Abstract
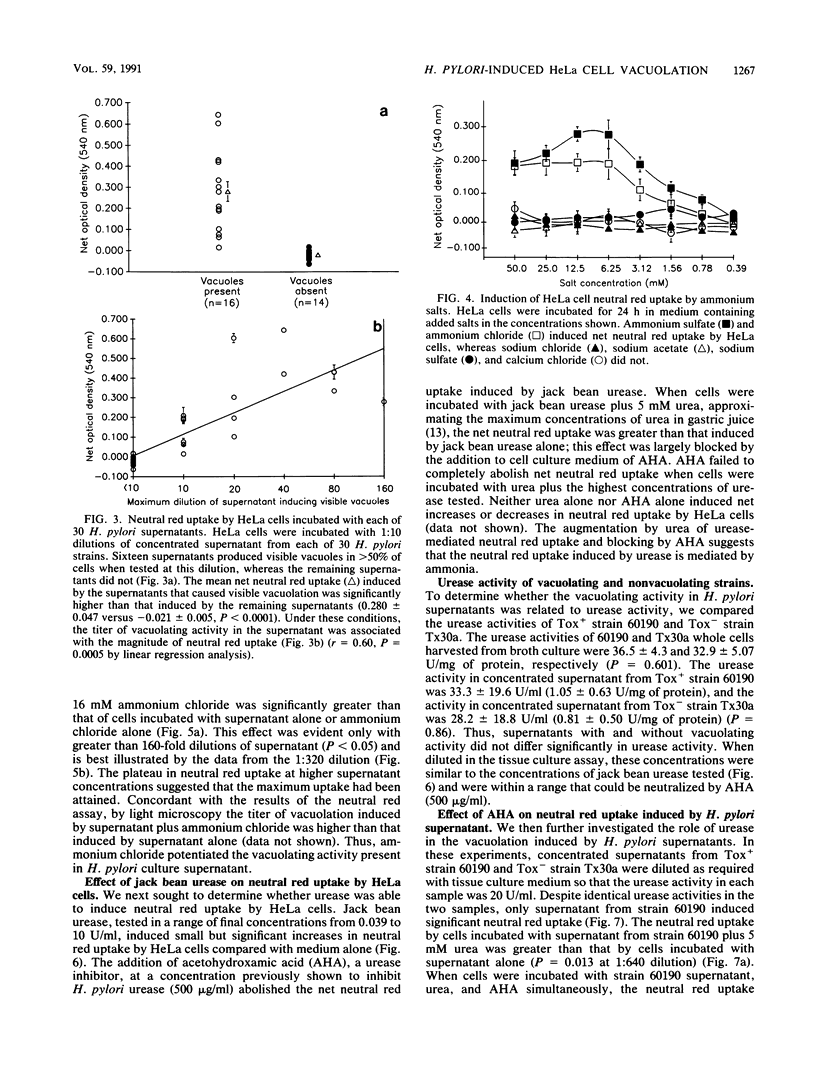
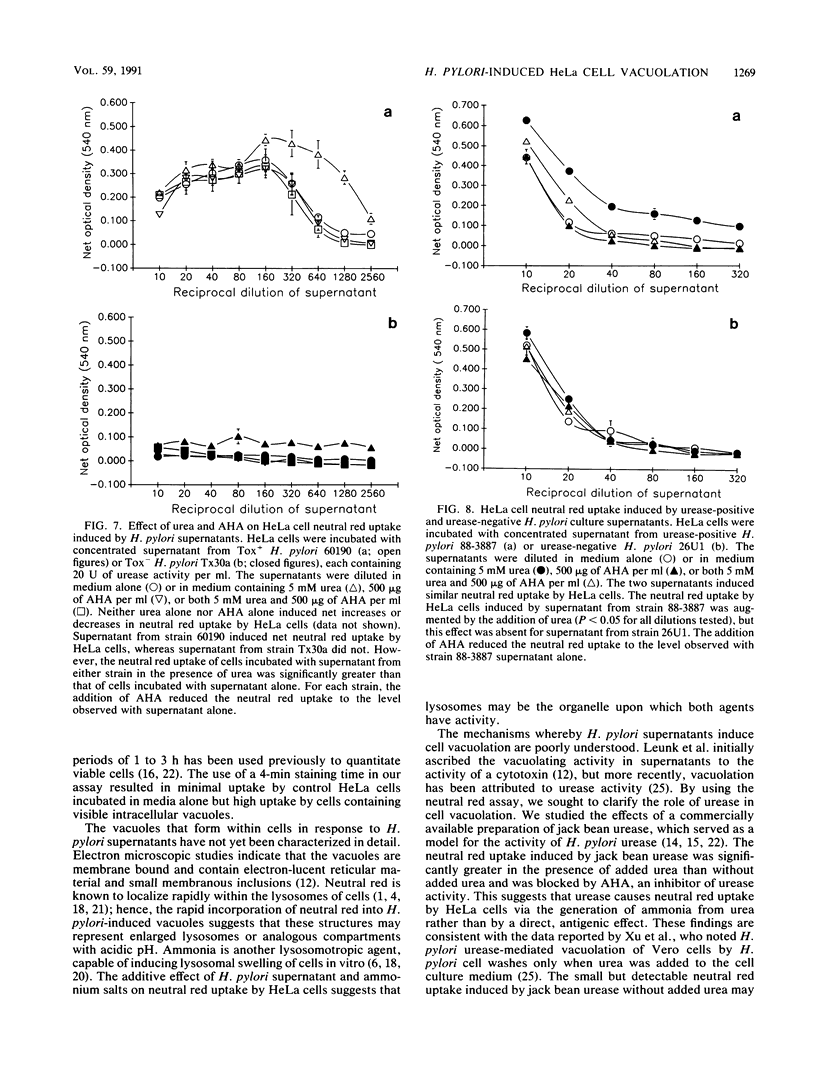
Concentrated broth culture supernatants from 50 to 60% of Helicobacter pylori strains induce eukaryotic cell vacuolation in vitro. A quantitative assay for cell vacuolation was developed on the basis of the rapid uptake of visibly vacuolated HeLa cells was significantly greater than that of nonvacuolated cells. By using the rapid NRU assay, we sought to determine the roles of H. pylori cytotoxin, urease, and ammonia in the vacuolation of HeLa cells. The NRU of HeLa cells incubated in medium containing ammonium chloride or ammonium sulfate was significantly greater than that of cells incubated in medium alone. In addition, ammonium salts augmented the NRU induced by H. pylori supernatants. The NRU induced by jack bean urease was augmented by the addition of urea to cell culture medium; this suggests that urease-mediated NRU occurs via the generation of ammonia. Acetohydroxamic acid blocked the NRU induced by jack bean urease and urea but failed to block the uptake induced by H. pylori supernatants. Supernatant from a non-urease-producing H. pylori mutant strain induced NRU identical to that of the urease-positive parental strain. These observations indicate that the vacuolating activity in H. pylori supernatants is not mediated solely by urease activity but that it may be potentiated by urease-mediated ammonia production.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLISON A. C., YOUNG M. R. UPTAKE OF DYES AND DRUGS BY LIVING CELLS IN CULTURE. Life Sci. 1964 Dec;3:1407–1414. doi: 10.1016/0024-3205(64)90082-7. [DOI] [PubMed] [Google Scholar]
- Barer M. R., Elliott T. S., Berkeley D., Thomas J. E., Eastham E. J. Cytopathic effects of Campylobacter pylori urease. J Clin Pathol. 1988 May;41(5):597–597. doi: 10.1136/jcp.41.5.597-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaser M. J. Helicobacter pylori and the pathogenesis of gastroduodenal inflammation. J Infect Dis. 1990 Apr;161(4):626–633. doi: 10.1093/infdis/161.4.626. [DOI] [PubMed] [Google Scholar]
- Bulychev A., Trouet A., Tulkens P. Uptake and intracellular distribution of neutral red in cultured fibroblasts. Exp Cell Res. 1978 Sep;115(2):343–355. doi: 10.1016/0014-4827(78)90288-4. [DOI] [PubMed] [Google Scholar]
