulus and drug. An additional within-subjects factor for the intensity of the emotion in the blend, that is, the mean of the stimulus intensity for the 2 continua in which the emotion is present, or the balance in the stimulus (5 levels for 10%–90%) was used in the analyses of the emotional hexagon accuracy and response-time data. The subjective data were analyzed using factors for drug, time and their interaction. A between-subjects factor for the order of drug administration was included initially for all analyses, but because the order effects were not significant, data were then collapsed across order. The significance level was set at a 2-tailed alpha of p = 0.05. Where indicated by the main analysis, repeated measures ANOVA was used post hoc to test for differences between emotions or intensities of emotion. Paired t tests were used to compare drug effects for specific emotions and were set at a 2-tailed alpha of p < 0.01 to control for multiple comparisons. Two-tailed rather than unidirectional tests were applied uniformly, because although a unidirectional effect on facial anger could be predicted for diazepam, based on earlier studies, this was not the case for other emotions. The intention was therefore to avoid demonstrating selectivity spuriously as a consequence of differential statistical power. Data are given as means and 95% confidence intervals (95% CI).
Results
All subjects completed the 2 test sessions, but there were missing data for 3 subjects for the multimorph task because of failures of the “save to file” step in the program on 3 tests. Diazepam significantly reduced subjective ratings of alertness and liveliness on the POMS in comparison with placebo, without significant effects on the other ratings (Table 1). For response accuracy on the multimorph task there were significant effects for emotion and trends for a main effect of drug and for an emotion х drug interaction (Fig. 1A; Table 2). Happy faces were more accurately recognized than other expressions under both drug conditions. Diazepam had least effect on the recognition of happy or angry faces, but differences between diazepam and placebo for the other emotions were not significant after correction for multiple comparisons. In particular, there was no difference in the recognition of angry expressions between diazepam and placebo (mean difference 0.16, 95% CI –0.7 to 1.0; p = 0.6). Tests for identification thresholds on the multimorph task (Fig. 1B) showed significant effects for emotion and drug, with a trend for an emotion х drug interaction (Table 2). Diazepam tended to increase the identification threshold needed to recognize all expressions, although this was significant only for surprised faces after correction for multiple comparisons (mean difference 2.9, 95% CI 0.2–5.6; df 24; p = 0.006). The mean difference in identification threshold for angry faces between diazepam and placebo was 1.4 (95% CI –0.5 to 3.3, p = 0.13).
Table 1
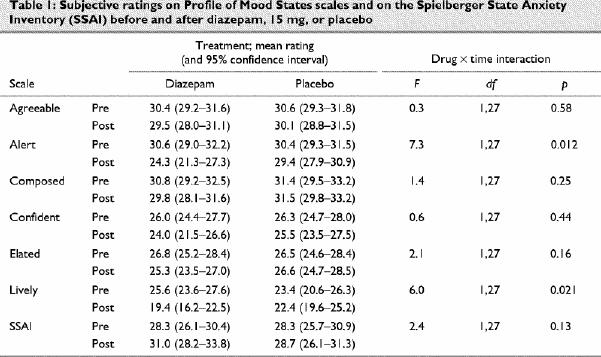
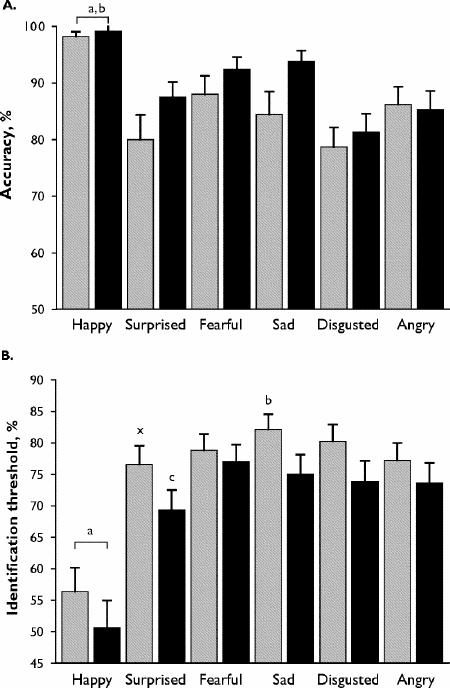
Fig. 1: Results of multimorph task of facial emotion recognition. Diazepam, 15 mg (grey bars); placebo (black bars).
