Abstract
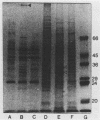
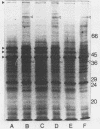
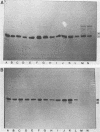
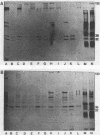
The cell envelope protein profiles of Staphylococcus epidermidis cultured in used human peritoneal dialysate (HPD) differed markedly from those of cells cultured in nutrient broth. Compared with broth-grown cells, many cell wall proteins were repressed in HPD, although three proteins of 42, 48, and 54 kDa predominated and an iron-repressible 130-kDa protein was induced. Growth in HPD also resulted in expression of two cell membrane proteins of 32 and 36 kDa which were iron repressible. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblot analysis using monospecific polyclonal antisera raised against the 32- and 36-kDa proteins revealed considerable antigenic and molecular mass homology among 12 S. epidermidis isolates from patients with continuous ambulatory peritoneal dialysis-related peritonitis. The 32-kDa antiserum also cross-reacted with a 32-kDa S. aureus cell membrane protein. Immunoblots of S. epidermidis cell walls and membranes were also probed with normal human serum and serum and HPD from continuous ambulatory peritoneal dialysis patients. While the cell wall proteins of S. epidermidis appeared to be relatively poorly immunogenic, the 32- and 36-kDa membrane proteins reacted strongly with antibodies present in each of the body fluids evaluated. These results suggest that the highly conserved 32- and 36-kDa iron-repressible proteins are expressed during growth in vivo and may be involved in iron transport, since all 12 S. epidermidis strains examined also produced iron chelators.
Full text
PDF


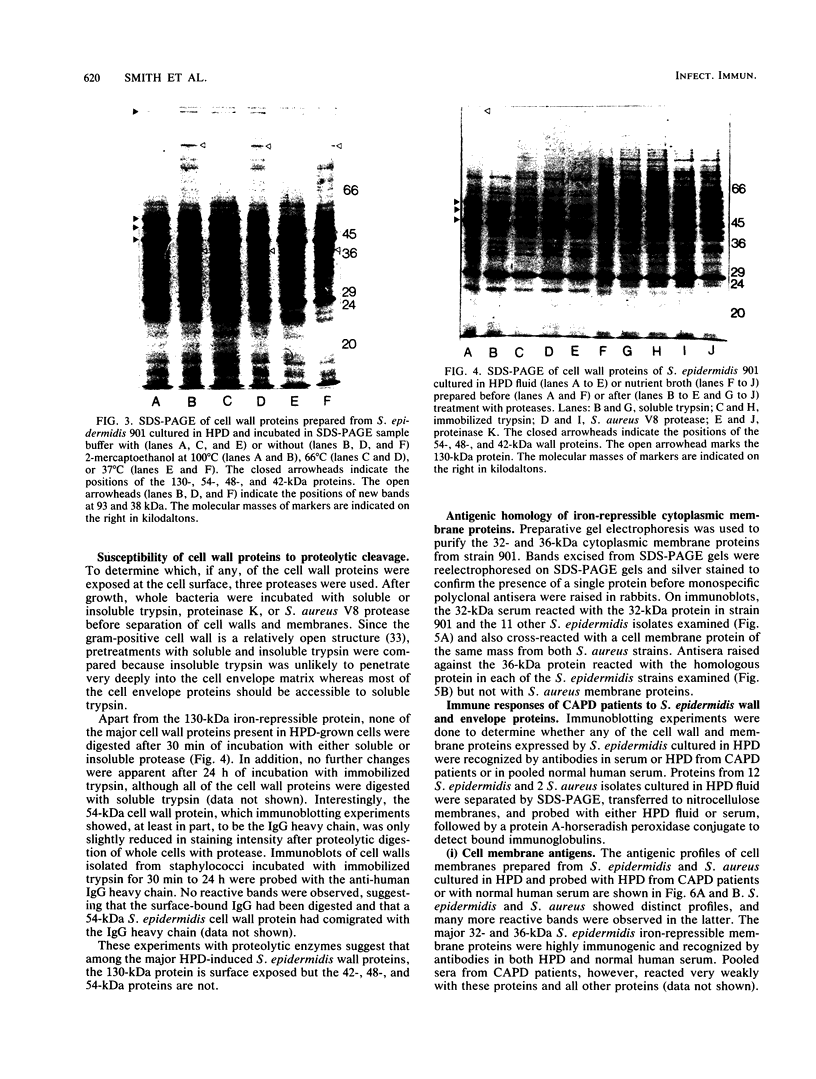


Images in this article
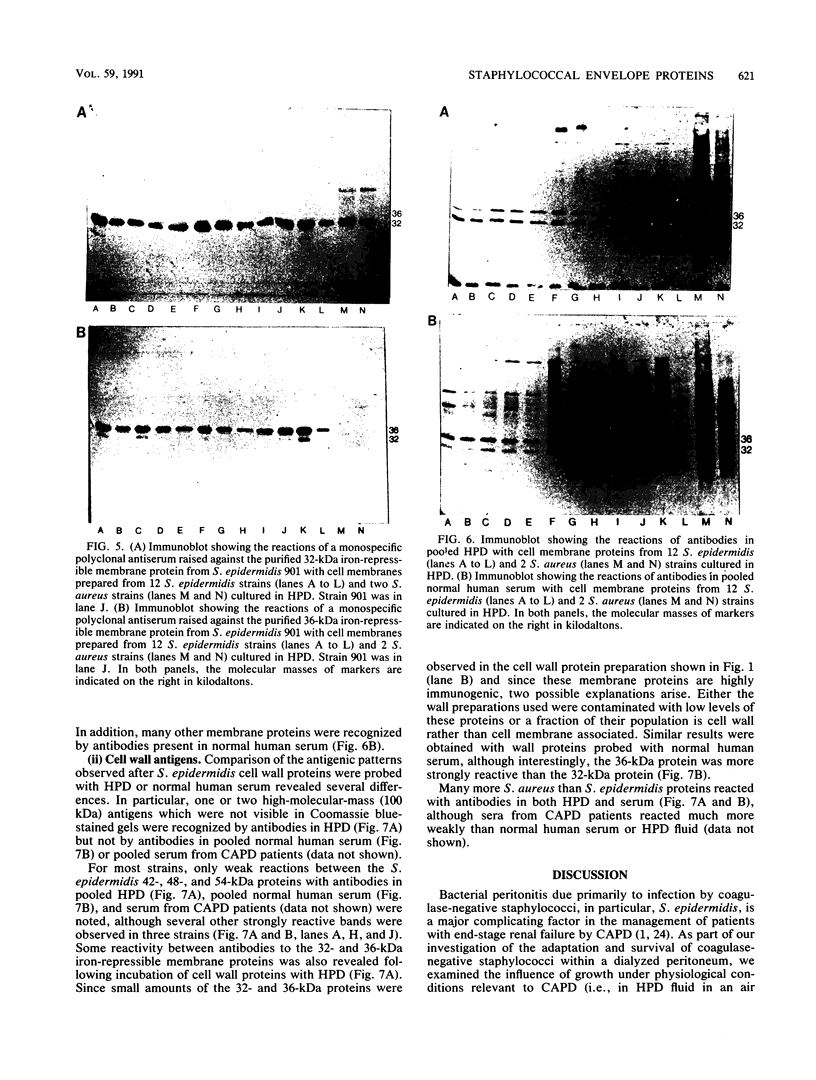
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown M. R., Williams P. The influence of environment on envelope properties affecting survival of bacteria in infections. Annu Rev Microbiol. 1985;39:527–556. doi: 10.1146/annurev.mi.39.100185.002523. [DOI] [PubMed] [Google Scholar]
