Abstract
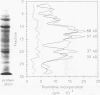
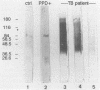
Little is known about the immunodominant or protective antigens of Mycobacterium tuberculosis in humans. Cell-mediated immunity is necessary for protection, and healthy tuberculin-positive individuals are relatively resistant to exogenous reinfection. We compared the targets of the cell-mediated immune response in healthy tuberculin-positive individuals to those of tuberculosis patients and tuberculin-negative persons. By using T-cell Western blotting (immunoblotting) of nitrocellulose-bound M. tuberculosis culture filtrate, peaks of T-cell blastogenic activity were identified in the healthy tuberculin reactors at 30, 37, 44, 57, 64, 71 and 88 kDa. Three of these fractions (30, 64, and 71 kDa) coincided with previously characterized proteins: antigen 6/alpha antigen, HSP60, and HSP70, respectively. The blastogenic responses to purified M. tuberculosis antigen 6/alpha antigen and BCG HSP60 were assessed. When cultured with purified antigen 6/alpha antigen, lymphocytes of healthy tuberculin reactors demonstrated greater [3H]thymidine incorporation than either healthy tuberculin-negative controls or tuberculous patients (8,113 +/- 1,939 delta cpm versus 645 +/- 425 delta cpm and 1,019 +/- 710 delta cpm, respectively; P less than 0.01). Healthy reactors also responded to HSP60, although to a lesser degree than antigen 6/alpha antigen (4,276 +/- 1,095 delta cpm; P less than 0.05). Partially purified HSP70 bound to nitrocellulose paper elicited a significant lymphocyte blastogenic response in two of six of the tuberculous patients but in none of the eight healthy tuberculin reactors. Lymphocytes of none of five tuberculin-negative controls responded to recombinant antigens at 14 or 19 kDa or to HSP70. Antibody reactivity generally was inversely correlated with blastogenic response: tuberculous sera had high titer antibody to M. tuberculosis culture filtrate in a range from 35 to 180 kDa. This is the first systematic evaluation of the human response to a panel of native and recombinant antigens in healthy tuberculin reactors and tuberculous patients. Antigens which stimulated prominent lymphocyte blastogenic responses were identified in seven fractions on T-cell Western blot analysis. Two of these may represent previously characterized proteins; the others may contain immunodominant proteins that will require further characterization.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abou-Zeid C., Filley E., Steele J., Rook G. A. A simple new method for using antigens separated by polyacrylamide gel electrophoresis to stimulate lymphocytes in vitro after converting bands cut from Western blots into antigen-bearing particles. J Immunol Methods. 1987 Apr 2;98(1):5–10. doi: 10.1016/0022-1759(87)90429-7. [DOI] [PubMed] [Google Scholar]
- Andersen A. B., Worsaae A., Chaparas S. D. Isolation and characterization of recombinant lambda gt11 bacteriophages expressing eight different mycobacterial antigens of potential immunological relevance. Infect Immun. 1988 May;56(5):1344–1351. doi: 10.1128/iai.56.5.1344-1351.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes P. F., Mehra V., Hirschfield G. R., Fong S. J., Abou-Zeid C., Rook G. A., Hunter S. W., Brennan P. J., Modlin R. L. Characterization of T cell antigens associated with the cell wall protein-peptidoglycan complex of Mycobacterium tuberculosis. J Immunol. 1989 Oct 15;143(8):2656–2662. [PubMed] [Google Scholar]
- Boom W. H., Husson R. N., Young R. A., David J. R., Piessens W. F. In vivo and in vitro characterization of murine T-cell clones reactive to Mycobacterium tuberculosis. Infect Immun. 1987 Sep;55(9):2223–2229. doi: 10.1128/iai.55.9.2223-2229.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coates A. R., Nicolai H., Pallen M. J., Guy A., Chaparas S. D., Mitchison D. A. The 45 kilodalton molecule of Mycobacterium tuberculosis identified by immunoblotting and monoclonal antibodies as antigenic in patients with tuberculosis. Br J Exp Pathol. 1989 Apr;70(2):215–225. [PMC free article] [PubMed] [Google Scholar]
- Collins F. M., Lamb J. R., Young D. B. Biological activity of protein antigens isolated from Mycobacterium tuberculosis culture filtrate. Infect Immun. 1988 May;56(5):1260–1266. doi: 10.1128/iai.56.5.1260-1266.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel T. M., Ferguson L. E. Purification and Characterization of Two Proteins from Culture Filtrates of Mycobacterium tuberculosis H(37)Ra Strain. Infect Immun. 1970 Feb;1(2):164–168. doi: 10.1128/iai.1.2.164-168.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmrich F., Thole J., van Embden J., Kaufmann S. H. A recombinant 64 kilodalton protein of Mycobacterium bovis bacillus Calmette-Guerin specifically stimulates human T4 clones reactive to mycobacterial antigens. J Exp Med. 1986 Apr 1;163(4):1024–1029. doi: 10.1084/jem.163.4.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine P. E. The BCG story: lessons from the past and implications for the future. Rev Infect Dis. 1989 Mar-Apr;11 (Suppl 2):S353–S359. doi: 10.1093/clinids/11.supplement_2.s353. [DOI] [PubMed] [Google Scholar]