A: Mean (and standard error) percentage accuracy of response. aDiazepam: happy > surprised, fearful, sad, disgusted, angry; bplacebo: happy > surprised, disgusted, angry (paired t tests, p < 0.01).
B: Mean (and standard error) threshold for emotion recognition. aDiazepam and placebo: happy < surprised, fearful, sad, disgusted, angry; bdiazepam: sad > surprised, angry; cplacebo: surprised < sad, fearful; xsurprised: diazepam > placebo (paired t tests, p < 0.01).
Table 2
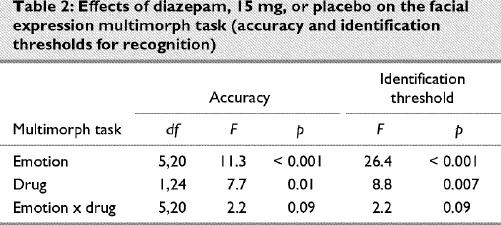
On the emotional hexagon task there was a sigmoid relation between the intensity of emotion and the percentage of stimuli in which the emotion was identified (Fig. 2A). The ANOVA showed significant main effects on identification for emotion, intensity and drug, plus significant intensity х drug and intensity х emotion interactions. Neither the drug х emotion interaction, nor the triple interaction with intensity was significant (Table 3). When responses for all emotions were combined in order to examine intensity effects, diazepam decreased identifications at intensities of 70% (mean difference 4.3%, 95% CI 0.6–8.0; p = 0.004) or 90% (mean difference 5.2%, 95% CI 0.7–9.8; p = 0.004), but not at lower intensities. For specific emotions, the only comparison between diazepam and placebo that survived adjustment for multiple comparisons was a reduction in accuracy for disgusted faces at 90% intensity (mean difference 8.2%, 95% CI 0.4–16.0; p = 0.007). The emotion х intensity interaction was related to sad expressions being identified more frequently at moderate intensities (30%–70%) and happy expressions more frequently at high intensities, compared with disgusted or angry expressions (Fig. 2A). The identification of angry faces was not significantly reduced at 50% intensity (mean difference 5.7%, 95% CI –8.0 to 19.4; p = 0.26) or at 90% intensity (mean difference 2.9%, 95% CI –9.2 to 14.0; p = 0.52), although there was a trend at 70% intensity (mean difference 8.2%, 95% CI –2.3 to 19.0; p = 0.051).
