Abstract
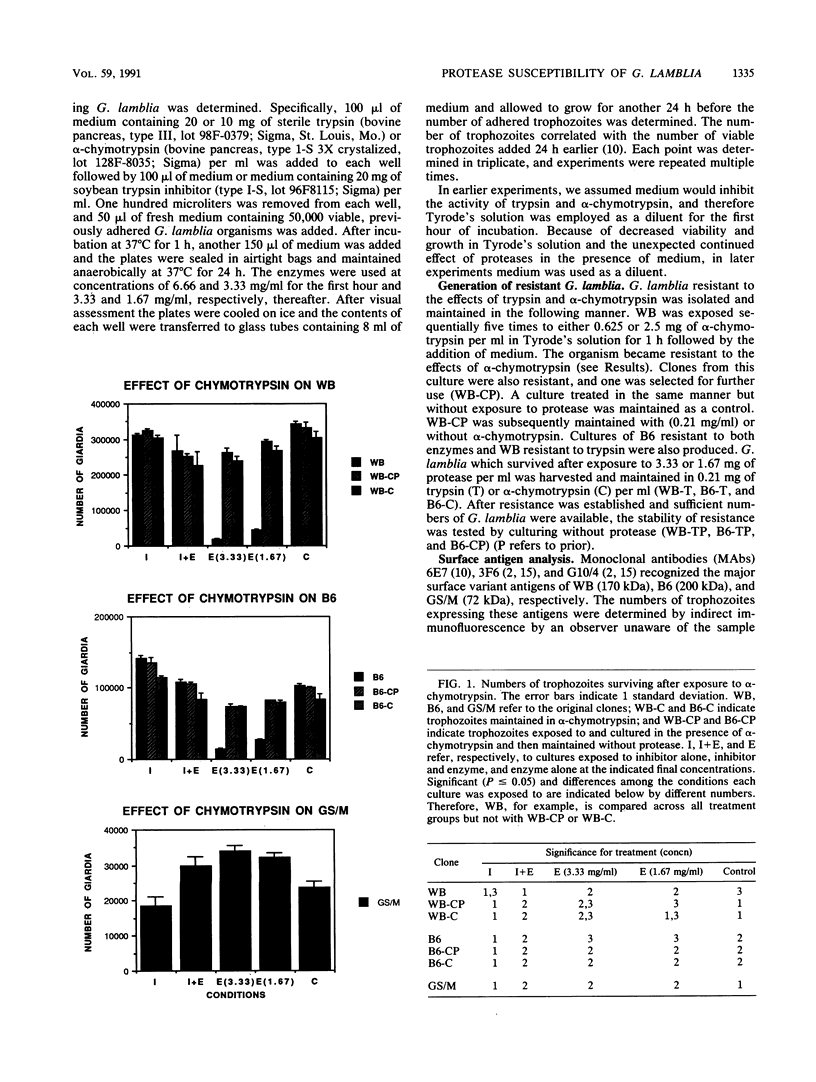
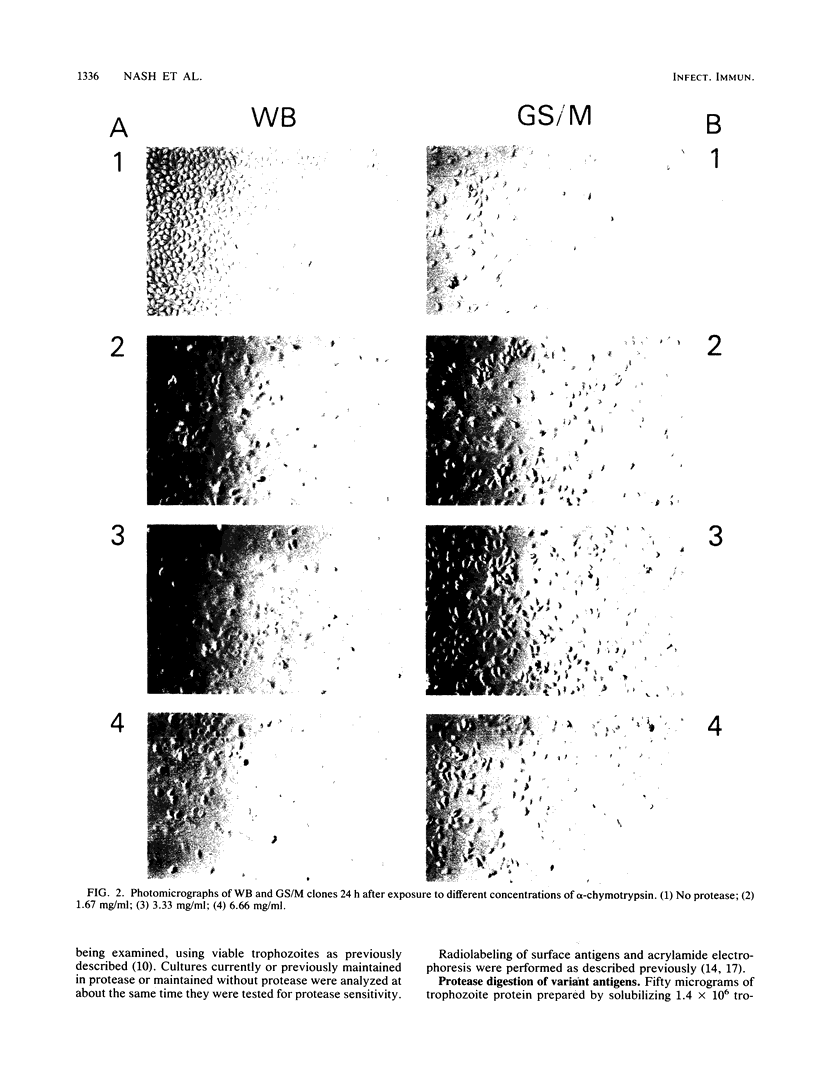
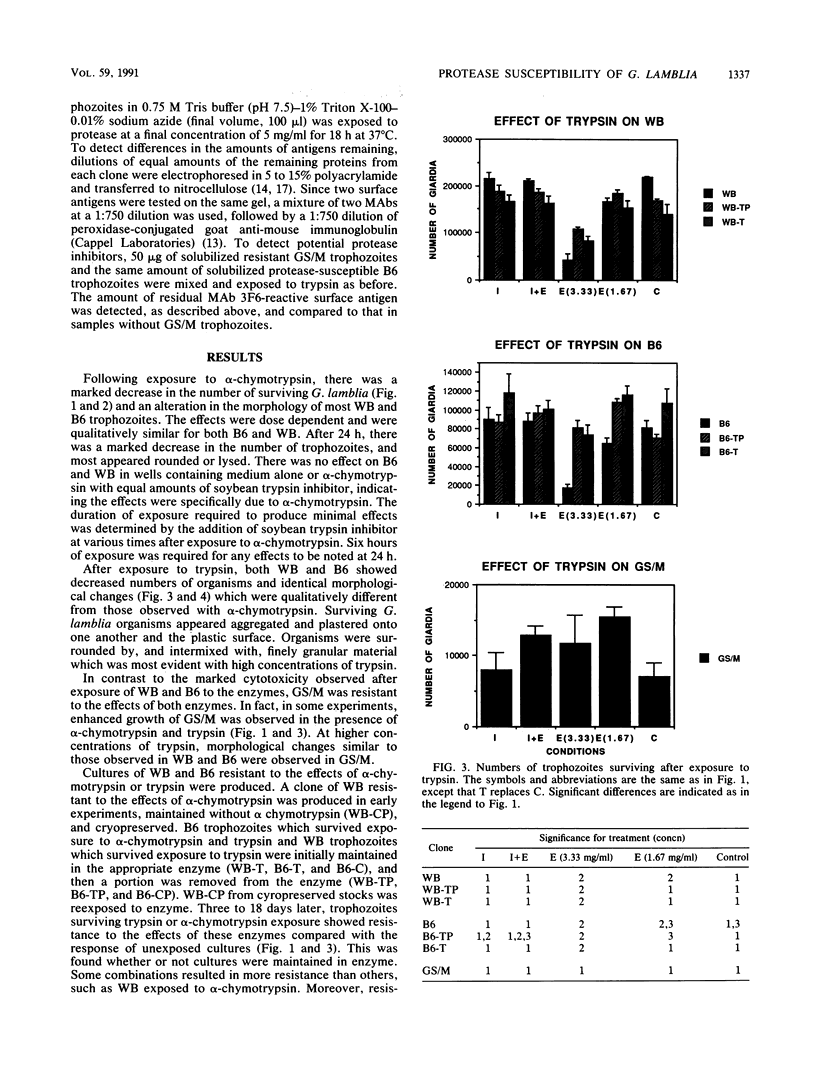
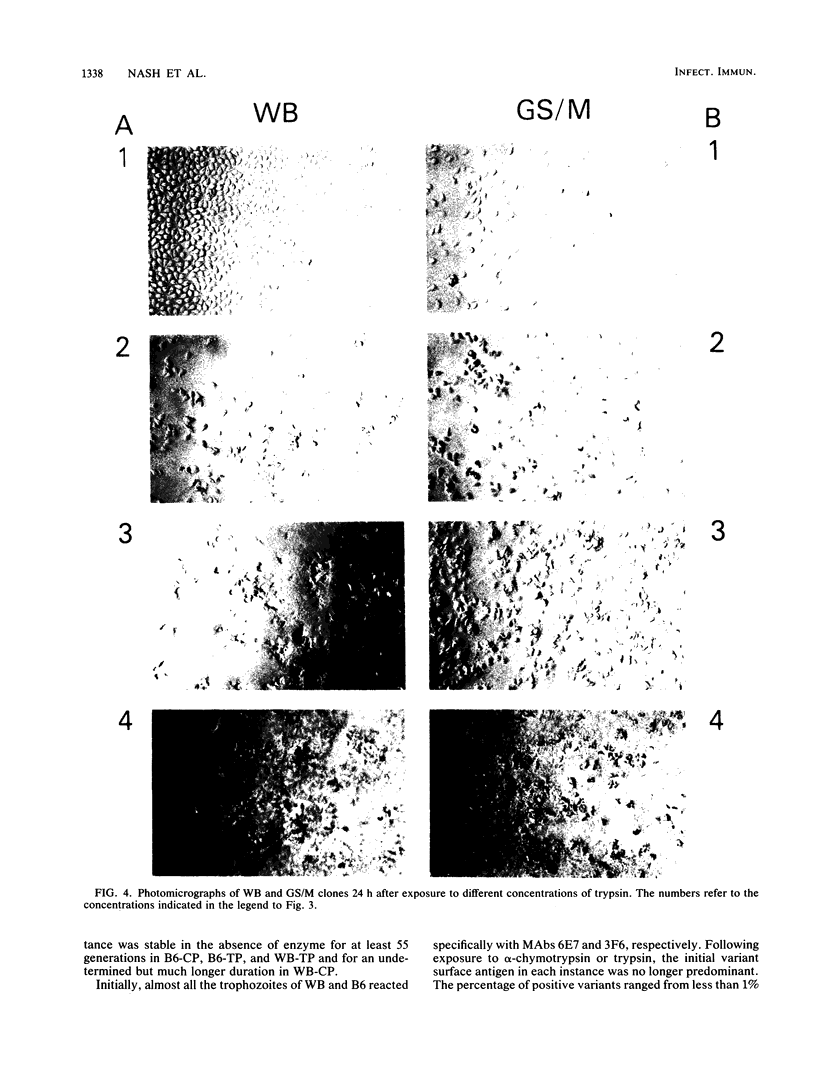
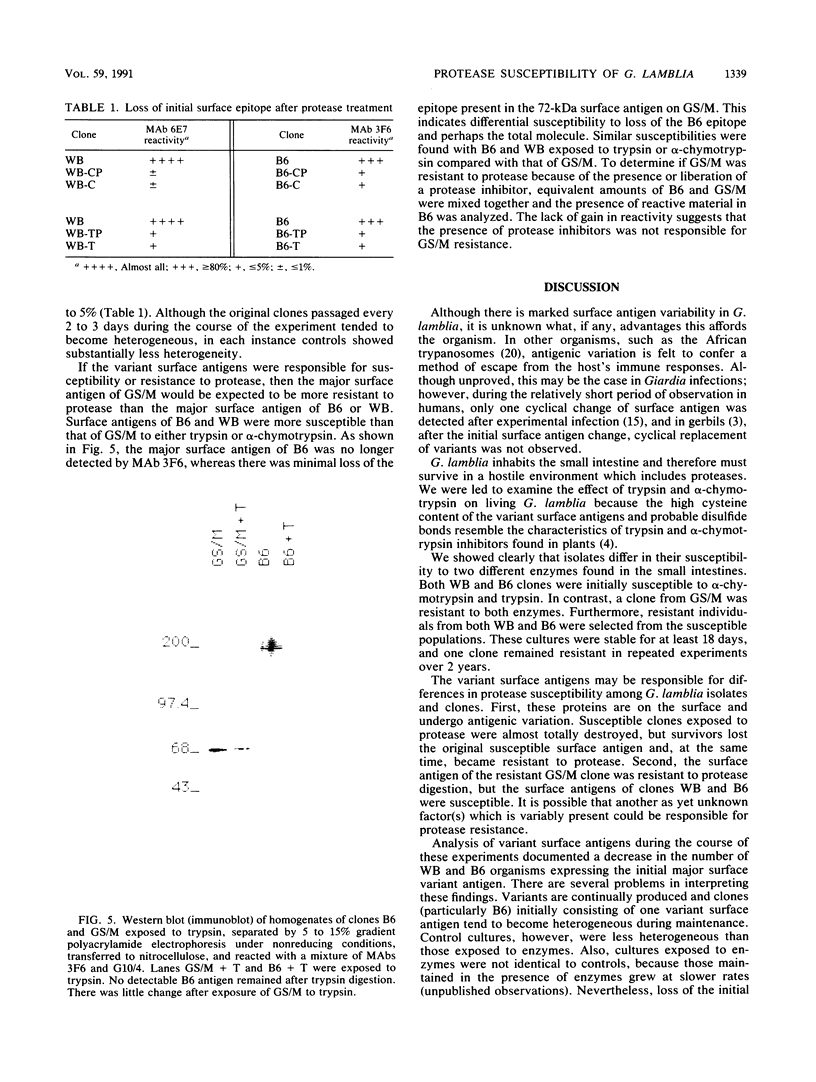
The surface antigens of Giardia lamblia differ. To determine whether the unique surface antigens found in variants and isolates could differentially protect the parasite from digestion by intestinal protease, G. lamblia clones WB-2X (WB), GS/M-H7 (GS/M), and B6, each of which expresses a unique surface variant antigen, were exposed to alpha-chymotrypsin and trypsin at