- Grange J. M. The humoral immune response in tuberculosis: its nature, biological role and diagnostic usefulness. Adv Tuberc Res. 1984;21:1–78. [PubMed] [Google Scholar]
- Huygen K., Van Vooren J. P., Turneer M., Bosmans R., Dierckx P., De Bruyn J. Specific lymphoproliferation, gamma interferon production, and serum immunoglobulin G directed against a purified 32 kDa mycobacterial protein antigen (P32) in patients with active tuberculosis. Scand J Immunol. 1988 Feb;27(2):187–194. doi: 10.1111/j.1365-3083.1988.tb02338.x. [DOI] [PubMed] [Google Scholar]
- Ivanyi J., Bothamley G. H., Jackett P. S. Immunodiagnostic assays for tuberculosis and leprosy. Br Med Bull. 1988 Jul;44(3):635–649. doi: 10.1093/oxfordjournals.bmb.a072273. [DOI] [PubMed] [Google Scholar]
- Lamb J. R., Ivanyi J., Rees A., Young R. A., Young D. B. The identification of T cell epitopes in Mycobacterium tuberculosis using human T lymphocyte clones. Lepr Rev. 1986 Dec;57 (Suppl 2):131–137. [PubMed] [Google Scholar]
- Matsuo K., Yamaguchi R., Yamazaki A., Tasaka H., Yamada T. Cloning and expression of the Mycobacterium bovis BCG gene for extracellular alpha antigen. J Bacteriol. 1988 Sep;170(9):3847–3854. doi: 10.1128/jb.170.9.3847-3854.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafa A. S., Oftung F., Gill H. K., Natvig I. Characteristics of human T-cell clones from BCG and killed M. leprae vaccinated subjects and tuberculosis patients. Recognition of recombinant mycobacterial antigens. Lepr Rev. 1986 Dec;57 (Suppl 2):123–130. [PubMed] [Google Scholar]
- Oftung F., Mustafa A. S., Husson R., Young R. A., Godal T. Human T cell clones recognize two abundant Mycobacterium tuberculosis protein antigens expressed in Escherichia coli. J Immunol. 1987 Feb 1;138(3):927–931. [PubMed] [Google Scholar]
- Orme I. M., Collins F. M. Protection against Mycobacterium tuberculosis infection by adoptive immunotherapy. Requirement for T cell-deficient recipients. J Exp Med. 1983 Jul 1;158(1):74–83. doi: 10.1084/jem.158.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orme I. M. Induction of nonspecific acquired resistance and delayed-type hypersensitivity, but not specific acquired resistance in mice inoculated with killed mycobacterial vaccines. Infect Immun. 1988 Dec;56(12):3310–3312. doi: 10.1128/iai.56.12.3310-3312.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Results of a World Health Organization-sponsored workshop to characterize antigens recognized by mycobacterium-specific monoclonal antibodies. Infect Immun. 1986 Feb;51(2):718–720. doi: 10.1128/iai.51.2.718-720.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sada E., Ferguson L. E., Daniel T. M. An ELISA for the serodiagnosis of tuberculosis using a 30,000-Da native antigen of Mycobacterium tuberculosis. J Infect Dis. 1990 Oct;162(4):928–931. doi: 10.1093/infdis/162.4.928. [DOI] [PubMed] [Google Scholar]
- Selwyn P. A., Hartel D., Lewis V. A., Schoenbaum E. E., Vermund S. H., Klein R. S., Walker A. T., Friedland G. H. A prospective study of the risk of tuberculosis among intravenous drug users with human immunodeficiency virus infection. N Engl J Med. 1989 Mar 2;320(9):545–550. doi: 10.1056/NEJM198903023200901. [DOI] [PubMed] [Google Scholar]
- Shinnick T. M., Krat C., Schadow S. Isolation and restriction site maps of the genes encoding five Mycobacterium tuberculosis proteins. Infect Immun. 1987 Jul;55(7):1718–1721. doi: 10.1128/iai.55.7.1718-1721.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiratsuchi H., Tsuyuguchi I. Analysis of T cell subsets by monoclonal antibodies in patients with tuberculosis after in vitro stimulation with purified protein derivative of tuberculin. Clin Exp Immunol. 1984 Aug;57(2):271–278. [PMC free article] [PubMed] [Google Scholar]
- Stead W. W., Lofgren J. P., Warren E., Thomas C. Tuberculosis as an endemic and nosocomial infection among the elderly in nursing homes. N Engl J Med. 1985 Jun 6;312(23):1483–1487. doi: 10.1056/NEJM198506063122304. [DOI] [PubMed] [Google Scholar]
- Wallis R. S., Alde S. L., Havlir D. V., Amir-Tahmasseb M. H., Daniel T. M., Ellner J. J. Identification of antigens of Mycobacterium tuberculosis using human monoclonal antibodies. J Clin Invest. 1989 Jul;84(1):214–219. doi: 10.1172/JCI114143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiker H. G., Harboe M., Nagai S., Patarroyo M. E., Ramirez C., Cruz N. MPB59, a widely cross-reacting protein of Mycobacterium bovis BCG. Int Arch Allergy Appl Immunol. 1986;81(4):307–314. doi: 10.1159/000234154. [DOI] [PubMed] [Google Scholar]