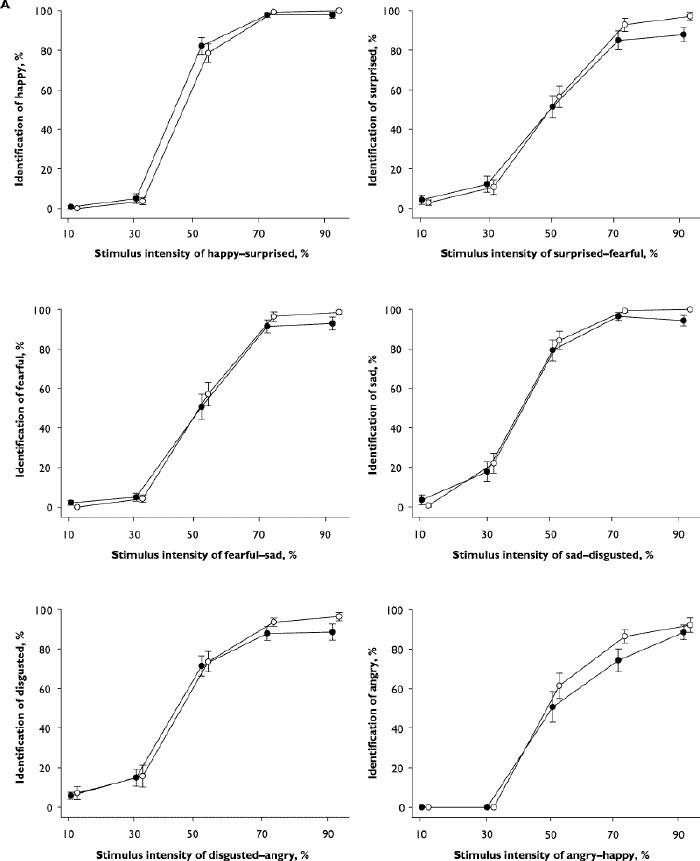
Fig. 2: Results for the emotional hexagon task of facial emotion recognition. Diazepam, 15 mg (black circles), placebo (white circles). A: Mean (and standard error) percentage identification of each basic emotion plotted against stimulus intensity for the 2 continua in which the emotion is present in the morphed stimuli.
Fig. 2 (continued): Results for the emotional hexagon task of facial emotion recognition. Diazepam, 15 mg (black circles), placebo (white circles). B: Mean (and standard error) response times (ms) for each stimulus in the 6 continua.
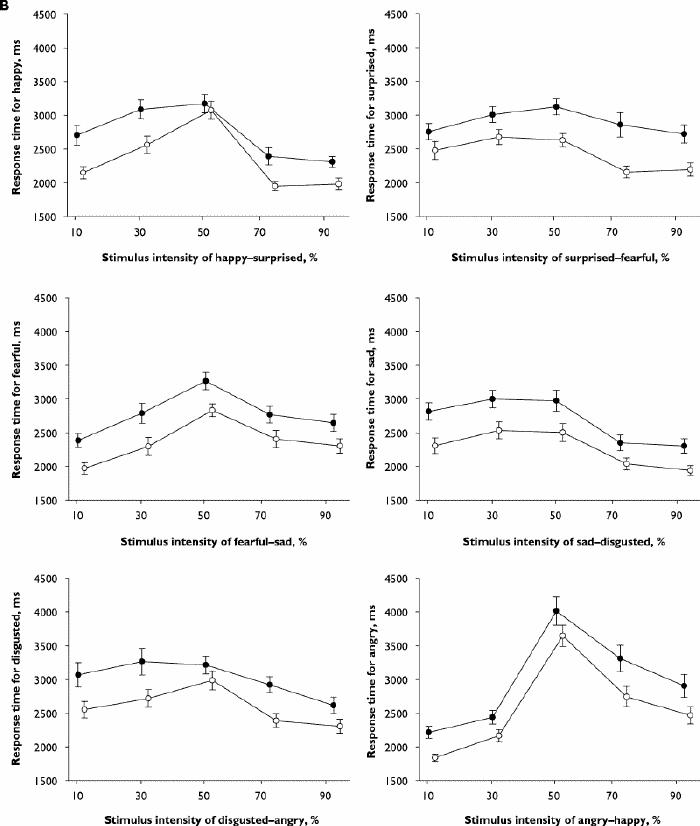
Fig. 2: Results for the emotional hexagon task of facial emotion recognition. Diazepam, 15 mg (black circles), placebo (white circles). A: Mean (and standard error) percentage identification of each basic emotion plotted against stimulus intensity for the 2 continua in which the emotion is present in the morphed stimuli.
Fig. 2 (continued): Results for the emotional hexagon task of facial emotion recognition. Diazepam, 15 mg (black circles), placebo (white circles). B: Mean (and standard error) response times (ms) for each stimulus in the 6 continua.
