Abstract
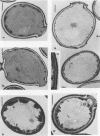
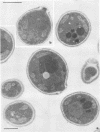
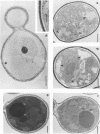
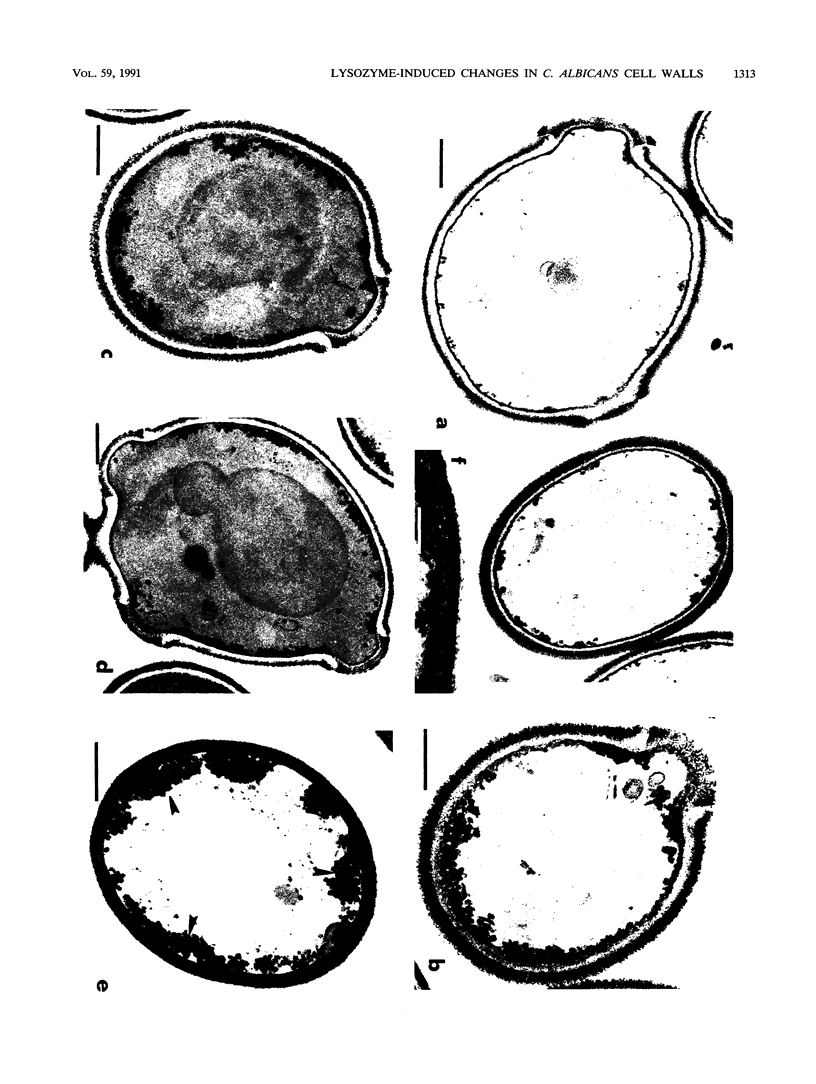
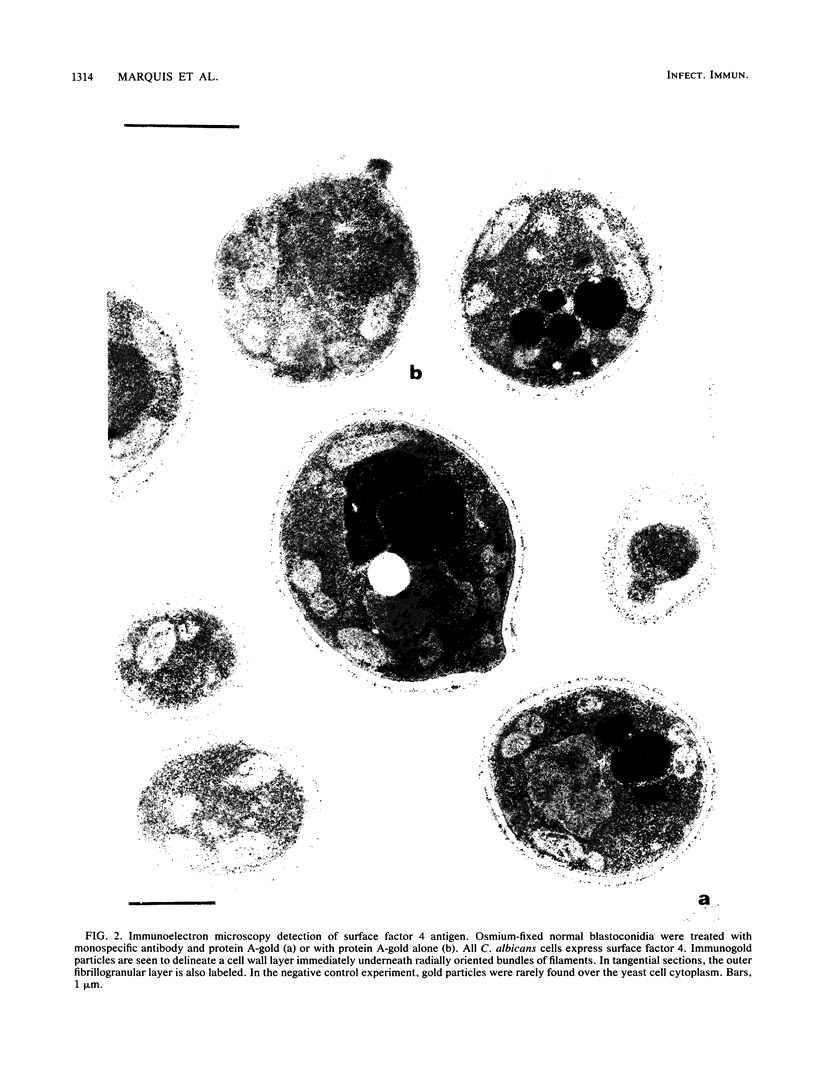
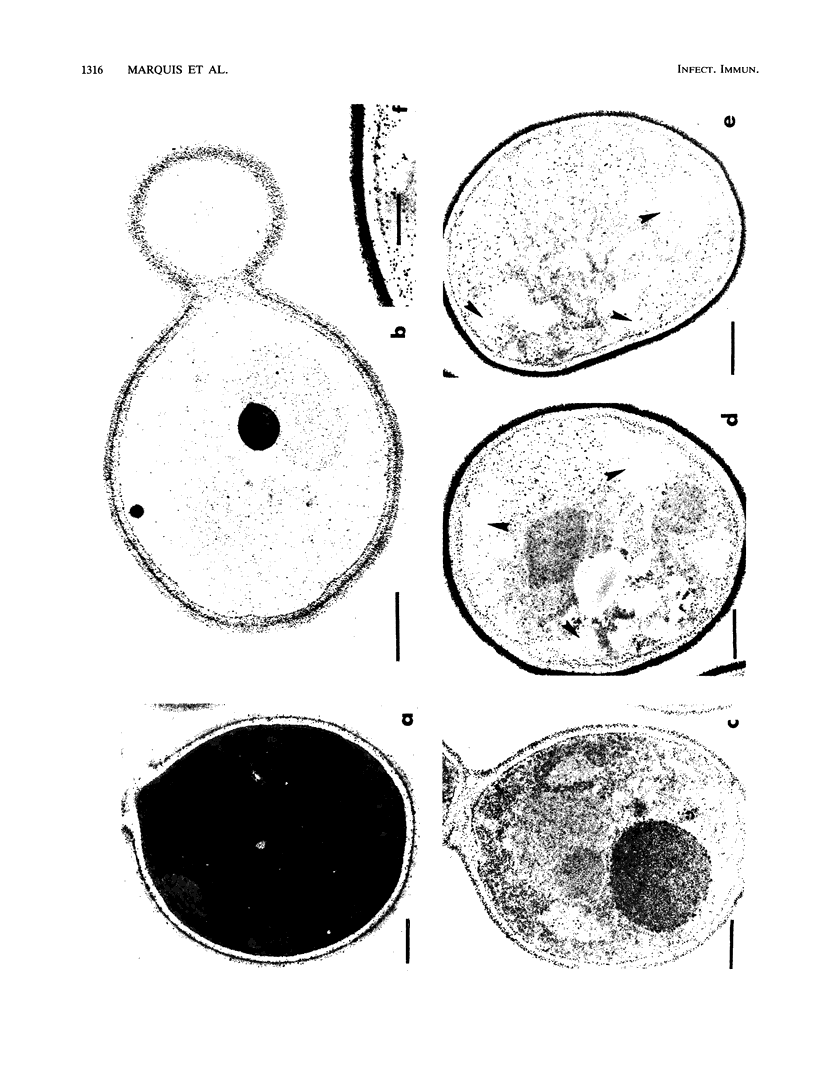
The fungicidal effect of lysozyme on Candida albicans involves ultrastructural modifications previously described (G. Marquis, S. Montplaisir, S. Garzon, H. Strykowski, and P. Auger, Lab. Invest. 46:627-636, 1982). To further define the action of lysozyme on the yeast cell wall, we used the following: (i) the periodic acid-thiocarbohydrazide-silver proteinate (PA-TCH-SP) method to highlight vicinal-glycol-reactive sites of complex carbohydrates; (ii) a monospecific antiserum and a protein A-gold complex to study the expression of surface factor 4, a major Candida antigen; and (iii) the periodic acid-silver methenamine method to stain cell wall glycoproteins. All Candida cells were found to express surface factor 4 antigen. In normal blastoconidia, surface factor 4 was located in a glycoprotein-rich cell wall layer, underneath radially oriented bundles of filaments which form the outermost wall layer. In lysozyme-treated blastoconidia, this glycoprotein-rich layer was lost and the regular brushlike organization of the outer fibrillogranular layer was disrupted. PA-TCH-SP staining and localization of surface factor 4 antigen demonstrated an altered arrangement of bundles of filaments in the outer wall layers of blastoconidia which were morphologically intact but had abnormal cell wall appearance. Next, there was a reduction in thickness of the outer layer and the expression of surface factor 4 antigen was limited to the cytoplasmic membrane area. Later on, the cell wall was almost uniformly highlighted by PA-TCH-SP staining. These data evinced a highly plastic architecture of the cell wall in C. albicans.