Abstract
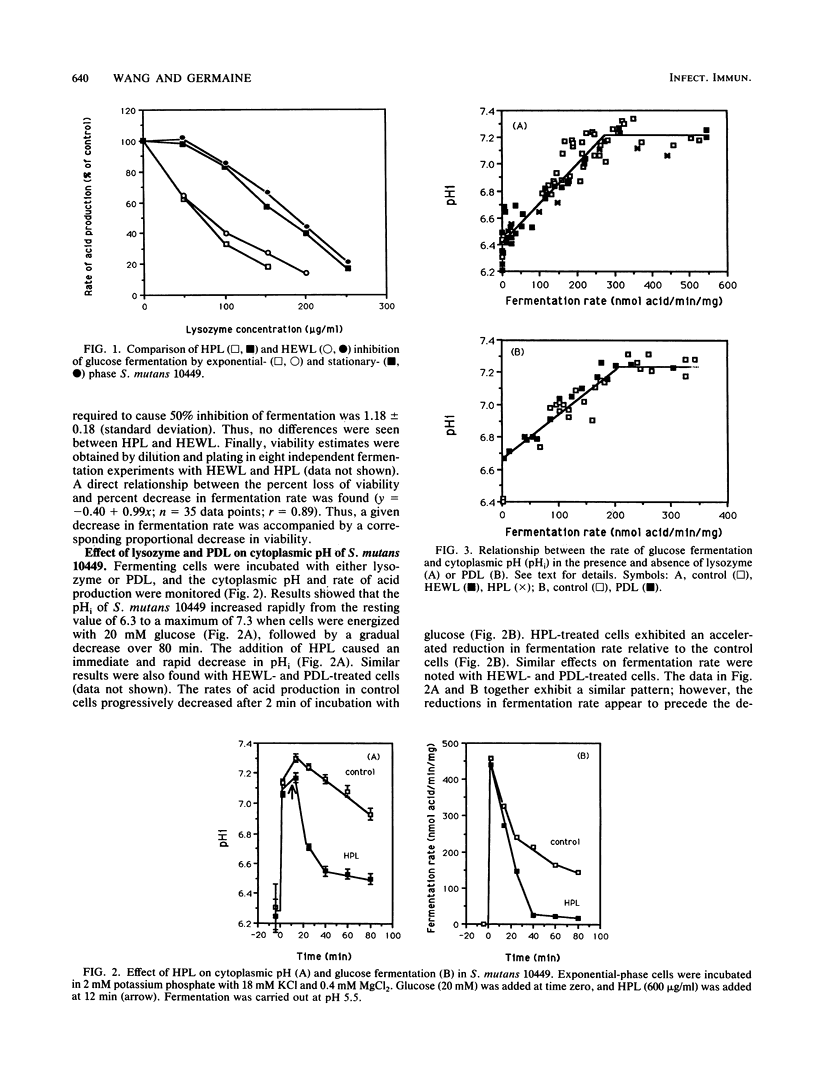
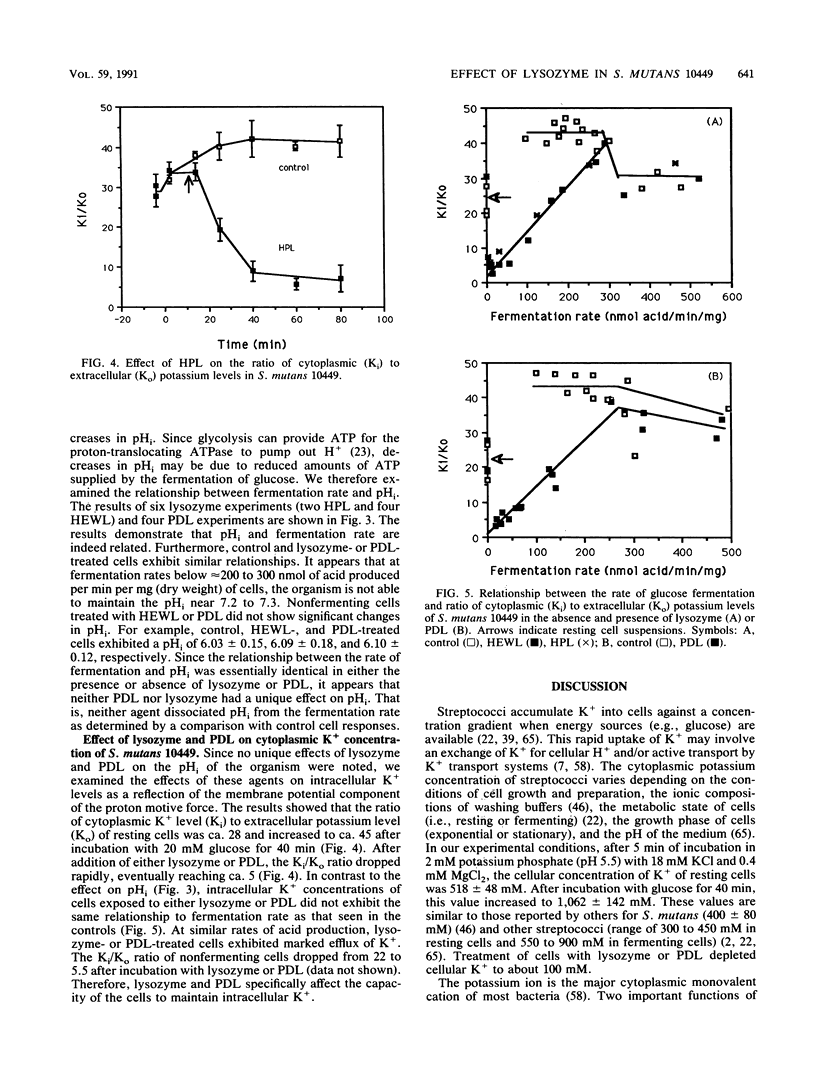
Several previous findings have suggested that the cationic nature of lysozyme is a major factor in its bactericidal activity. Since a number of cationic proteins or peptides have been reported to cause membrane damage in bacteria, we investigated the effect of lysozyme on glucose fermentation and intracellular pH and K+ in Streptococcus mutans under conditions in which lysis does not occur. Results showed that lysozyme and poly-D-lysine (PDL) cause inhibition of glucose fermentation at pH 5.5 in a dose-dependent manner. Human placental lysozyme and hen egg-white lysozyme exhibited similar inhibitory potency on glucose fermentation. Both lysozyme and PDL caused a marked acidification of the cytoplasm of S. mutans. However, when cytoplasmic pH was examined as a function of fermentation rate, the relationship was similar regardless of the presence or absence of lysozyme or PDL. Therefore, acidification of the cytoplasm appeared to not depend specifically on lysozyme or PDL. In contrast, the same relationship between the profound loss of intracellular K+, when fermenting cells were exposed to either lysozyme or PDL, and the fermentation rate was not exhibited in the controls. These results indicate that lysozyme and PDL specifically affected the ability of the cells to maintain intracellular K+. We concluded that lysozyme and PDL indeed perturb membrane function, perhaps in a selective manner. Furthermore, the similarity in action of lysozyme and the cationic homopolypeptide PDL supports the notion that the cationic property of lysozyme indeed plays a significant role in its antibacterial activity.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bakker E. P., Harold F. M. Energy coupling to potassium transport in Streptococcus faecalis. Interplay of ATP and the protonmotive force. J Biol Chem. 1980 Jan 25;255(2):433–440. [PubMed] [Google Scholar]
- Chipman D. M., Sharon N. Mechanism of lysozyme action. Science. 1969 Aug 1;165(3892):454–465. doi: 10.1126/science.165.3892.454. [DOI] [PubMed] [Google Scholar]
- Cole M. F., Hsu S. D., Baum B. J., Bowen W. H., Sierra L. I., Aquirre M., Gillespie G. Specific and nonspecific immune factors in dental plaque fluid and saliva from young and old populations. Infect Immun. 1981 Mar;31(3):998–1002. doi: 10.1128/iai.31.3.998-1002.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer W. A., Dankert J. R., Uratani Y. The membrane channel-forming bacteriocidal protein, colicin El. Biochim Biophys Acta. 1983 Mar 21;737(1):173–193. doi: 10.1016/0304-4157(83)90016-3. [DOI] [PubMed] [Google Scholar]