Table 3
The response-time data for the hexagon task showed stimulus, intensity and drug effects, plus a stimulus х intensity interaction (Table 3). Responses were slower for diazepam than placebo across all stimuli. For all stimuli and both drugs combined, the mean response times at different intensities were ordered as follows: 50% intensity (mean 3119 [95% CI 2969–3270] ms) > 70% (mean 2617 [95% CI 2460–2774] ms) > 90% (mean 2414 [95% CI 2265–2562] ms). Stimuli in the angry–happy continuum contributed disproportionately to the significant stimulus х intensity interaction. If this continuum was excluded from the analysis, the stimulus х intensity interaction was no longer significant (F = 2.1, df 16,12; p = 0.12), but the interaction remained highly significant when each of the other continua was excluded in turn (data not shown). Inspection of the data showed that the longest response times for any stimulus were for the angry 50%–happy 50% morph (Fig. 2B). The response times for correct identifications of stimuli showing 70% or 90% angry faces were also slower than for other stimuli of the same intensity, being significantly slower than for sad or happy expressions (Fig. 3).
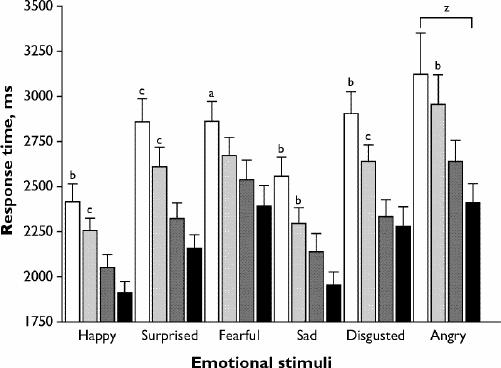
Fig. 3: Mean (and standard error) reaction times for correct responses to emotional hexagon stimuli at 2 different intensities (70% or 90%). Diazepam, 15 mg, 70% intensity (white bars); diazepam, 15 mg, 90% intensity (light-grey bars); placebo 70% intensity (dark-grey bars); placebo 90% intensity (black bars). Diazepam, 15 mg, > placebo: ap < 0.01, bp < 0.005, cp < 0.001. zAngry > happy = sad: p < 0.01.
Discussion
We investigated the impact of diazepam on the recognition of emotional expressions using the multimorph and hexagon paradigms and found that diazepam produced broad impairments in recognition accuracy, recognition thresholds and response speed. The processing of surprise (identification threshold in multimorph task) and of disgust (accuracy in hexagon task at 90%) were most affected by diazepam.
These findings apparently contrast with the results of 2 other investigations that used the hexagon task to test the effects of diazepam on recognition accuracy for emotional expressions, both of which showed that the recognition of angry expressions was significantly impaired. However, trend effects were found in the current sample for angry expressions (70% intensity, p = 0.051) and also for surprised (70% and 90% intensity, p = 0.031) and sad (90% intensity, p = 0.03) expressions. This broader than predicted impact of diazepam was not entirely inconsistent with the previous studies, which, in addition to the impairment of anger recognition, showed some effects of diazepam on fearful12 and, at a trend level, disgusted expressions.11 In the present study, the multimorph task also showed relatively broad effects of diazepam, with a significantly increased identification threshold for surprised expressions and trends in this direction for happy (p = 0.04), sad (p = 0.011) and disgusted expressions (p = 0.032), together with trends for impaired accuracy for surprised (p = 0.044) and sad (p = 0.034) expressions. Overall, the results show a more general impact of diazepam than on anger expressions, with the significant findings being limited to 2 expressions partly as a consequence of adjustment for multiple comparisons and type II error.
