Abstract
We describe here the development of a mouse subcutaneous chamber model that allows for the examination of host-parasite interactions as well as the determination of gross pathology with Porphyromonas (Bacteroides) gingivalis challenge. When inoculated into stainless-steel chambers implanted subcutaneously in female BALB/c mice, P. gingivalis W83, W50, and A7436 (10(8) to 10(10) CFU) caused cachexia, ruffling, general erythema and phlegmonous, ulcerated, necrotic lesions, and death. P. gingivalis W50/BEI, HG405, and 33277 (10(10) CFU) produced localized abscesses in the mouse chamber model with rejection of chambers at the injection site. Analysis of chamber fluid from 33277-, HG405-, and W50/BEI-infected mice by cytocentrifugation revealed inflammatory cell debris, polymorphonuclear leukocytes, and high numbers of dead bacteria. In contrast, fluid from A7436-, W50-, and W83-infected mice revealed infiltration predominantly of polymorphonuclear leukocytes and live bacteria. Bacteria were found primarily associated with polymorphonuclear leukocytes in the fluid from W50-, HG405-, and W83-infected mice but not from A7436-infected mice. Viable isolates were recoverable from the chamber fluid through day 3 for W50/BEI, day 5 for 33277, day 6 for HG405, day 7 for W50, day 14 for W83, and day 26 for A7436. All strains induced a systemic immunoglobulin G response in serum and chamber fluid samples. The use of this model will allow us to examine the virulence of P. gingivalis as defined by the interaction of host response to localized infection with P. gingivalis.
Full text
PDF


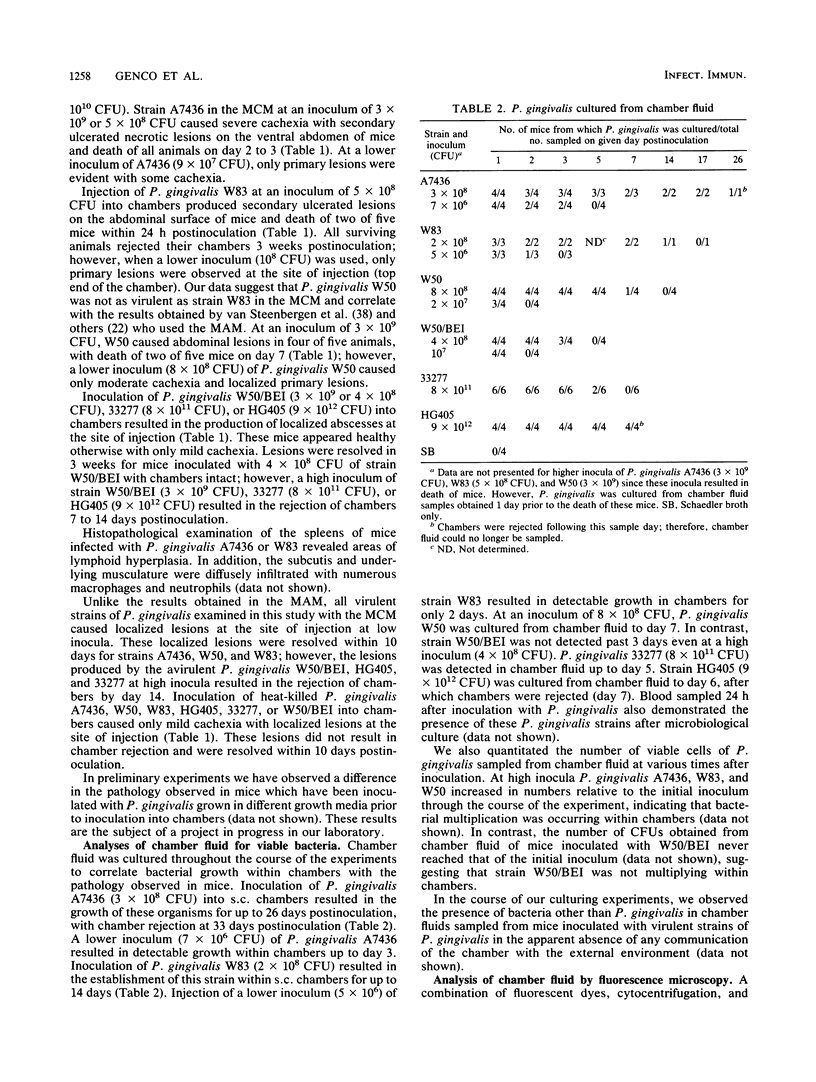
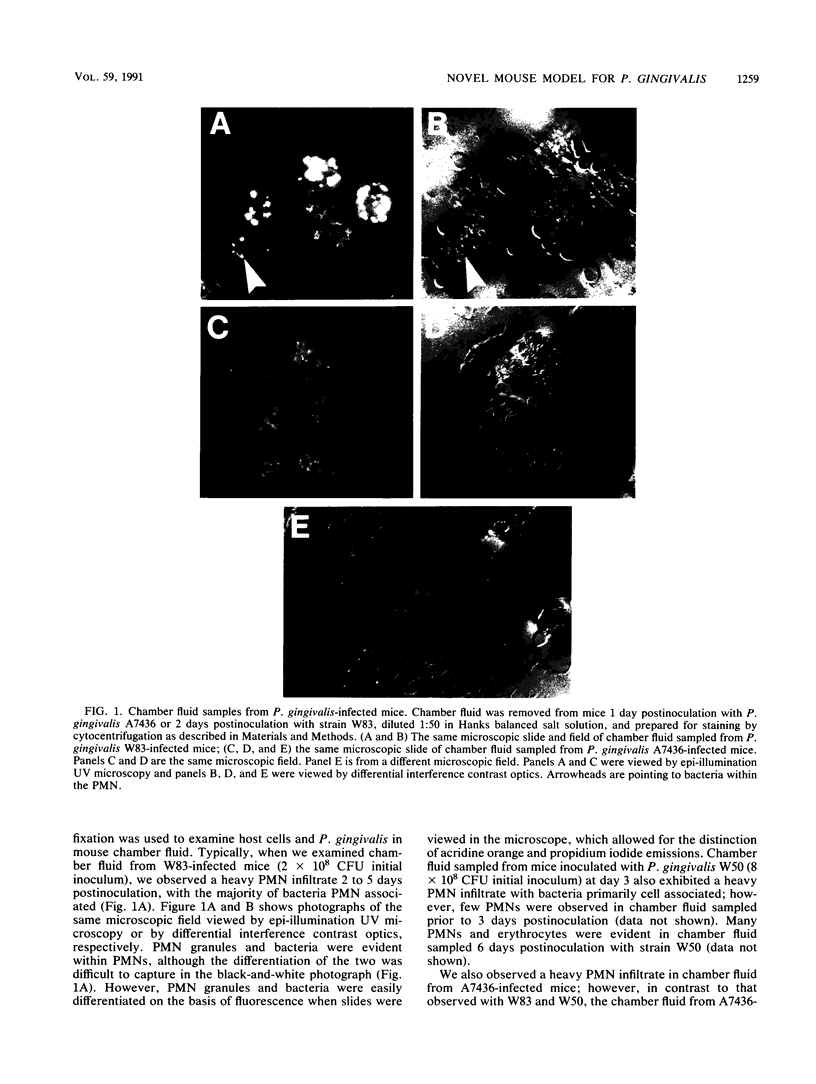




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman L. C., Page R. C., Ebersole J. L., Vandesteen E. G. Assessment of host defenses and serum antibodies to suspected periodontal pathogens in patients with various types of periodontitis. J Periodontal Res. 1982 Sep;17(5):495–497. doi: 10.1111/j.1600-0765.1982.tb02037.x. [DOI] [PubMed] [Google Scholar]
- Anderson D. C., Schmalstieg F. C., Arnaout M. A., Kohl S., Tosi M. F., Dana N., Buffone G. J., Hughes B. J., Brinkley B. R., Dickey W. D. Abnormalities of polymorphonuclear leukocyte function associated with a heritable deficiency of high molecular weight surface glycoproteins (GP138): common relationship to diminished cell adherence. J Clin Invest. 1984 Aug;74(2):536–551. doi: 10.1172/JCI111451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson D. C., Springer T. A. Leukocyte adhesion deficiency: an inherited defect in the Mac-1, LFA-1, and p150,95 glycoproteins. Annu Rev Med. 1987;38:175–194. doi: 10.1146/annurev.me.38.020187.001135. [DOI] [PubMed] [Google Scholar]
