Abstract
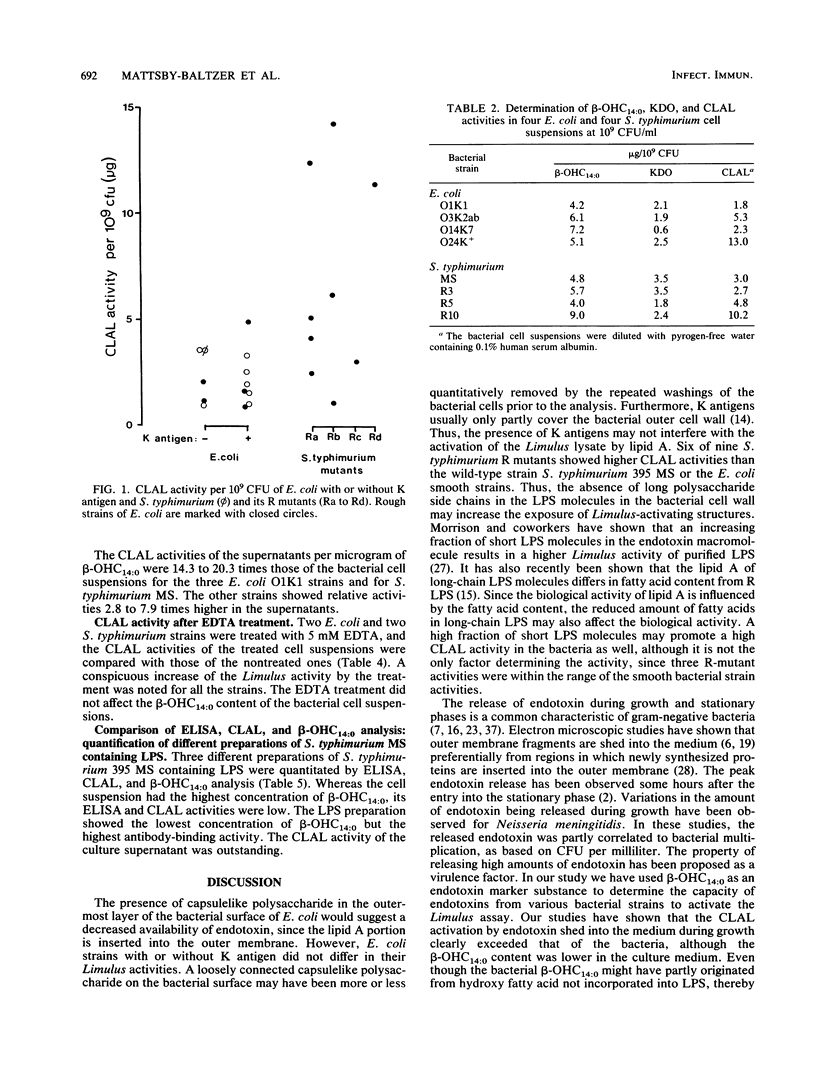
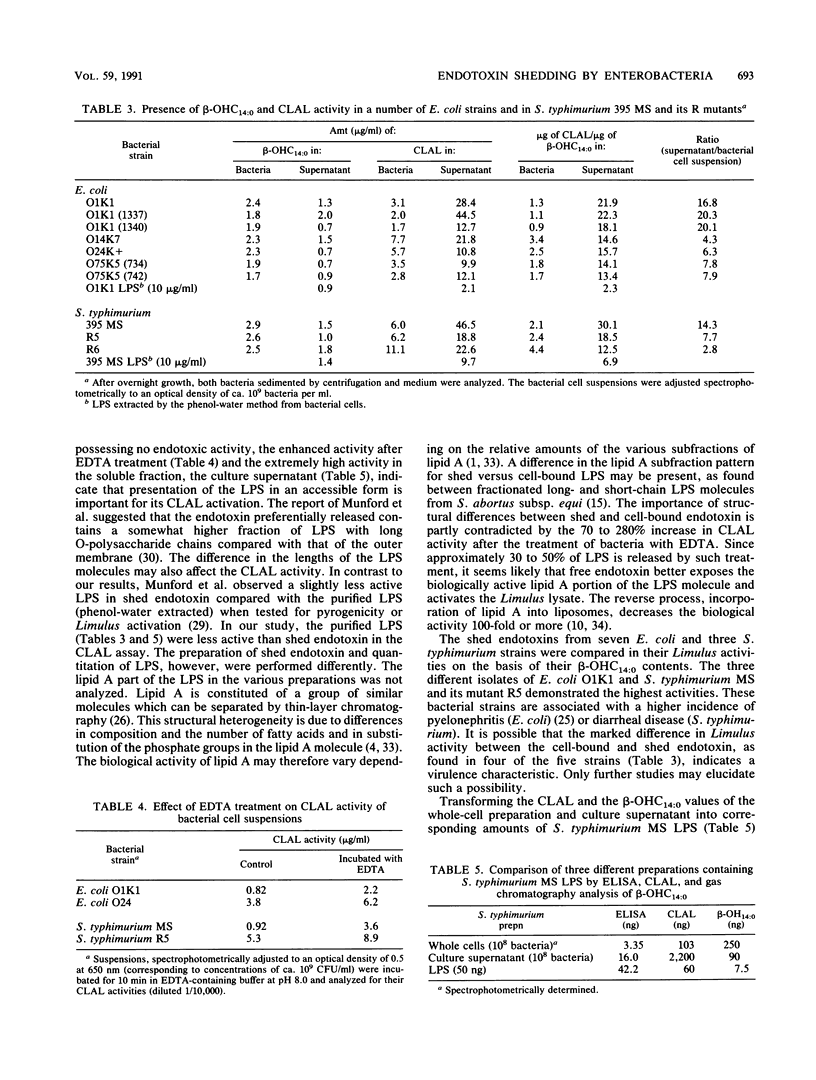
The endotoxin activities of gram-negative bacteria and their lipopolysaccharides (LPS) have been quantitated by a chromogenic Limulus amocbocyte lysate (CLAL) assay. When bacterial cell exposing various cell surface structures were compared, the highest Limulus activities were found in R strains of Escherichia coli and Salmonella typhimurium mutants. E. coli with K antigens did not differ from K-negative strains. By measuring beta-hydroxymyristic acid (3-OH tetradecanoic acid, beta-OHC14:0), it was possible to compare the CLAL activities of LPS bound to bacterial cells, LPS shed into the culture medium, and purified LPS. After 16 h of growth, the cell-free culture supernatants of three E. coli O1K1 strains and S. typhimurium showed CLAL activities 14.3 to 20.3 times higher than did the corresponding bacterial cell suspensions in relation to their beta-OHC14:0 contents. Four other E. coli strains (O serotypes O14, O24, and O75) and the S. typhimurium 395 R mutants MR5 and MR6 showed CLAL values 2.8 to 7.9 times higher in their culture supernatants. LPS of E. coli O1K1 and S. typhimurium had lower CLAL activities than the culture supernatants (1/10 and 1/4, respectively). Although the beta-OHC14:0 concentrations of the culture supernatants were approximately half those of the corresponding bacterial cells, all had CLAL values that were 2 to 21 times higher. The bacterial cell suspension, culture supernatant, and purified LPS of S. typhimurium MS were compared by CLAL assay and a quantitative enzyme-linked immunosorbent assay based on monoclonal antibodies to the O5 antigen. Endotoxin shed into the culture medium was the most CLAL-active form of LPS, while purified LPS was the most antigen-active form. The results emphasize the importance of appropriate standards when quantifying endotoxin in various states. In conclusion, E. coli and S. typhimurium bacteria shed significant amounts of endotoxin into the surrounding medium during growth. This form of LPS is more CLAL active than the cell-bound or purified LPS.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alving C. R., Richardson E. C. Mitogenic activities of lipid A and liposome-associated lipid A: effects of epitope density. Rev Infect Dis. 1984 Jul-Aug;6(4):493–496. doi: 10.1093/clinids/6.4.493. [DOI] [PubMed] [Google Scholar]
- Andersen B. M., Solberg O. Endotoxin liberation and invasivity of Neisseria meningitidis. Scand J Infect Dis. 1984;16(3):247–254. doi: 10.3109/00365548409070397. [DOI] [PubMed] [Google Scholar]