concentrations up to 20 mg/ml in culture medium. The number of surviving trophozoites and morphologic changes were assessed over time. After 24 h, there was a significant decrease in the number of surviving trophozoites of WB (80.5 and 94.2% for trypsin and alpha-chymotrypsin treatments, respectively, compared with controls) and B6 (78.9 and 95.5% for trypsin and alpha-chymotrypsin treatments, respectively, compared with controls) at 10 mg of enzyme per ml compared with culture medium alone. Cytotoxicity was prevented by the presence of soybean trypsin inhibitor, indicating the effects were due to protease activity. In contrast, there was no significant cytotoxicity after exposure of GS/M to either enzyme at the same enzyme concentration. After exposure to alpha-chymotrypsin, susceptible G. lamblia became rounded and then lysed, but after exposure to trypsin, G. lamblia appeared plastered onto the surface of the well and was intertwined and surrounded by finely granular material. Effects were concentration and time dependent; at least 6 h of treatment was required to observe changes 12 to 18 h later. Trophozoites surviving alpha-chymotrypsin or trypsin exposure became stably resistant to protease treatment. In vitro, the variant surface antigen of GS/M, but not those of WB or B6, resisted digestion by trypsin or alpha-chymotrypsin, suggesting that the variant surface antigens impart susceptibility or resistance to digestion. The initial surface variant antigens of WB and B6 were replaced in resistant cultures. Trophozoites differ in their ability to survive after exposure to intestinal proteases, which may enable certain G. lamblia isolates or isolates possessing certain surface variant antigens to survive in the small intestine.