- Chen P. B., Davern L. B., Schifferle R., Zambon J. J. Protective immunization against experimental Bacteroides (Porphyromonas) gingivalis infection. Infect Immun. 1990 Oct;58(10):3394–3400. doi: 10.1128/iai.58.10.3394-3400.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P. B., Neiders M. E., Millar S. J., Reynolds H. S., Zambon J. J. Effect of immunization on experimental Bacteroides gingivalis infection in a murine model. Infect Immun. 1987 Oct;55(10):2534–2537. doi: 10.1128/iai.55.10.2534-2537.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlén G., Slots J. Experimental infections by Bacteroides gingivalis in non-immunized and immunized rabbits. Oral Microbiol Immunol. 1989 Mar;4(1):6–11. doi: 10.1111/j.1399-302x.1989.tb00399.x. [DOI] [PubMed] [Google Scholar]
- Ebersole J. L., Frey D. E., Taubman M. A., Smith D. J. An ELISA for measuring serum antibodies to Actinobacillus actinomycetemcomitans. J Periodontal Res. 1980 Nov;15(6):621–632. doi: 10.1111/j.1600-0765.1980.tb00321.x. [DOI] [PubMed] [Google Scholar]
- Grenier D., Mayrand D. Etudes d'infections mixtes anaérobies comportant Bacteroides gingivalis. Can J Microbiol. 1983 May;29(5):612–618. [PubMed] [Google Scholar]
- Grenier D., Mayrand D. Selected characteristics of pathogenic and nonpathogenic strains of Bacteroides gingivalis. J Clin Microbiol. 1987 Apr;25(4):738–740. doi: 10.1128/jcm.25.4.738-740.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haapasalo M., Shah H., Gharbia S., Seddon S., Lounatmaa K. Surface properties and ultrastructure of Porphyromonas gingivalis W50 and pleiotropic mutants. Scand J Dent Res. 1989 Aug;97(4):355–360. doi: 10.1111/j.1600-0722.1989.tb01623.x. [DOI] [PubMed] [Google Scholar]
- Holt S. C., Ebersole J., Felton J., Brunsvold M., Kornman K. S. Implantation of Bacteroides gingivalis in nonhuman primates initiates progression of periodontitis. Science. 1988 Jan 1;239(4835):55–57. doi: 10.1126/science.3336774. [DOI] [PubMed] [Google Scholar]
- Kalmar J. R., Arnold R. R., van Dyke T. E. Direct interaction of Actinobacillus actinomycetemcomitans with normal and defective (LJP) neutrophils. J Periodontal Res. 1987 May;22(3):179–181. doi: 10.1111/j.1600-0765.1987.tb01561.x. [DOI] [PubMed] [Google Scholar]
- Kastelein P., van Steenbergen T. J., Bras J. M., de Graaff J. An experimentally induced phlegmonous abscess by a strain of Bacteroides gingivalis in guinea pigs and mice. Antonie Van Leeuwenhoek. 1981 Mar;47(1):1–9. doi: 10.1007/BF00399062. [DOI] [PubMed] [Google Scholar]
- Marsh P. D., McKee A. S., McDermid A. S., Dowsett A. B. Ultrastructure and enzyme activities of a virulent and an avirulent variant of Bacteroides gingivalis W50. FEMS Microbiol Lett. 1989 May;50(1-2):181–185. doi: 10.1016/0378-1097(89)90482-5. [DOI] [PubMed] [Google Scholar]