Blair and Curran11 argued that diazepam, 15 mg, was sufficient to disrupt a neurocognitive circuit responding to angry expressions that involves the orbital-frontal cortex. Their argument was based on a functional imaging study in which activation of the lateral orbital-frontal cortex correlated with the intensity of angry expressions viewed.22 However, it should be noted that although this region was activated by angry expressions, it has also been activated by other expressions such as disgust and fear.27,28 No functional imaging studies have investigated the neural response to surprised expressions. Moreover, neuropsychologic studies of neurologic patients with orbital-frontal cortex lesions or psychiatric patients with impulsive aggressive disorder,29 where orbital-frontal cortex pathology is suggested, report generalized expression recognition impairment and, where examined, find this impairment most notable for anger, disgust or surprise, or some combination thereof.30,31,32 This suggests the following amendment to the Blair and Curran11 claim: diazepam, 15 mg, may disrupt the functioning of the orbital-frontal cortex, and this can affect processing of the emotional expressions that rely on this circuitry. This is more general than the original claim, although it appears likely that some expressions may be disproportionately affected. In keeping with this, a recent neuroimaging study found that diazepam, 15 mg, attenuated the response of the orbital-frontal cortex to emotional expressions, particularly those of anger (Dr. R. James Blair: unpublished data, 2002).
Of course, despite the evidence for general effects of diazepam in this study, it remains unclear why the previous effects of diazepam on angry expressions in the hexagon task were not replicated. Several factors should be considered. First, the previous studies both used between-subjects designs and were not fully balanced in terms of subject sex, but it seems unlikely that individual or sex differences would have led to consistent impairments for facial anger in both samples.11,12 Second, pharmacokinetic differences could be relevant. All of the studies to date have used the same dose of diazepam and have started the tasks during the time range reported for peak diazepam plasma levels (30–90 min).33 However, the hexagon task was started at 75 minutes following diazepam administration in the present study, compared with 45 minutes in the other studies. It is, therefore, possible that diazepam had reached higher plasma levels by the time of the test, producing less selective effects on facial emotion recognition as a consequence of sedation. This could be clarified by doing a dose–response study and measuring plasma levels. Third, modification of the task parameters from the previous studies might have influenced the results. In the other studies, the hexagon stimuli were presented onscreen for 3000 ms, then when the stimulus ceased the subjects had a further 4000–6000 ms to respond by naming the emotion out loud to the researcher. In the current study, a 5000-ms stimulus duration was used and subjects had to respond within 7000 ms of stimulus onset using the mouse. In highly socially anxious volunteers, social threat due to being observed influences attention to emotional faces.34 However, the requirement to interact with an observer is unlikely to explain the previous results with diazepam, because social threat did not influence attention to different emotions selectively and our subjects were not selected for high social anxiety. Differences in the duration of stimulus might have contributed to the differences in findings, however, because in the reaction-time component of the present study we observed that reaction times were greater for the 70% or 90% angry faces than for other emotions (Fig. 2B, Fig. 3), reaching a mean of 3287 ms for the 70% anger stimuli. In order to explore the reaction times further, we plotted the proportion of subjects who had completed a response for each emotion (70% and 90% intensities combined) against a range of cut-offs for reaction time (Fig. 4). This plot suggests that although the increase in reaction times following diazepam was not stimulus or intensity dependent (Fig. 3), the proportion of subjects who completed a response within a specific time cut-off shows a differential effect of diazepam for angry expressions at timings between 3000 and 4000 ms. In the earlier studies, therefore, diazepam might have had a relatively selective effect on the angry face blends because of an interaction between the 3000-ms stimulus duration and processing speed. Most subjects had responded to all stimuli by 5000 ms in the current study, so that a similar interaction would not have occurred.