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auger P., Marquis G., Dallaire L. Viability assessment by dye exclusion. A fluorescent method for fungal cells. Arch Dermatol. 1979 Oct;115(10):1195–1196. [PubMed] [Google Scholar]
- Cassone A., Kerridge D., Gale E. F. Ultrastructural changes in the cell wall of Candida albicans following cessation of growth and their possible relationship to the development of polyene resistance. J Gen Microbiol. 1979 Feb;110(2):339–349. doi: 10.1099/00221287-110-2-339. [DOI] [PubMed] [Google Scholar]
- Chaffin W. L., Ringler L., Larsen H. S. Interactions of monospecific antisera with cell surface determinants of Candida albicans. Infect Immun. 1988 Dec;56(12):3294–3296. doi: 10.1128/iai.56.12.3294-3296.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garzon S., Marquis G., Montplaisir S., Kurstak E., Benhamou N. Antigenic structure of Candida albicans. Electronmicroscopic localization of polysaccharide and immunodeterminants in the cell wall. Immunol Ser. 1989;47:3–36. [PubMed] [Google Scholar]
- Kamaya T. Lytic action of lysozyme on Candida albicans. Mycopathol Mycol Appl. 1970 Dec 29;42(3):197–207. doi: 10.1007/BF02051947. [DOI] [PubMed] [Google Scholar]
- Marquis G., Montplaisir S., Garzon S., Strykowski H., Auger P. Fungitoxicity of muramidase. Ultrastructural damage to Candida albicans. Lab Invest. 1982 Jun;46(6):627–636. [PubMed] [Google Scholar]
- McCourtie J., Douglas L. J. Relationship between cell surface composition of Candida albicans and adherence to acrylic after growth on different carbon sources. Infect Immun. 1981 Jun;32(3):1234–1241. doi: 10.1128/iai.32.3.1234-1241.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister H., Heymer B., Schäfer H., Haferkamp O. Role of Candida albicans in granulomatous tissue reactions. I. In vitro degradation of C. albicans and immunospecificity of split products. J Infect Dis. 1977 Feb;135(2):224–234. doi: 10.1093/infdis/135.2.224. [DOI] [PubMed] [Google Scholar]