- Cover T. L., Dooley C. P., Blaser M. J. Characterization of and human serologic response to proteins in Helicobacter pylori broth culture supernatants with vacuolizing cytotoxin activity. Infect Immun. 1990 Mar;58(3):603–610. doi: 10.1128/iai.58.3.603-610.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn B. E., Campbell G. P., Perez-Perez G. I., Blaser M. J. Purification and characterization of urease from Helicobacter pylori. J Biol Chem. 1990 Jun 5;265(16):9464–9469. [PubMed] [Google Scholar]
- Eaton K. A., Morgan D. R., Krakowka S. Campylobacter pylori virulence factors in gnotobiotic piglets. Infect Immun. 1989 Apr;57(4):1119–1125. doi: 10.1128/iai.57.4.1119-1125.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figura N., Guglielmetti P., Rossolini A., Barberi A., Cusi G., Musmanno R. A., Russi M., Quaranta S. Cytotoxin production by Campylobacter pylori strains isolated from patients with peptic ulcers and from patients with chronic gastritis only. J Clin Microbiol. 1989 Jan;27(1):225–226. doi: 10.1128/jcm.27.1.225-226.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leunk R. D., Ferguson M. A., Morgan D. R., Low D. E., Simor A. E. Antibody to cytotoxin in infection by Helicobacter pylori. J Clin Microbiol. 1990 Jun;28(6):1181–1184. doi: 10.1128/jcm.28.6.1181-1184.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leunk R. D., Johnson P. T., David B. C., Kraft W. G., Morgan D. R. Cytotoxic activity in broth-culture filtrates of Campylobacter pylori. J Med Microbiol. 1988 Jun;26(2):93–99. doi: 10.1099/00222615-26-2-93. [DOI] [PubMed] [Google Scholar]
- Marshall B. J., Langton S. R. Urea hydrolysis in patients with Campylobacter pyloridis infection. Lancet. 1986 Apr 26;1(8487):965–966. doi: 10.1016/s0140-6736(86)91060-3. [DOI] [PubMed] [Google Scholar]
- Mobley H. L., Cortesia M. J., Rosenthal L. E., Jones B. D. Characterization of urease from Campylobacter pylori. J Clin Microbiol. 1988 May;26(5):831–836. doi: 10.1128/jcm.26.5.831-836.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobley H. L., Hausinger R. P. Microbial ureases: significance, regulation, and molecular characterization. Microbiol Rev. 1989 Mar;53(1):85–108. doi: 10.1128/mr.53.1.85-108.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montefiori D. C., Robinson W. E., Jr, Schuffman S. S., Mitchell W. M. Evaluation of antiviral drugs and neutralizing antibodies to human immunodeficiency virus by a rapid and sensitive microtiter infection assay. J Clin Microbiol. 1988 Feb;26(2):231–235. doi: 10.1128/jcm.26.2.231-235.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan W. S., Fernando J., Alousi M. A. Studies of the biological activity of neutral red. Exp Mol Pathol. 1966 Oct;5(5):491–503. doi: 10.1016/0014-4800(66)90028-1. [DOI] [PubMed] [Google Scholar]