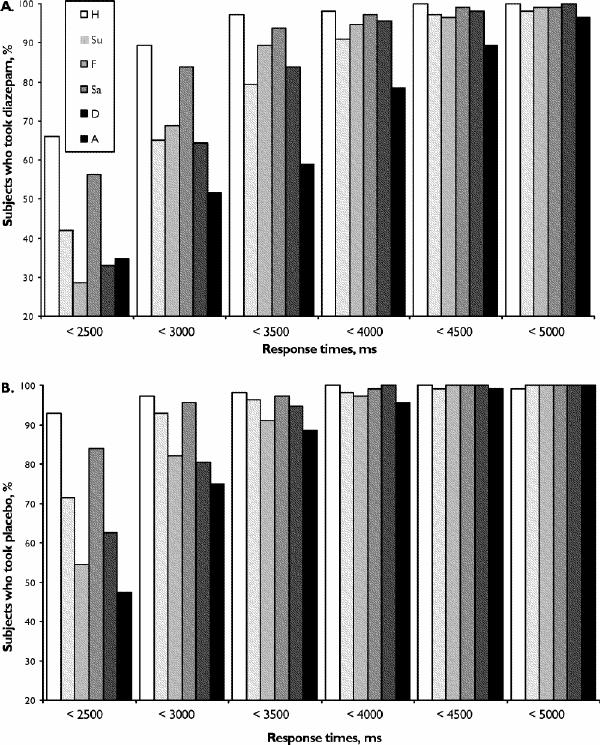
Fig. 4: Proportions of subjects who completed a response on the emotional hexagon tasks within the specified time interval at combined intensities of 70% and 90% (A) after diazepam, 15 mg, and (B) after placebo. H = happy; Su = surprised; F = fearful; Sa = sad; D = disgusted; A = angry.
Diazepam produced broader decreases on timed measures than on accuracy. On the multimorph identification threshold and hexagon task reaction-time measures, diazepam produced deficits even when accuracy was not decreased, such as for happy faces. However, a slowing of simple reaction times alone would not fully explain the results, because the overall mean 5% increase in identification threshold represents a 1000-ms delay, compared with an overall mean 420-ms increase in reaction times in the hexagon task. Diazepam, therefore, appears likely to have affected stimulus evaluation as well as simple reaction times. The present study design could not in itself distinguish whether the effects of diazepam were specific to facial emotion processing, as opposed to facial or visual processing more generally. However, diazepam at a similar dose (0.3 mg/kg) did not impair the recognition of ambiguous visual objects,18 and a functional neuroimaging study showed that diazepam exerted its effect in the lateral orbital-frontal cortex, a region that appears to be involved in processing affective qualities of faces rather than more basic stimulus properties.1,2 Diazepam could also impair visual attention, but it may be noted that very high levels of distraction were required to disrupt facial emotion processing at the neural level.35,36 In future studies, a facial identity matching task might be used to control for more general effects of diazepam on face processing,10 although a new study has shown important deficits in the 2 standard tests most commonly used for this purpose.37 Famous face identification has also been used as a control task, but this would be less suitable given the negative effects of benzodiazepines on explicit memory.19,20
Taken together, the findings from the studies of diazepam to date suggest that caution should be exercised before ascribing a decrease in performance on a facial recognition task to a selective deficit in an emotion-specific neural network. It is important to exclude the possibility that the decrease is task specific, rather than emotion specific, particularly because the hexagon task, which has been used most often to date, uses only the expressions of a single model. The present study found more general impairments of facial emotion recognition following diazepam, 15 mg, than suggested previously, and studies are indicated to determine whether the degree of selectivity for diazepam is dose related or concentration related. The incorporation of control tasks for simple reaction times and non-affective processing would clarify the interpretation of the results.
Acknowledgments
We gratefully acknowledge the assistance of Misha Hartfeil with data collection/entry and John McIntyre with programming for the hexagon task. We thank Dr. Andrew Calder, MRC Cognition and Brain Sciences Unit, Cambridge, United Kingdom, for providing the emotional hexagon images and Professor Paul Ekman, University of California, for permission to use the images derived from the Pictures of Facial Affect series. Support was received from the Alberta Heritage Foundation for Medical Research (N.C., R.S.), the University of Alberta Hospital Foundation (N.C.) and the University of Alberta Faculty of Medicine and Dentistry 75th anniversary award fund (A.S.).
Footnotes
Competing interests: None declared.
