Abstract
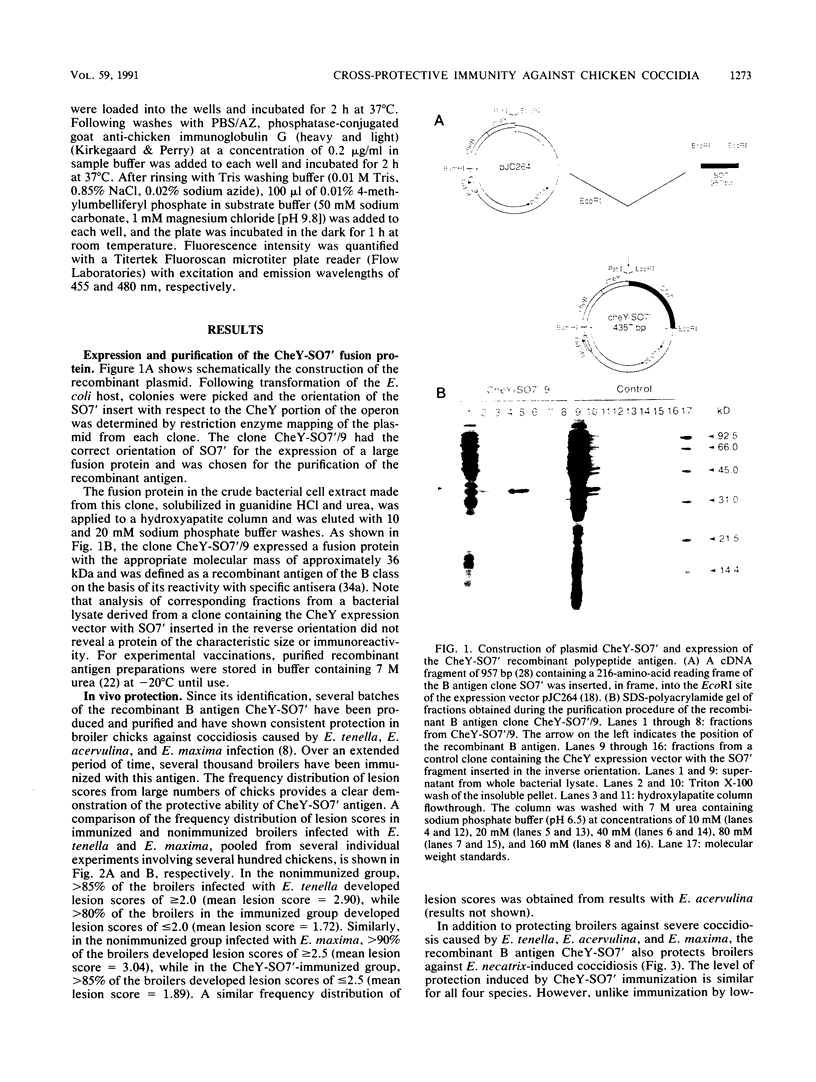
A cDNA clone, SO7', from an Eimeria tenella cDNA library was inserted into the high-expression vector pJC264 and was expressed in Escherichia coli as a fusion protein, CheY-SO7', with a molecular mass of approximately 36 kDa. By using the purified recombinant antigen to immunize young chicks, it was demonstrated that a single dose, without adjuvant, not only protected against severe coccidiosis induced by infection with E. tenella but also protected chicks challenged with the heterologous species Eimeria acervulina, E. maxima, and E. necatrix. By using rabbit antiserum raised against recombinant CheY-SO7', Western blot (immunoblot) analysis of sporulated oocysts of all seven major species of chicken coccidia showed that all species tested contained proteins characteristic of the B class of antigens, of which CheY-SO7' is representative. It seems likely that a single B antigen could protect chickens against severe coccidiosis caused by infection with any of these Eimeria species. Although chicks exposed to prolonged, natural infection develop antibodies to B antigen, active immunization of young chicks with a protective dose of CheY-SO7' does not elicit a humoral antibody response, suggesting that the partial protection results from cell-mediated effector mechanisms. In addition, the cross-protective nature of the immunity indicates that the response to B antigen is different from that induced by natural infection, which elicits a species-specific immunity. To date, the protection induced by B antigen immunization, although remarkable for a single recombinant protein, is not sufficient to compete with prophylactic chemotherapy.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blake M. S., Johnston K. H., Russell-Jones G. J., Gotschlich E. C. A rapid, sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on Western blots. Anal Biochem. 1984 Jan;136(1):175–179. doi: 10.1016/0003-2697(84)90320-8. [DOI] [PubMed] [Google Scholar]
- Brothers V. M., Kuhn I., Paul L. S., Gabe J. D., Andrews W. H., Sias S. R., McCaman M. T., Dragon E. A., Files J. G. Characterization of a surface antigen of Eimeria tenella sporozoites and synthesis from a cloned cDNA in Escherichia coli. Mol Biochem Parasitol. 1988 Apr;28(3):235–247. doi: 10.1016/0166-6851(88)90008-4. [DOI] [PubMed] [Google Scholar]
- Crane M. S., Gnozzio M. J., Murray P. K. Eimeria tenella (Eucoccidiorida): a quantitative assay for sporozoite infectivity in vivo. J Protozool. 1986 Feb;33(1):94–98. doi: 10.1111/j.1550-7408.1986.tb05566.x. [DOI] [PubMed] [Google Scholar]