- Germaine G. R., Tellefson L. M. Potential role of lysozyme in bactericidal activity of in vitro-acquired salivary pellicle against Streptococcus faecium 9790. Infect Immun. 1986 Dec;54(3):846–854. doi: 10.1128/iai.54.3.846-854.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germaine G. R., Tellefson L. M. Simple and rapid procedure for the selective removal of lysozyme from human saliva. Infect Immun. 1979 Dec;26(3):991–995. doi: 10.1128/iai.26.3.991-995.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsburg I., Lahav M., Giesbrecht P. Effect of leukocyte hydrolases on bacteria XVI. Activation by leukocyte factors and cationic substances of autolytic enzymes in Staphylococcus aureus: modulation by anionic polyelectrolytes in relation to survival of bacteria in inflammatory exudates. Inflammation. 1982 Sep;6(3):269–284. doi: 10.1007/BF00916408. [DOI] [PubMed] [Google Scholar]
- Ginsburg I. The role of lysosomal factors of leukocytes in the biodegradation and storage of microbial constituents in infectious granulomas. Front Biol. 1979;48:327–406. [PubMed] [Google Scholar]
- Hamada S., Slade H. D. Biology, immunology, and cariogenicity of Streptococcus mutans. Microbiol Rev. 1980 Jun;44(2):331–384. doi: 10.1128/mr.44.2.331-384.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harold F. M. Conservation and transformation of energy by bacterial membranes. Bacteriol Rev. 1972 Jun;36(2):172–230. doi: 10.1128/br.36.2.172-230.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacono V. J., Byrnes T. P., Crawford I. T., Grossbard B. L., Pollock J. J., MacKay B. J. Lysozyme-mediated de-chaining of Streptococcus mutans and its antibacterial significance in an acidic environment. J Dent Res. 1985 Jan;64(1):48–53. doi: 10.1177/00220345850640010901. [DOI] [PubMed] [Google Scholar]
- Iacono V. J., Zove S. M., Grossbard B. L., Pollock J. J., Fine D. H., Greene L. S. Lysozyme-mediated aggregation and lysis of the periodontal microorganism Capnocytophaga gingivalis 2010. Infect Immun. 1985 Feb;47(2):457–464. doi: 10.1128/iai.47.2.457-464.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto Y., Nakamura R., Watanabe T., Tsunemitsu A. Purification and amino acid analysis of human parotid saliva lysozyme. J Dent Res. 1970 Sep-Oct;49(5):1104–1110. doi: 10.1177/00220345700490051801. [DOI] [PubMed] [Google Scholar]
- Kagan B. L., Selsted M. E., Ganz T., Lehrer R. I. Antimicrobial defensin peptides form voltage-dependent ion-permeable channels in planar lipid bilayer membranes. Proc Natl Acad Sci U S A. 1990 Jan;87(1):210–214. doi: 10.1073/pnas.87.1.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashket E. R. Proton motive force in growing Streptococcus lactis and Staphylococcus aureus cells under aerobic and anaerobic conditions. J Bacteriol. 1981 Apr;146(1):369–376. doi: 10.1128/jb.146.1.369-376.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashket E. R. Stoichiometry of the H+-ATPase of growing and resting, aerobic Escherichia coli. Biochemistry. 1982 Oct 26;21(22):5534–5538. doi: 10.1021/bi00265a024. [DOI] [PubMed] [Google Scholar]