- Andersen B. M., Solberg O. Liberation of endotoxin during growth of Neisseria meningitidis in a chemically-defined medium. Acta Pathol Microbiol Scand B. 1978 Oct;86B(5):275–281. doi: 10.1111/j.1699-0463.1978.tb00044.x. [DOI] [PubMed] [Google Scholar]
- Baltzer L. H., Mattsby-Baltzer I. Heterogeneity of lipid A: structural determination by 13C and 31P NMR of lipid A fractions from lipopolysaccharide of Escherichia coli 0111. Biochemistry. 1986 Jun 17;25(12):3570–3575. doi: 10.1021/bi00360a015. [DOI] [PubMed] [Google Scholar]
- Bayer E. A., Wilchek M. The use of the avidin-biotin complex as a tool in molecular biology. Methods Biochem Anal. 1980;26:1–45. doi: 10.1002/9780470110461.ch1. [DOI] [PubMed] [Google Scholar]
- Burdett I. D., Murray R. G. Electron microscope study of septum formation in Escherichia coli strains B and B-r during synchronous growth. J Bacteriol. 1974 Sep;119(3):1039–1056. doi: 10.1128/jb.119.3.1039-1056.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadieux J. E., Kuzio J., Milazzo F. H., Kropinski A. M. Spontaneous release of lipopolysaccharide by Pseudomonas aeruginosa. J Bacteriol. 1983 Aug;155(2):817–825. doi: 10.1128/jb.155.2.817-825.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crutchley M. J., Marsh D. G., Cameron J. Free Endotoxin. Nature. 1967 Jun 3;214(5092):1052–1052. doi: 10.1038/2141052a0. [DOI] [PubMed] [Google Scholar]
- Demonty J., De Graeve J. Release of endotoxic lipopolysaccharide by sensitive strains of Escherichia coli submitted to the bactericidal action of human serum. Med Microbiol Immunol. 1982;170(4):265–277. doi: 10.1007/BF02123317. [DOI] [PubMed] [Google Scholar]
- Dijkstra J., Mellors J. W., Ryan J. L., Szoka F. C. Modulation of the biological activity of bacterial endotoxin by incorporation into liposomes. J Immunol. 1987 Apr 15;138(8):2663–2670. [PubMed] [Google Scholar]
- Elin R. J., Sandberg A. L., Rosentreich D. L. Comparison of the pyrogenicity, Limulus activity mitogenicity and complement reactivity of several bacterial endotoxins and related compounds. J Immunol. 1976 Oct;117(4):1238–1242. [PubMed] [Google Scholar]
- Friberger P., Knös M., Mellstam L. A quantitative endotoxin assay utilizing LAL and a chromogenic substrate. Prog Clin Biol Res. 1982;93:195–206. [PubMed] [Google Scholar]
- Hoekstra D., van der Laan J. W., de Leij L., Witholt B. Release of outer membrane fragments from normally growing Escherichia coli. Biochim Biophys Acta. 1976 Dec 14;455(3):889–899. doi: 10.1016/0005-2736(76)90058-4. [DOI] [PubMed] [Google Scholar]
- Jiao B. H., Freudenberg M., Galanos C. Characterization of the lipid A component of genuine smooth-form lipopolysaccharide. Eur J Biochem. 1989 Apr 1;180(3):515–518. doi: 10.1111/j.1432-1033.1989.tb14676.x. [DOI] [PubMed] [Google Scholar]