Correspondence to: Dr. Nick J. Coupland, 1E7.16 WMC, Psychopharmacology Research Unit, Department of Psychiatry, University of Alberta, 8440–112 St., Edmonton AB T6G 2B7; fax 780 407-6672; nc2@ualberta.ca
Submitted Aug. 28, 2002 Revised Feb. 26, 2003 Accepted Apr. 23, 2003
References
- 1.Adolphs R. Neural systems for recognizing emotion. Curr Opin Neurobiol 2002;12:169-77. [DOI] [PubMed]
- 2.Haxby JV, Hoffman EA, Gobbini MI. The distributed human neural system for face perception. Trends Cogn Sci 2000;4:223-33. [DOI] [PubMed]
- 3.Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage 2002;16:331-48. [DOI] [PubMed]
- 4.Blair R, Colledge E, Murray L, Mitchell D. A selective impairment in the processing of sad and fearful expressions in children with psychopathic tendencies. J Abnorm Child Psychol 2001;29:491-8. [DOI] [PubMed]
- 5.Sprengelmeyer R, Young AW, Calder AJ, Karnat A, Lange H, Homberg V, et al. Loss of disgust. Perception of faces and emotions in Huntington's disease. Brain 1996;119:1647-65. [DOI] [PubMed]
- 6.Pollak SD, Kistler DJ. Early experience is associated with the development of categorical representations for facial expressions of emotion. Proc Natl Acad Sci U S A 2002;99:9072-6. [DOI] [PMC free article] [PubMed]
- 7.Borrill JA, Rosen BK, Summerfield AB. The influence of alcohol on judgement of facial expression of emotion. Br J Med Psychol 1987;60:71-7. [PubMed]
- 8.Calder A, Young A, Perrett D, Etcoff N, Rowland D. Categorical perception of morphed facial expressions. Vis Cogn 1996; 3:81-117.
- 9.Harmer CJ, Perrett DI, Cowen PJ, Goodwin GM. Administration of the beta-adrenoceptor blocker propranolol impairs the processing of facial expressions of sadness. Psychopharmacol 2001;154:383-9. [DOI] [PubMed]
- 10.Lawrence AD, Calder AJ, McGowan SW, Grasby PM. Selective disruption of the recognition of facial expressions of anger. Neuroreport 2002;13:881-4. [DOI] [PubMed]
- 11.Blair RJ, Curran HV. Selective impairment in the recognition of anger induced by diazepam. Psychopharmacol 1999;147:335-8. [DOI] [PubMed]
- 12.Zangara A, Blair RJ, Curran HV. A comparison of the effects of a beta-adrenergic blocker and a benzodiazepine upon the recognition of human facial expressions. Psychopharmacol 2002;163:36-41. [DOI] [PubMed]
- 13.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 1998;59:22-33. [PubMed]
- 14.Lorr M, McNair DM, Fisher SU. Evidence for bipolar mood states. J Personality Assess 1982;46:432-6. [DOI] [PubMed]
- 15.Johanson CE, Uhlenhuth EH. Drug preference and mood in humans: diazepam. Psychopharmacol 1980;71:269-73. [DOI] [PubMed]
- 16.Spielberger C, Gorsuch RL, Lushene RE. Manual for the state-trait anxiety inventory. Palo Alto: Consulting Psychologist Press; 1970.
- 17.Monteiro MG, Schuckit MA, Irwin M. Subjective feelings of anxiety in young men after ethanol and diazepam infusions. J Clin Psychiatry 1990;51:12-6. [PubMed]
- 18.Wagemans J, Notebaert W, Boucart M. Lorazepam but not diazepam impairs identification of pictures on the basis of specific contour fragments. Psychopharmacol 1998;138:326-33. [DOI] [PubMed]
- 19.Danion JM, Zimmermann MA, Willard-Schroeder D, Grange D, Singer L. Diazepam induces a dissociation between explicit and implicit memory. Psychopharmacol 1989;99:238-43. [DOI] [PubMed]
- 20.Legrand F, Vidailhet P, Danion JM, Grange D, Giersch A, Van der Linden M, et al. Time course of the effects of diazepam and lorazepam on perceptual priming and explicit memory. Psychopharmacol 1995;118:475-9. [DOI] [PubMed]
- 21.Ekman P, Friesen W. Pictures of facial affect. Palo Alto: Consulting Psychologist Press; 1976.