- Kashket E. R. The proton motive force in bacteria: a critical assessment of methods. Annu Rev Microbiol. 1985;39:219–242. doi: 10.1146/annurev.mi.39.100185.001251. [DOI] [PubMed] [Google Scholar]
- Kobayashi H. A proton-translocating ATPase regulates pH of the bacterial cytoplasm. J Biol Chem. 1985 Jan 10;260(1):72–76. [PubMed] [Google Scholar]
- Kobayashi H. Second system for potassium transport in Streptococcus faecalis. J Bacteriol. 1982 May;150(2):506–511. doi: 10.1128/jb.150.2.506-511.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konisky J. Colicins and other bacteriocins with established modes of action. Annu Rev Microbiol. 1982;36:125–144. doi: 10.1146/annurev.mi.36.100182.001013. [DOI] [PubMed] [Google Scholar]
- Kordel M., Benz R., Sahl H. G. Mode of action of the staphylococcinlike peptide Pep 5: voltage-dependent depolarization of bacterial and artificial membranes. J Bacteriol. 1988 Jan;170(1):84–88. doi: 10.1128/jb.170.1.84-88.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laible N. J., Germaine G. R. Adsorption of lysozyme from human whole saliva by Streptococcus sanguis 903 and other oral microorganisms. Infect Immun. 1982 Apr;36(1):148–159. doi: 10.1128/iai.36.1.148-159.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laible N. J., Germaine G. R. Bactericidal activity of human lysozyme, muramidase-inactive lysozyme, and cationic polypeptides against Streptococcus sanguis and Streptococcus faecalis: inhibition by chitin oligosaccharides. Infect Immun. 1985 Jun;48(3):720–728. doi: 10.1128/iai.48.3.720-728.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loesche W. J. Role of Streptococcus mutans in human dental decay. Microbiol Rev. 1986 Dec;50(4):353–380. doi: 10.1128/mr.50.4.353-380.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloney P. C. Relationship between phosphorylation potential and electrochemical H+ gradient during glycolysis in Streptococcus lactis. J Bacteriol. 1983 Mar;153(3):1461–1470. doi: 10.1128/jb.153.3.1461-1470.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf R. H., Deibel R. H. Differential lytic response of enterococci associated with addition order of lysozyme and anions. J Bacteriol. 1969 Sep;99(3):674–680. doi: 10.1128/jb.99.3.674-680.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odeberg H., Olsson I. Mechanisms for the microbicidal activity of cationic proteins of human granulocytes. Infect Immun. 1976 Dec;14(6):1269–1275. doi: 10.1128/iai.14.6.1269-1275.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PETIT J. F., JOLLES P. PURIFICATION AND ANALYSIS OF HUMAN SALIVA LYSOZYME. Nature. 1963 Oct 12;200:168–169. doi: 10.1038/200168a0. [DOI] [PubMed] [Google Scholar]