- Cheung A. L., Fischetti V. A. Role of surface proteins in staphylococcal adherence to fibers in vitro. J Clin Invest. 1989 Jun;83(6):2041–2049. doi: 10.1172/JCI114115. [DOI] [PMC free article] [PubMed] [Google Scholar]
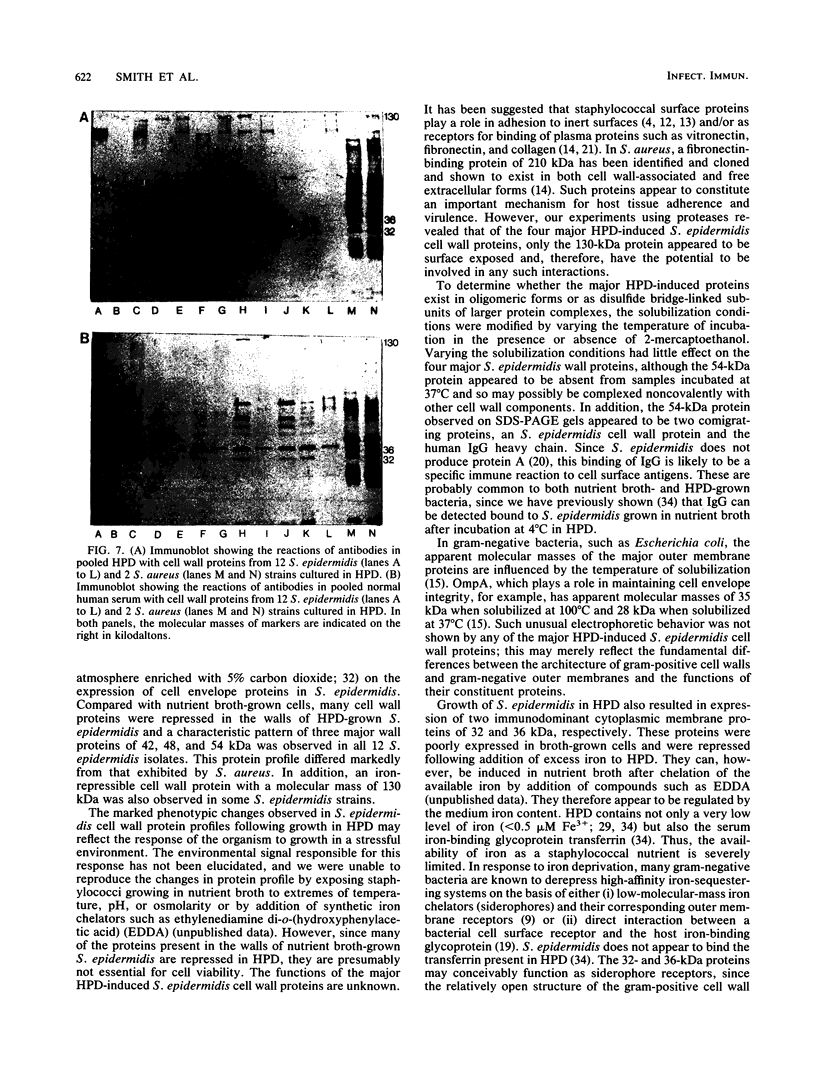
- Cheung A. L., Fischetti V. A. Variation in the expression of cell wall proteins of Staphylococcus aureus grown on solid and liquid media. Infect Immun. 1988 May;56(5):1061–1065. doi: 10.1128/iai.56.5.1061-1065.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clink J., Pennington T. H. Staphylococcal whole-cell polypeptide analysis: evaluation as a taxonomic and typing tool. J Med Microbiol. 1987 Feb;23(1):41–44. doi: 10.1099/00222615-23-1-41. [DOI] [PubMed] [Google Scholar]
- Coagulase-negative staphylococci. J Med Microbiol. 1986 Dec;22(4):285–295. doi: 10.1099/00222615-22-4-285. [DOI] [PubMed] [Google Scholar]
- Fry R. J., Sinclair W. K. New dosimetry of atomic bomb radiations. Lancet. 1987 Oct 10;2(8563):845–848. doi: 10.1016/s0140-6736(87)91027-0. [DOI] [PubMed] [Google Scholar]
- Griffiths E., Stevenson P., Thorpe R., Chart H. Naturally occurring antibodies in human sera that react with the iron-regulated outer membrane proteins of Escherichia coli. Infect Immun. 1985 Mar;47(3):808–813. doi: 10.1128/iai.47.3.808-813.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey D. M., Sheppard K. J., Morgan A. G., Fletcher J. Effect of dialysate fluids on phagocytosis and killing by normal neutrophils. J Clin Microbiol. 1987 Aug;25(8):1424–1427. doi: 10.1128/jcm.25.8.1424-1427.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogt A. H., Dankert J., Hulstaert C. E., Feijen J. Cell surface characteristics of coagulase-negative staphylococci and their adherence to fluorinated poly(ethylenepropylene). Infect Immun. 1986 Jan;51(1):294–301. doi: 10.1128/iai.51.1.294-301.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogt A. H., Dankert J., de Vries J. A., Feijen J. Adhesion of coagulase-negative staphylococci to biomaterials. J Gen Microbiol. 1983 Sep;129(9):2959–2968. doi: 10.1099/00221287-129-9-2959. [DOI] [PubMed] [Google Scholar]