Full text
PDF

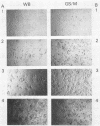
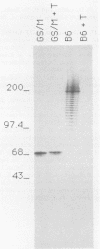
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam R. D., Aggarwal A., Lal A. A., de La Cruz V. F., McCutchan T., Nash T. E. Antigenic variation of a cysteine-rich protein in Giardia lamblia. J Exp Med. 1988 Jan 1;167(1):109–118. doi: 10.1084/jem.167.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggarwal A., Merritt J. W., Jr, Nash T. E. Cysteine-rich variant surface proteins of Giardia lamblia. Mol Biochem Parasitol. 1989 Jan 1;32(1):39–47. doi: 10.1016/0166-6851(89)90127-8. [DOI] [PubMed] [Google Scholar]
- Aggarwal A., Nash T. E. Antigenic variation of Giardia lamblia in vivo. Infect Immun. 1988 Jun;56(6):1420–1423. doi: 10.1128/iai.56.6.1420-1423.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birk Y. Trypsin and chymotrypsin inhibitors from soybeans. Methods Enzymol. 1976;45:700–707. doi: 10.1016/s0076-6879(76)45061-9. [DOI] [PubMed] [Google Scholar]
- Farthing M. J., Pereira M. E., Keusch G. T. Description and characterization of a surface lectin from Giardia lamblia. Infect Immun. 1986 Feb;51(2):661–667. doi: 10.1128/iai.51.2.661-667.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottstein B., Harriman G. R., Conrad J. T., Nash T. E. Antigenic variation in Giardia lamblia: cellular and humoral immune response in a mouse model. Parasite Immunol. 1990 Nov;12(6):659–673. doi: 10.1111/j.1365-3024.1990.tb00995.x. [DOI] [PubMed] [Google Scholar]
- Inge P. M., Edson C. M., Farthing M. J. Attachment of Giardia lamblia to rat intestinal epithelial cells. Gut. 1988 Jun;29(6):795–801. doi: 10.1136/gut.29.6.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keister D. B. Axenic culture of Giardia lamblia in TYI-S-33 medium supplemented with bile. Trans R Soc Trop Med Hyg. 1983;77(4):487–488. doi: 10.1016/0035-9203(83)90120-7. [DOI] [PubMed] [Google Scholar]
- Lev B., Ward H., Keusch G. T., Pereira M. E. Lectin activation in Giardia lamblia by host protease: a novel host-parasite interaction. Science. 1986 Apr 4;232(4746):71–73. doi: 10.1126/science.3513312. [DOI] [PubMed] [Google Scholar]
- Nash T. E., Aggarwal A., Adam R. D., Conrad J. T., Merritt J. W., Jr Antigenic variation in Giardia lamblia. J Immunol. 1988 Jul 15;141(2):636–641. [PubMed] [Google Scholar]
- Nash T. E., Aggarwal A. Cytotoxicity of monoclonal antibodies to a subset of Giardia isolates. J Immunol. 1986 Apr 1;136(7):2628–2632. [PubMed] [Google Scholar]
- Nash T. E., Conrad J. T., Merritt J. W., Jr Variant specific epitopes of Giardia lamblia. Mol Biochem Parasitol. 1990 Aug;42(1):125–132. doi: 10.1016/0166-6851(90)90120-b. [DOI] [PubMed] [Google Scholar]
- Nash T. E., Gillin F. D., Smith P. D. Excretory-secretory products of Giardia lamblia. J Immunol. 1983 Oct;131(4):2004–2010. [PubMed] [Google Scholar]
- Nash T. E., Herrington D. A., Levine M. M., Conrad J. T., Merritt J. W., Jr Antigenic variation of Giardia lamblia in experimental human infections. J Immunol. 1990 Jun 1;144(11):4362–4369. [PubMed] [Google Scholar]
- Nash T. E., Herrington D. A., Losonsky G. A., Levine M. M. Experimental human infections with Giardia lamblia. J Infect Dis. 1987 Dec;156(6):974–984. doi: 10.1093/infdis/156.6.974. [DOI] [PubMed] [Google Scholar]
- Nash T. E., Keister D. B. Differences in excretory-secretory products and surface antigens among 19 isolates of Giardia. J Infect Dis. 1985 Dec;152(6):1166–1171. doi: 10.1093/infdis/152.6.1166. [DOI] [PubMed] [Google Scholar]
- Nash T. E., McCutchan T., Keister D., Dame J. B., Conrad J. D., Gillin F. D. Restriction-endonuclease analysis of DNA from 15 Giardia isolates obtained from humans and animals. J Infect Dis. 1985 Jul;152(1):64–73. doi: 10.1093/infdis/152.1.64. [DOI] [PubMed] [Google Scholar]
- Smith P. D., Gillin F. D., Spira W. M., Nash T. E. Chronic giardiasis: studies on drug sensitivity, toxin production, and host immune response. Gastroenterology. 1982 Oct;83(4):797–803. [PubMed] [Google Scholar]
- Ward H. D., Lev B. I., Kane A. V., Keusch G. T., Pereira M. E. Identification and characterization of taglin, a mannose 6-phosphate binding, trypsin-activated lectin from Giardia lamblia. Biochemistry. 1987 Dec 29;26(26):8669–8675. doi: 10.1021/bi00400a027. [DOI] [PubMed] [Google Scholar]