- Patterson-Delafield J., Martinez R. J., Lehrer R. I. Microbicidal cationic proteins in rabbit alveolar macrophages: a potential host defense mechanism. Infect Immun. 1980 Oct;30(1):180–192. doi: 10.1128/iai.30.1.180-192.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoads D. B., Epstein W. Energy coupling to net K+ transport in Escherichia coli K-12. J Biol Chem. 1977 Feb 25;252(4):1394–1401. [PubMed] [Google Scholar]
- Rottenberg H. The measurement of membrane potential and deltapH in cells, organelles, and vesicles. Methods Enzymol. 1979;55:547–569. doi: 10.1016/0076-6879(79)55066-6. [DOI] [PubMed] [Google Scholar]
- Ruhr E., Sahl H. G. Mode of action of the peptide antibiotic nisin and influence on the membrane potential of whole cells and on cytoplasmic and artificial membrane vesicles. Antimicrob Agents Chemother. 1985 May;27(5):841–845. doi: 10.1128/aac.27.5.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahl H. G. Bactericidal cationic peptides involved in bacterial antagonism and host defence. Microbiol Sci. 1985 Jul;2(7):212–217. [PubMed] [Google Scholar]
- Sahl H. G., Brandis H. Mode of action of the staphylococcin-like peptide Pep 5 and culture conditions effecting its activity. Zentralbl Bakteriol Mikrobiol Hyg A. 1982 Jun;252(2):166–175. [PubMed] [Google Scholar]
- Sahl H. G. Influence of the staphylococcinlike peptide Pep 5 on membrane potential of bacterial cells and cytoplasmic membrane vesicles. J Bacteriol. 1985 May;162(2):833–836. doi: 10.1128/jb.162.2.833-836.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y., Noji S., Suzuki R., Taniguchi S. Dual mechanism for stimulation of glutamate transport by potassium ions in Streptococcus mutans. J Bacteriol. 1989 Sep;171(9):4963–4966. doi: 10.1128/jb.171.9.4963-4966.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schein S. J., Kagan B. L., Finkelstein A. Colicin K acts by forming voltage-dependent channels in phospholipid bilayer membranes. Nature. 1978 Nov 9;276(5684):159–163. doi: 10.1038/276159a0. [DOI] [PubMed] [Google Scholar]
- Spitznagel J. K. Nonoxidative antimicrobial reactions of leukocytes. Contemp Top Immunobiol. 1984;14:283–343. doi: 10.1007/978-1-4757-4862-8_10. [DOI] [PubMed] [Google Scholar]
- Stuchell R. N., Mandel I. D. A comparative study of salivary lysozyme in caries-resistant and caries-susceptible adults. J Dent Res. 1983 May;62(5):552–554. doi: 10.1177/00220345830620050701. [DOI] [PubMed] [Google Scholar]
- Tatevossian A., Gould C. T. The composition of the aqueous phase in human dental plaque. Arch Oral Biol. 1976;21(5):319–323. doi: 10.1016/0003-9969(76)90055-8. [DOI] [PubMed] [Google Scholar]