- Johnson K. G., Perry M. B., McDonald I. J., Russel R. R. Cellular and free lipopolysaccharides of some species of Neisseria. Can J Microbiol. 1975 Dec;21(12):1969–1980. doi: 10.1139/m75-285. [DOI] [PubMed] [Google Scholar]
- Kanegasaki S., Kojima Y., Matsuura M., Homma J. Y., Yamamoto A., Kumazawa Y., Tanamoto K., Yasuda T., Tsumita T., Imoto M. Biological activities of analogues of lipid A based chemically on the revised structural model. Comparison of mediator-inducing, immunomodulating and endotoxic activities. Eur J Biochem. 1984 Sep 3;143(2):237–242. doi: 10.1111/j.1432-1033.1984.tb08364.x. [DOI] [PubMed] [Google Scholar]
- Karkhanis Y. D., Zeltner J. Y., Jackson J. J., Carlo D. J. A new and improved microassay to determine 2-keto-3-deoxyoctonate in lipopolysaccharide of Gram-negative bacteria. Anal Biochem. 1978 Apr;85(2):595–601. doi: 10.1016/0003-2697(78)90260-9. [DOI] [PubMed] [Google Scholar]
- Knox K. W., Vesk M., Work E. Relation between excreted lipopolysaccharide complexes and surface structures of a lysine-limited culture of Escherichia coli. J Bacteriol. 1966 Oct;92(4):1206–1217. doi: 10.1128/jb.92.4.1206-1217.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Leive L. Release of lipopolysaccharide by EDTA treatment of E. coli. Biochem Biophys Res Commun. 1965 Nov 22;21(4):290–296. doi: 10.1016/0006-291x(65)90191-9. [DOI] [PubMed] [Google Scholar]
- Lindsay S. S., Wheeler B., Sanderson K. E., Costerton J. W., Cheng K. J. The release of alkaline phosphatase and of lipopolysaccharide during the growth of rough and smooth strains of Salmonella typhimurium. Can J Microbiol. 1973 Mar;19(3):335–343. doi: 10.1139/m73-056. [DOI] [PubMed] [Google Scholar]
- Mattsby-Baltzer I., Gemski P., Alving C. R. Heterogeneity of lipid A. Rev Infect Dis. 1984 Jul-Aug;6(4):444–448. doi: 10.1093/clinids/6.4.444. [DOI] [PubMed] [Google Scholar]
- Morrison D. C., Vukajlovich S. W., Ryan J. L., Levin J. Structural requirements for gelation of the Limulus amebocyte lysate by endotoxin. Prog Clin Biol Res. 1987;231:55–73. [PubMed] [Google Scholar]
- Mug-Opstelten D., Witholt B. Preferential release of new outer membrane fragments by exponentially growing Escherichia coli. Biochim Biophys Acta. 1978 Apr 4;508(2):287–295. doi: 10.1016/0005-2736(78)90331-0. [DOI] [PubMed] [Google Scholar]