- Mleczko J., Litke L. L., Larsen H. S., Chaffin W. L. Effect of glutaraldehyde fixation on cell surface binding capacity of Candida albicans. Infect Immun. 1989 Oct;57(10):3247–3249. doi: 10.1128/iai.57.10.3247-3249.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulain D., Tronchin G., Dubremetz J. F., Biguet J. Ultrastructure of the cell wall of Candida albicans blastospores: study of its constitutive layers by the use of a cytochemical technique revealing polysaccharides. Ann Microbiol (Paris) 1978 Feb-Mar;129(2):141–153. [PubMed] [Google Scholar]
- Poulain D., Tronchin G., Jouvert S., Herbaut J., Biguet J. Architecture pariétale des blastospores de Candida albicans: localisation de composants chimiques et antigéniques. Ann Microbiol (Paris) 1981 May-Jun;132(3):219–238. [PubMed] [Google Scholar]
- Rambourg A. An improved silver methenamine technique for the detection of periodic acid-reactive complex carbohydrates with the electron microscope. J Histochem Cytochem. 1967 Jul;15(7):409–412. doi: 10.1177/15.7.409. [DOI] [PubMed] [Google Scholar]
- Shinoda T., Kaufman L., Padhye A. A. Comparative evaluation of the Iatron serological Candida check kit and the API 20C kit for identification of medically important Candida species. J Clin Microbiol. 1981 Mar;13(3):513–518. doi: 10.1128/jcm.13.3.513-518.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya T., Fukazawa Y., Taguchi M., Nakase T., Shinoda T. Serologic aspects on yeast classification. Mycopathol Mycol Appl. 1974 Aug 30;53(1):77–91. doi: 10.1007/BF02127199. [DOI] [PubMed] [Google Scholar]