- Lugtenberg B., Van Alphen L. Molecular architecture and functioning of the outer membrane of Escherichia coli and other gram-negative bacteria. Biochim Biophys Acta. 1983 Mar 21;737(1):51–115. doi: 10.1016/0304-4157(83)90014-x. [DOI] [PubMed] [Google Scholar]
- McGregor S. J., Brock J. H., Briggs J. D., Junor B. J. Longitudinal study of peritoneal defence mechanisms in patients on continuous ambulatory peritoneal dialysis (CAPD). Perit Dial Int. 1989;9(2):115–119. [PubMed] [Google Scholar]
- McGregor S. J., Brock J. H., Briggs J. D., Junor B. J. Properties of human peritoneal macrophages from continuous ambulatory peritoneal dialysis (CAPD) patients. FEMS Microbiol Immunol. 1989 Apr;1(5):303–304. doi: 10.1111/j.1574-6968.1989.tb02400.x. [DOI] [PubMed] [Google Scholar]
- Meiwes J., Fiedler H. P., Haag H., Zähner H., Konetschny-Rapp S., Jung G. Isolation and characterization of staphyloferrin A, a compound with siderophore activity from Staphylococcus hyicus DSM 20459. FEMS Microbiol Lett. 1990 Jan 15;55(1-2):201–205. doi: 10.1111/j.1574-6968.1990.tb13863.x. [DOI] [PubMed] [Google Scholar]
- Morton D. J., Williams P. Siderophore-independent acquisition of transferrin-bound iron by Haemophilus influenzae type b. J Gen Microbiol. 1990 May;136(5):927–933. doi: 10.1099/00221287-136-5-927. [DOI] [PubMed] [Google Scholar]
- Paulsson M., Wadström T. Vitronectin and type-I collagen binding by Staphylococcus aureus and coagulase-negative staphylococci. FEMS Microbiol Immunol. 1990 May;2(1):55–62. doi: 10.1111/j.1574-6968.1990.tb03479.x. [DOI] [PubMed] [Google Scholar]
- Smith H. Pathogenicity and the microbe in vivo. The 1989 Fred Griffith Review Lecture. J Gen Microbiol. 1990 Mar;136(3):377–393. doi: 10.1099/00221287-136-3-377. [DOI] [PubMed] [Google Scholar]