- Crane M. S., Murray P. K., Gnozzio M. J., MacDonald T. T. Passive protection of chickens against Eimeria tenella infection by monoclonal antibody. Infect Immun. 1988 Apr;56(4):972–976. doi: 10.1128/iai.56.4.972-976.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane M. S., Norman D. J., Gnozzio M. J., Tate A. C., Gammon M., Murray P. K. Eimeria tenella: quantitative in vitro and in vivo studies on the effects of mouse polyclonal and monoclonal antibodies on sporozoites. Parasite Immunol. 1986 Sep;8(5):467–480. doi: 10.1111/j.1365-3024.1986.tb00862.x. [DOI] [PubMed] [Google Scholar]
- Danforth H. D., Augustine P. C., Ruff M. D., McCandliss R., Strausberg R. L., Likel M. Genetically engineered antigen confers partial protection against avian coccidial parasites. Poult Sci. 1989 Dec;68(12):1643–1652. doi: 10.3382/ps.0681643. [DOI] [PubMed] [Google Scholar]
- Danforth H. D., Augustine P. C. Use of hybridoma antibodies and recombinant DNA technology in protozoan vaccine development. Avian Dis. 1986 Jan-Mar;30(1):37–42. [PubMed] [Google Scholar]
- Engvall E., Perlmann P. Enzyme-linked immunosorbent assay (ELISA). Quantitative assay of immunoglobulin G. Immunochemistry. 1971 Sep;8(9):871–874. doi: 10.1016/0019-2791(71)90454-x. [DOI] [PubMed] [Google Scholar]
- Gan Z. R., Condra J. H., Gould R. J., Zivin R. A., Bennett C. D., Jacobs J. W., Friedman P. A., Polokoff M. A. High-level expression in Escherichia coli of a chemically synthesized gene for [Leu-28]echistatin. Gene. 1989 Jun 30;79(1):159–166. doi: 10.1016/0378-1119(89)90101-7. [DOI] [PubMed] [Google Scholar]
- Johnson J., Reid W. M. Anticoccidial drugs: lesion scoring techniques in battery and floor-pen experiments with chickens. Exp Parasitol. 1970 Aug;28(1):30–36. doi: 10.1016/0014-4894(70)90063-9. [DOI] [PubMed] [Google Scholar]
- Johnson J., Reid W. M., Jeffers T. K. Practical immunization of chickens against coccidiosis using an attenuated strain of Eimeria tenella. Poult Sci. 1979 Jan;58(1):37–41. doi: 10.3382/ps.0580037. [DOI] [PubMed] [Google Scholar]
- Karkhanis Y. D., Nollstadt K. A., Bhogal B. S., Ravino O., Pellegrino R., Crane M. S., Murray P. K., Turner M. J. Purification and characterization of a protective antigen from Eimeria tenella. Infect Immun. 1991 Mar;59(3):983–989. doi: 10.1128/iai.59.3.983-989.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C. S., Bowers J. A. Thiamin content of chicken muscle and effect of purification variables on determined values. Poult Sci. 1988 Jan;67(1):72–77. doi: 10.3382/ps.0670072. [DOI] [PubMed] [Google Scholar]
- Kim K. S., Jenkins M. C., Lillehoj H. S. Immunization of chickens with live Escherichia coli expressing Eimeria acervulina merozoite recombinant antigen induces partial protection against coccidiosis. Infect Immun. 1989 Aug;57(8):2434–2440. doi: 10.1128/iai.57.8.2434-2440.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee E. H. Vaccination against coccidiosis in commercial roaster chickens. Can Vet J. 1987 Jul;28(7):434–436. [PMC free article] [PubMed] [Google Scholar]