- 22.Blair RJ, Morris JS, Frith CD, Perrett DI, Dolan RJ. Dissociable neural responses to facial expressions of sadness and anger. Brain 1999;122:883-93. [DOI] [PubMed]
- 23.Hess U, Blairy S, Kleck R. The intensity of emotional facial expressions and decoding accuracy. J Nonverbal Behav 1997;21:241-57.
- 24.Coupland NJ, Sustrik R, Ting P, Li D, Hartfeil M, Singh AJ, et al. Positive and negative affect differentially influence identification of facial emotions. Depress Anxiety. In press. [DOI] [PubMed]
- 25.Sprengelmeyer R, Young AW, Pundt I, Sprengelmeyer A, Calder AJ, Berrios G, et al. Disgust implicated in obsessive-compulsive disorder. Proc R Soc Lond B Biol Sci 1997;264:1767-73. [DOI] [PMC free article] [PubMed]
- 26.Calder AJ, Keane J, Manes F, Antoun N, Young AW. Impaired recognition and experience of disgust following brain injury. Nat Neurosci 2000;3:1077-8. [DOI] [PubMed]
- 27.Kesler-West ML, Andersen AH, Smith CD, Avison MJ, Davis CE, Kryscio RJ, et al. Neural substrates of facial emotion processing using fMRI. Brain Res Cogn Brain Res 2001;11:213-26. [DOI] [PubMed]
- 28.Sprengelmeyer R, Rausch M, Eysel UT, Przuntek H. Neural structures associated with recognition of facial expressions of basic emotions. Proc R Soc Lond B Biol Sci 1998;265:1927-31. [DOI] [PMC free article] [PubMed]
- 29.Coccaro EF. Impulsive aggression: a behavior in search of clinical definition. Harv Rev Psychiatry 1998;5:336-9. [DOI] [PubMed]
- 30.Best M, Williams JM, Coccaro EF. Evidence for a dysfunctional prefrontal circuit in patients with an impulsive aggressive disorder. Proc Natl Acad Sci U S A 2002;99:8448-53. [DOI] [PMC free article] [PubMed]
- 31.Blair RJ, Cipolotti L. Impaired social response reversal. A case of “acquired sociopathy.” Brain 2000;123:1122-41. [DOI] [PubMed]
- 32.Hornak J, Rolls ET, Wade D. Face and voice expression identification in patients with emotional and behavioural changes following ventral frontal lobe damage. Neuropsychologia 1996;34:247-61. [DOI] [PubMed]
- 33.Mandelli M, Tognoni G, Garattini S. Clinical pharmacokinetics of diazepam. Clin Pharmacokinet 1978;3:72-91. [DOI] [PubMed]
- 34.Mansell W, Clark DM, Ehlers A, Chen YP. Social anxiety and attention away from emotional faces. Cogn Emotion 1999;13:673-90.
- 35.Vuilleumier P, Armony JL, Driver J, Dolan RJ. Effects of attention and emotion on face processing in the human brain: an event-related fMRI study. Neuron 2001;30:829-41. [DOI] [PubMed]
- 36.Pessoa L, McKenna M, Gutierrez E, Ungerleider LG. Neural processing of emotional faces requires attention. Proc Natl Acad Sci U S A 2002;99:11458-63. [DOI] [PMC free article] [PubMed]
- 37.Duchaine BC, Weidenfeld A. An evaluation of two commonly used tests of unfamiliar face recognition. Neuropsychologia 2003;41:713-20. [DOI] [PubMed]