- Tobgi R. S., Samaranayake L. P., MacFarlane T. W. In vitro susceptibility of Candida species to lysozyme. Oral Microbiol Immunol. 1988 Mar;3(1):35–39. doi: 10.1111/j.1399-302x.1988.tb00603.x. [DOI] [PubMed] [Google Scholar]
- Tokuda H., Konisky J. Effect of colicins Ia and E1 on ion permeability of liposomes. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6167–6171. doi: 10.1073/pnas.76.12.6167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuda H., Konisky J. Mode of action of colicin Ia: effect of colicin on the Escherichia coli proton electrochemical gradient. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2579–2583. doi: 10.1073/pnas.75.6.2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tompkins G. R., O'Neill M. M., Cafarella T. G., Germaine G. R. Inhibition of bactericidal and bacteriolytic activities of poly-D-lysine and lysozyme by chitotriose and ferric iron. Infect Immun. 1991 Feb;59(2):655–664. doi: 10.1128/iai.59.2.655-664.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twetman S., Lindqvist L. Effect of salivary lysozyme on glucose incorporation and acid production in Streptococcus mutans. Caries Res. 1985;19(5):414–421. doi: 10.1159/000260876. [DOI] [PubMed] [Google Scholar]
- Twetman S., Lindqvist L., Sund M. L. Effect of human lysozyme on 2-deoxyglucose uptake by Streptococcus mutans and other oral microorganisms. Caries Res. 1986;20(3):223–229. doi: 10.1159/000260939. [DOI] [PubMed] [Google Scholar]
- Vasstrand E. N., Jensen H. B. Affinity chromatography of human saliva lysozyme and effect of pH and ionic strength on lytic activity. Scand J Dent Res. 1980 Jun;88(3):219–228. doi: 10.1111/j.1600-0722.1980.tb01218.x. [DOI] [PubMed] [Google Scholar]
- Weiss M. J., Luria S. E. Reduction of membrane potential, an immediate effect of colicin K. Proc Natl Acad Sci U S A. 1978 May;75(5):2483–2487. doi: 10.1073/pnas.75.5.2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimoto T., Tobiishi M., Tsuru D. Affinity chromatographic purification of human lysozyme, with special reference to human leukemia lysozyme. J Biochem. 1976 Oct;80(4):703–709. doi: 10.1093/oxfordjournals.jbchem.a131329. [DOI] [PubMed] [Google Scholar]
- Yphantis D. A., Dainko J. L., Schlenk F. Effect of some proteins on the yeast cell membrane. J Bacteriol. 1967 Nov;94(5):1509–1515. doi: 10.1128/jb.94.5.1509-1515.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarlengo M. H., Schultz S. G. Cation transport and metabolism in Streptococcus fecalis. Biochim Biophys Acta. 1966 Oct 10;126(2):308–320. doi: 10.1016/0926-6585(66)90068-9. [DOI] [PubMed] [Google Scholar]
- van Palenstein Helderman W. H. Lysozyme concentrations in the gingival crevice and at other oral sites in human subjects with and without gingivitis. Arch Oral Biol. 1976;21(4):251–255. doi: 10.1016/0003-9969(76)90043-1. [DOI] [PubMed] [Google Scholar]


