Abstract
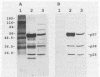
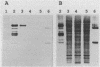
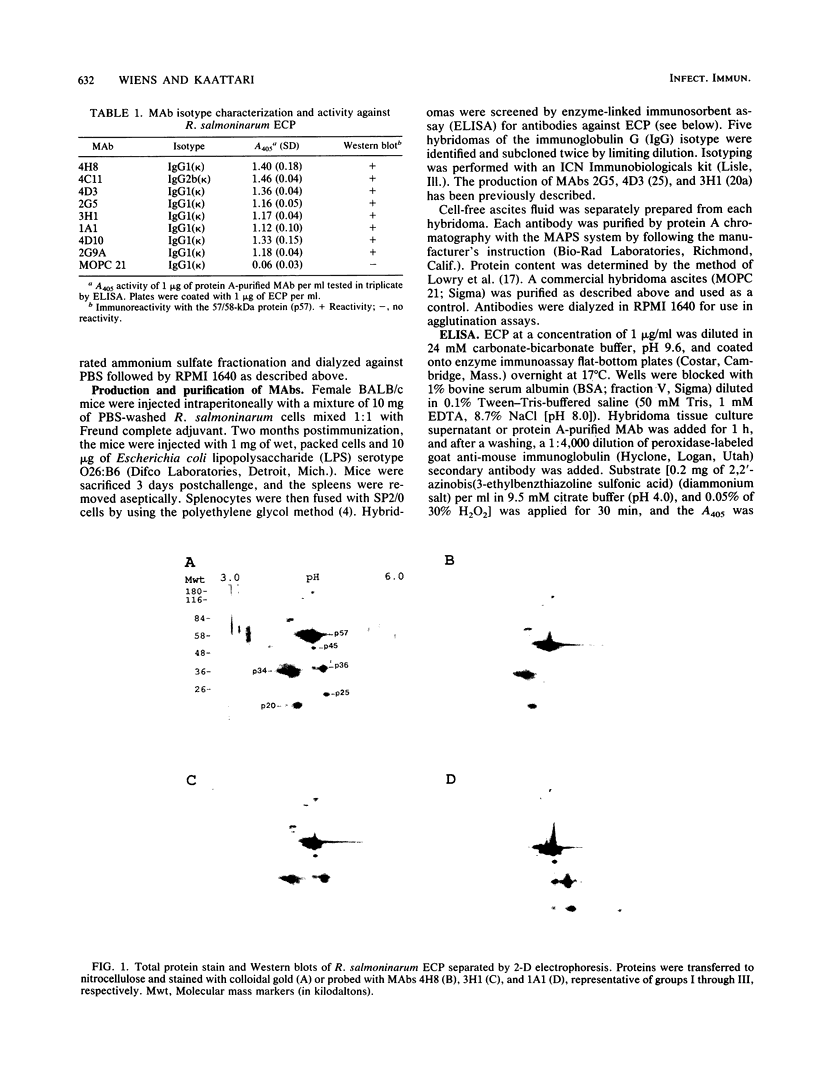
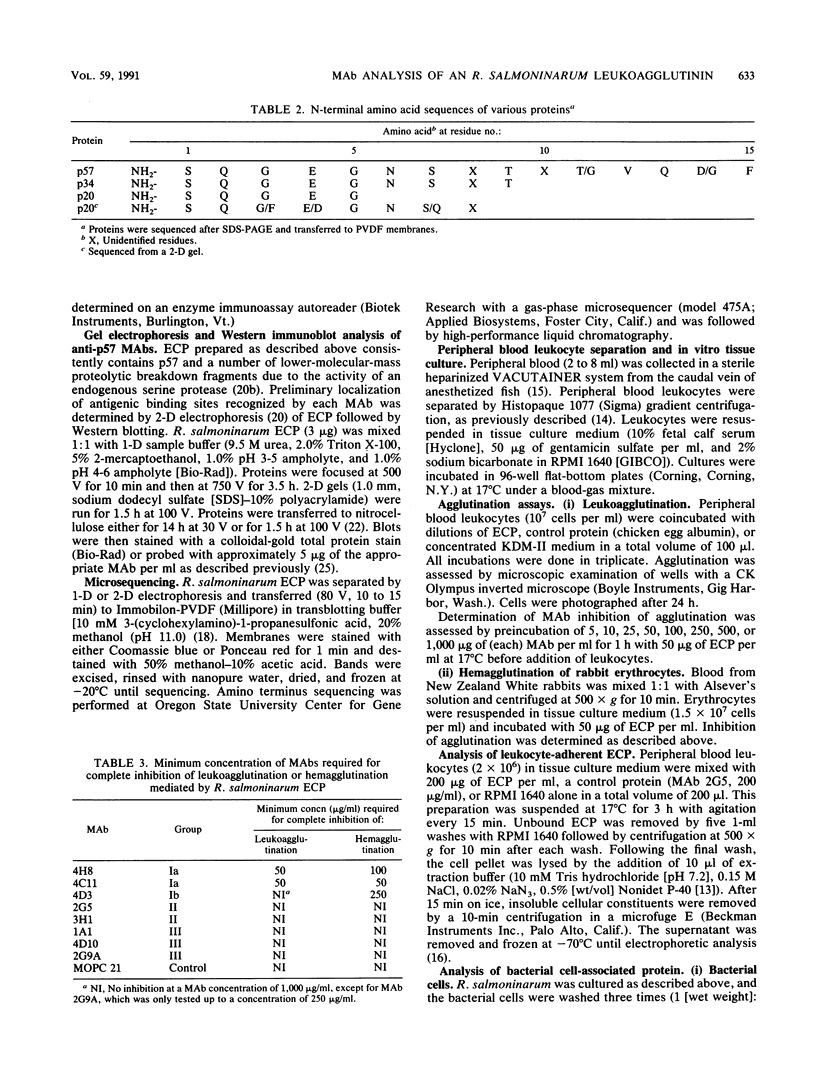
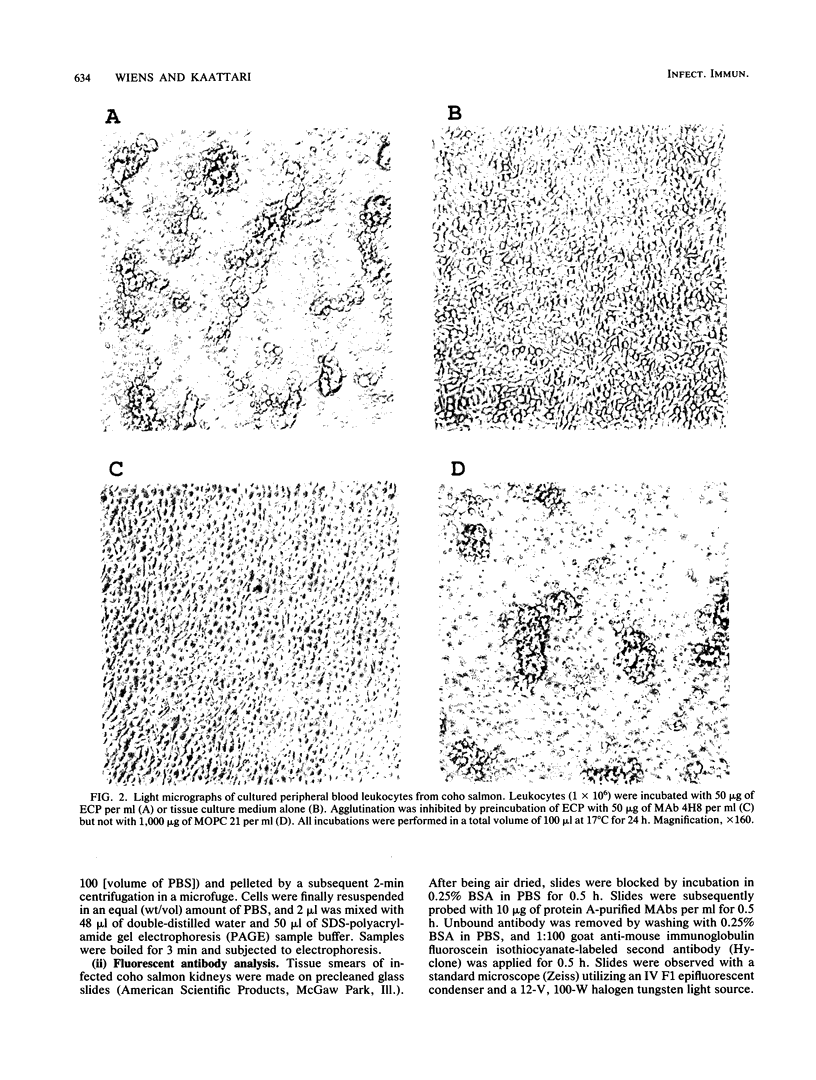
Renibacterium salmoninarum causes a chronic disease of salmonid fish known as bacterial kidney disease. High concentrations of bacterially produced extracellular protein (ECP) are present in plasma, kidney, and spleen tissue of naturally and experimentally infected fish. ECP agglutinated salmonid leukocytes in vitro at concentrations which correspond to levels found in highly infected fish. Association of biological activity with the structure of the major protein constituent of ECP, p57, was accomplished by monoclonal antibody (MAb) analysis. Location of the antigenic binding sites recognized by the MAbs was determined by two-dimensional electrophoresis and Western immunoblotting of the proteolytic breakdown fragments of p57. Eight MAbs have been classified into three groups on the basis of their differential recognition of these proteolytic breakdown products. Group I MAbs bound a region proximal to the amino terminus of the protein. Two of these MAbs were also able to block leukoagglutinating activity. Group III MAbs bound to a region associated with the bacterial cell surface, while group II MAbs bound a region between group I and group III. These analyses have allowed the identification of potential structural and functional regions of p57.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Daly J. G., Stevenson R. M. Characterization of the Renibacterium salmoninarum haemagglutinin. J Gen Microbiol. 1990 May;136(5):949–953. doi: 10.1099/00221287-136-5-949. [DOI] [PubMed] [Google Scholar]
- Fryer J. L., Sanders J. E. Bacterial kidney disease of salmonid fish. Annu Rev Microbiol. 1981;35:273–298. doi: 10.1146/annurev.mi.35.100181.001421. [DOI] [PubMed] [Google Scholar]
- Jones G. W., Isaacson R. E. Proteinaceous bacterial adhesins and their receptors. Crit Rev Microbiol. 1983;10(3):229–260. doi: 10.3109/10408418209113564. [DOI] [PubMed] [Google Scholar]
- Kaattari S. L., Irwin M. J. Salmonid spleen and anterior kidney harbor populations of lymphocytes with different B cell repertoires. Dev Comp Immunol. 1985 Summer;9(3):433–444. doi: 10.1016/0145-305x(85)90006-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]