- Mayrand D., McBride B. C. Exological relationships of bacteria involved in a simple, mixed anaerobic infection. Infect Immun. 1980 Jan;27(1):44–50. doi: 10.1128/iai.27.1.44-50.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee A. S., McDermid A. S., Baskerville A., Dowsett A. B., Ellwood D. C., Marsh P. D. Effect of hemin on the physiology and virulence of Bacteroides gingivalis W50. Infect Immun. 1986 May;52(2):349–355. doi: 10.1128/iai.52.2.349-355.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee A. S., McDermid A. S., Wait R., Baskerville A., Marsh P. D. Isolation of colonial variants of Bacteroides gingivalis W50 with a reduced virulence. J Med Microbiol. 1988 Sep;27(1):59–64. doi: 10.1099/00222615-27-1-59. [DOI] [PubMed] [Google Scholar]
- Neiders M. E., Chen P. B., Suido H., Reynolds H. S., Zambon J. J., Shlossman M., Genco R. J. Heterogeneity of virulence among strains of Bacteroides gingivalis. J Periodontal Res. 1989 May;24(3):192–198. doi: 10.1111/j.1600-0765.1989.tb02005.x. [DOI] [PubMed] [Google Scholar]
- Okuda K., Kato T., Naito Y., Ono M., Kikuchi Y., Takazoe I. Susceptibility of Bacteroides gingivalis to bactericidal activity of human serum. J Dent Res. 1986 Jul;65(7):1024–1027. doi: 10.1177/00220345860650070601. [DOI] [PubMed] [Google Scholar]
- Ryder M. I., Winkler J. R., Weinreb R. N. Elevated phagocytosis, oxidative burst, and F-actin formation in PMNs from individuals with intraoral manifestations of HIV infection. J Acquir Immune Defic Syndr. 1988;1(4):346–353. [PubMed] [Google Scholar]
- Salt W. G., Samara M. A., Stretton R. J. Experimentally induced Bacteroides infections in the rabbit. Microbios. 1987;50(204-205):163–175. [PubMed] [Google Scholar]
- Schenkein H. A. Failure of Bacteroides gingivalis W83 to accumulate bound C3 following opsonization with serum. J Periodontal Res. 1989 Jan;24(1):20–27. doi: 10.1111/j.1600-0765.1989.tb00853.x. [DOI] [PubMed] [Google Scholar]
- Schenkein H. A. The effect of periodontal proteolytic Bacteroides species on proteins of the human complement system. J Periodontal Res. 1988 May;23(3):187–192. doi: 10.1111/j.1600-0765.1988.tb01356.x. [DOI] [PubMed] [Google Scholar]
- Shah H. N., Seddon S. V., Gharbia S. E. Studies on the virulence properties and metabolism of pleiotropic mutants of Porphyromonas gingivalis (Bacteroides gingivalis) W50. Oral Microbiol Immunol. 1989 Mar;4(1):19–23. doi: 10.1111/j.1399-302x.1989.tb00401.x. [DOI] [PubMed] [Google Scholar]