- Spencer R. C. Infections in continuous ambulatory peritoneal dialysis. J Med Microbiol. 1988 Sep;27(1):1–9. doi: 10.1099/00222615-27-1-1. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbrugh H. A., Keane W. F., Conroy W. E., Peterson P. K. Bacterial growth and killing in chronic ambulatory peritoneal dialysis fluids. J Clin Microbiol. 1984 Aug;20(2):199–203. doi: 10.1128/jcm.20.2.199-203.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbrugh H. A., Keane W. F., Hoidal J. R., Freiberg M. R., Elliott G. R., Peterson P. K. Peritoneal macrophages and opsonins: antibacterial defense in patients undergoing chronic peritoneal dialysis. J Infect Dis. 1983 Jun;147(6):1018–1029. doi: 10.1093/infdis/147.6.1018. [DOI] [PubMed] [Google Scholar]
- Ward K. H., Anwar H., Brown R. W., Wale J., Gowar J. Antibody response to outer-membrane antigens of Pseudomonas aeruginosa in human burn wound infection. J Med Microbiol. 1988 Nov;27(3):179–190. doi: 10.1099/00222615-27-3-179. [DOI] [PubMed] [Google Scholar]
- Wilcox M. H., Edwards R., Finch R. G. Laboratory studies on coagulase-negative staphylococci from CAPD-associated peritonitis. J Antimicrob Chemother. 1985 Mar;15(3):297–303. doi: 10.1093/jac/15.3.297. [DOI] [PubMed] [Google Scholar]
- Wilcox M. H., Smith D. G., Evans J. A., Denyer S. P., Finch R. G., Williams P. Influence of carbon dioxide on growth and antibiotic susceptibility of coagulase-negative staphylococci cultured in human peritoneal dialysate. J Clin Microbiol. 1990 Oct;28(10):2183–2186. doi: 10.1128/jcm.28.10.2183-2186.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox M. H., Smith D. G., Evans J. A., Denyer S. P., Finch R. G., Williams P. Influence of carbon dioxide on growth and antibiotic susceptibility of coagulase-negative staphylococci cultured in human peritoneal dialysate. J Clin Microbiol. 1990 Oct;28(10):2183–2186. doi: 10.1128/jcm.28.10.2183-2186.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams P. Role of the cell envelope in bacterial adaptation to growth in vivo in infections. Biochimie. 1988 Aug;70(8):987–1011. doi: 10.1016/0300-9084(88)90263-5. [DOI] [PubMed] [Google Scholar]