- Munford R. S., Hall C. L., Lipton J. M., Dietschy J. M. Biological activity, lipoprotein-binding behavior, and in vivo disposition of extracted and native forms of Salmonella typhimurium lipopolysaccharides. J Clin Invest. 1982 Oct;70(4):877–888. doi: 10.1172/JCI110684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munford R. S., Hall C. L., Rick P. D. Size heterogeneity of Salmonella typhimurium lipopolysaccharides in outer membranes and culture supernatant membrane fragments. J Bacteriol. 1980 Nov;144(2):630–640. doi: 10.1128/jb.144.2.630-640.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mårild S., Jodal U., Orskov I., Orskov F., Svanborg Edén C. Special virulence of the Escherichia coli O1:K1:H7 clone in acute pyelonephritis. J Pediatr. 1989 Jul;115(1):40–45. doi: 10.1016/s0022-3476(89)80326-9. [DOI] [PubMed] [Google Scholar]
- Pearson F. C. A comparison of the pyrogenicity of environmental endotoxins and lipopolysaccharides. Prog Clin Biol Res. 1985;189:251–265. [PubMed] [Google Scholar]
- Postel J., Schloerb P. R., Furtado D. Pathophysiologic alterations during bacterial infusions for the study of bacteremic shock. Surg Gynecol Obstet. 1975 Nov;141(5):683–692. [PubMed] [Google Scholar]
- Qureshi N., Takayama K., Ribi E. Purification and structural determination of nontoxic lipid A obtained from the lipopolysaccharide of Salmonella typhimurium. J Biol Chem. 1982 Oct 10;257(19):11808–11815. [PubMed] [Google Scholar]
- Richardson E. C., Banerji B., Seid R. C., Jr, Levin J., Alving C. R. Interactions of lipid a and liposome-associated lipid A with Limulus polyphemus amoebocytes. Infect Immun. 1983 Mar;39(3):1385–1391. doi: 10.1128/iai.39.3.1385-1391.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rietschel E. T., Brade L., Schade U., Seydel U., Zähringer U., Brandenburg K., Helander I., Holst O., Kondo S., Kuhn H. M. Bacterial lipopolysaccharides: relationship of structure and conformation to endotoxic activity, serological specificity and biological function. Adv Exp Med Biol. 1990;256:81–99. doi: 10.1007/978-1-4757-5140-6_5. [DOI] [PubMed] [Google Scholar]
- Rothfield L., Pearlman-Kothencz M. Synthesis and assembly of bacterial membrane components. A lipopolysaccharide-phospholipid-protein complex excreted by living bacteria. J Mol Biol. 1969 Sep 28;44(3):477–492. doi: 10.1016/0022-2836(69)90374-x. [DOI] [PubMed] [Google Scholar]
- Røkke O., Revhaug A., Osterud B., Giercksky K. E. Increased plasma levels of endotoxin and corresponding changes in circulatory performance in a porcine sepsis model: the effect of antibiotic administration. Prog Clin Biol Res. 1988;272:247–262. [PubMed] [Google Scholar]
- Sjögren-Jansson E., Jeansson S. Large-scale production of monoclonal antibodies in dialysis tubing. J Immunol Methods. 1985 Nov 28;84(1-2):359–364. doi: 10.1016/0022-1759(85)90442-9. [DOI] [PubMed] [Google Scholar]
- Svanborg Edén C., Hull R., Falkow S., Leffler H. Target cell specificity of wild-type E. coli and mutants and clones with genetically defined adhesins. Prog Food Nutr Sci. 1983;7(3-4):75–89. [PubMed] [Google Scholar]