- Slots J., Genco R. J. Black-pigmented Bacteroides species, Capnocytophaga species, and Actinobacillus actinomycetemcomitans in human periodontal disease: virulence factors in colonization, survival, and tissue destruction. J Dent Res. 1984 Mar;63(3):412–421. doi: 10.1177/00220345840630031101. [DOI] [PubMed] [Google Scholar]
- Smalley J. W., Birss A. J., Kay H. M., McKee A. S., Marsh P. D. The distribution of trypsin-like enzyme activity in cultures of a virulent and an avirulent strain of Bacteroides gingivalis W50. Oral Microbiol Immunol. 1989 Sep;4(3):178–181. doi: 10.1111/j.1399-302x.1989.tb00249.x. [DOI] [PubMed] [Google Scholar]
- Sundqvist G. K., Carlsson J., Herrmann B. F., Höfling J. F., Vätäinen A. Degradation in vivo of the C3 protein of guinea-pig complement by a pathogenic strain of Bacteroides gingivalis. Scand J Dent Res. 1984 Feb;92(1):14–24. doi: 10.1111/j.1600-0722.1984.tb00854.x. [DOI] [PubMed] [Google Scholar]
- Sundqvist G. K., Eckerbom M. I., Larsson A. P., Sjögren U. T. Capacity of anaerobic bacteria from necrotic dental pulps to induce purulent infections. Infect Immun. 1979 Aug;25(2):685–693. doi: 10.1128/iai.25.2.685-693.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundqvist G., Bloom G. D., Enberg K., Johansson E. Phagocytosis of Bacteroides melaninogenicus and Bacteroides gingivalis in vitro by human neutrophils. J Periodontal Res. 1982 Mar;17(2):113–121. doi: 10.1111/j.1600-0765.1982.tb01137.x. [DOI] [PubMed] [Google Scholar]
- Van Steenbergen T. J., Delemarre F. G., Namavar F., De Graaff J. Differences in virulence within the species Bacteroides gingivalis. Antonie Van Leeuwenhoek. 1987;53(4):233–244. doi: 10.1007/BF00393930. [DOI] [PubMed] [Google Scholar]
- Wong K. H., Arko R. J., Schalla W. O., Steurer F. J. Immunological and serological diversity of Neisseria gonorrhoeae: identification of new immunotypes and highly protective strains. Infect Immun. 1979 Mar;23(3):717–722. doi: 10.1128/iai.23.3.717-722.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerli W., Waldvogel F. A., Vaudaux P., Nydegger U. E. Pathogenesis of foreign body infection: description and characteristics of an animal model. J Infect Dis. 1982 Oct;146(4):487–497. doi: 10.1093/infdis/146.4.487. [DOI] [PubMed] [Google Scholar]
- van Steenbergen T. J., Kastelein P., Touw J. J., de Graaff J. Virulence of black-pigmented Bacteroides strains from periodontal pockets and other sites in experimentally induced skin lesions in mice. J Periodontal Res. 1982 Jan;17(1):41–49. doi: 10.1111/j.1600-0765.1982.tb01129.x. [DOI] [PubMed] [Google Scholar]
- van Steenbergen T. J., van Winkelhoff A. J., de Graaff J. Pathogenic synergy: mixed infections in the oral cavity. Antonie Van Leeuwenhoek. 1984;50(5-6):789–798. doi: 10.1007/BF02386241. [DOI] [PubMed] [Google Scholar]