- Ohkuma S., Poole B. Cytoplasmic vacuolation of mouse peritoneal macrophages and the uptake into lysosomes of weakly basic substances. J Cell Biol. 1981 Sep;90(3):656–664. doi: 10.1083/jcb.90.3.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio C. A., Kato Y. Classification of vacuolated cells in the gastric mucosa. J Surg Oncol. 1987 Feb;34(2):128–132. doi: 10.1002/jso.2930340212. [DOI] [PubMed] [Google Scholar]
- Seglen P. O., Reith A. Ammonia inhibition of protein degradation in isolated rat hepatocytes. Quantitative ultrastructural alterations in the lysosomal system. Exp Cell Res. 1976 Jul;100(2):276–280. doi: 10.1016/0014-4827(76)90148-8. [DOI] [PubMed] [Google Scholar]
- Shau H., Dawson J. R. The role of the lysosome in natural killing: inhibition by lysosomotropic vital dyes. Immunology. 1984 Dec;53(4):745–751. [PMC free article] [PubMed] [Google Scholar]
- Smoot D. T., Mobley H. L., Chippendale G. R., Lewison J. F., Resau J. H. Helicobacter pylori urease activity is toxic to human gastric epithelial cells. Infect Immun. 1990 Jun;58(6):1992–1994. doi: 10.1128/iai.58.6.1992-1994.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tricottet V., Bruneval P., Vire O., Camilleri J. P., Bloch F., Bonte N., Roge J. Campylobacter-like organisms and surface epithelium abnormalities in active, chronic gastritis in humans: an ultrastructural study. Ultrastruct Pathol. 1986;10(2):113–122. doi: 10.3109/01913128609014587. [DOI] [PubMed] [Google Scholar]
- Xu J. K., Goodwin C. S., Cooper M., Robinson J. Intracellular vacuolization caused by the urease of Helicobacter pylori. J Infect Dis. 1990 Jun;161(6):1302–1304. doi: 10.1093/infdis/161.6.1302. [DOI] [PubMed] [Google Scholar]
- de Duve C., de Barsy T., Poole B., Trouet A., Tulkens P., Van Hoof F. Commentary. Lysosomotropic agents. Biochem Pharmacol. 1974 Sep 15;23(18):2495–2531. doi: 10.1016/0006-2952(74)90174-9. [DOI] [PubMed] [Google Scholar]
- van Anken H. C., Schiphorst M. E. A kinetic determination of ammonia in plasma. Clin Chim Acta. 1974 Oct 30;56(2):151–157. doi: 10.1016/0009-8981(74)90223-x. [DOI] [PubMed] [Google Scholar]