- Liberator P. A., Hsu J., Turner M. J. Tandem trinucleotide repeats throughout the nucleotide sequence of a cDNA encoding an Eimeria tenella sporozoite antigen. Nucleic Acids Res. 1989 Sep 12;17(17):7104–7104. doi: 10.1093/nar/17.17.7104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long P. L., Jeffers T. K. Control of chicken coccidiosis. Parasitol Today. 1986 Sep;2(9):236–240. doi: 10.1016/0169-4758(86)90002-5. [DOI] [PubMed] [Google Scholar]
- Miller G. A., Bhogal B. S., McCandliss R., Strausberg R. L., Jessee E. J., Anderson A. C., Fuchs C. K., Nagle J., Likel M. H., Strasser J. M. Characterization and vaccine potential of a novel recombinant coccidial antigen. Infect Immun. 1989 Jul;57(7):2014–2020. doi: 10.1128/iai.57.7.2014-2020.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nollstadt K. H., Karkhanis Y. D., Gnozzio M. J., Crane M. S., Gurnett A. M., Schmatz D. M., Turner M. J. Potential of the sulfobetaine detergent Zwittergent 3-12 as a desorbing agent in biospecific and bioselective affinity chromatography. J Chromatogr. 1989 Dec 29;497:87–100. doi: 10.1016/0378-4347(89)80008-8. [DOI] [PubMed] [Google Scholar]
- Profous-Juchelka H., Liberator P., Turner M. Identification and characterization of cDNA clones encoding antigens of Eimeria tenella. Mol Biochem Parasitol. 1988 Sep;30(3):233–241. doi: 10.1016/0166-6851(88)90092-8. [DOI] [PubMed] [Google Scholar]
- Rose M. E. Immunity to coccidiosis: protective effect of transferred serum in Eimeria maxima infections. Parasitology. 1971 Feb;62(1):11–25. doi: 10.1017/s0031182000071249. [DOI] [PubMed] [Google Scholar]
- Rubin R. W., Warren R. W. Quantitation of microgram amounts of protein in SDS-mercaptoethanol-tris electrophoresis sample buffer. Anal Biochem. 1977 Dec;83(2):773–777. doi: 10.1016/0003-2697(77)90084-7. [DOI] [PubMed] [Google Scholar]
- Shirley M. W. Development of a live attenuated vaccine against coccidiosis of poultry. Parasite Immunol. 1989 Mar;11(2):117–124. doi: 10.1111/j.1365-3024.1989.tb00653.x. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallach M., Pillemer G., Yarus S., Halabi A., Pugatsch T., Mencher D. Passive immunization of chickens against Eimeria maxima infection with a monoclonal antibody developed against a gametocyte antigen. Infect Immun. 1990 Feb;58(2):557–562. doi: 10.1128/iai.58.2.557-562.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweerink H. J., Gammon M. C., Hutchison C. F., Jackson J. J., Lombardo D., Miner K. M., Puckett J. M., Sewell T. J., Sigal N. H. Human monoclonal antibodies that protect mice against challenge with Pseudomonas aeruginosa. Infect Immun. 1988 Aug;56(8):1873–1879. doi: 10.1128/iai.56.8.1873-1879.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]