Abstract
In the companion paper (L. A. Borenstein, M. E. Selsted, R. I. Lehrer, and J. N. Miller, Infect. Immun. 59:1359-1367, 1991), we report that rabbit alveolar macrophage and neutrophil derived defensins possess antimicrobial activity against Treponema pallidum subsp. pallidum, the etiologic agent of syphilis. In this study, antisera specific for NP-1 and NP-2 (defensins present in certain macrophages and polymorphonuclear leukocytes) and NP-5 (a defensin produced only in neutrophils) were used to detect these peptides by immunoperoxidase staining in testicular lesions from infected rabbits. Profound amounts of cell-free and cell-associated defensins were detected in the tunica albuginea and interstitial spaces during the first 24 h of infection. The presence of defensins was transient and almost undetectable by day 4. Interstitial defensins were detected again at day 10 and increased through day 16, at which time lesion healing was evident by hematoxylin and eosin staining. The appearance and increase in detectable defensins between days 10 and 16 of infection correlated with a reduction in numbers and disappearance of T. pallidum, as demonstrated by using silver staining. The extent and pattern of immunostaining for NP-1 and NP-2 corresponded with immunostaining for NP-5 and identified neutrophils as the cellular source of the defensins. These findings indicate that defensins may contribute to the control of local T. pallidum infection and suggest a role for acute inflammatory processes in the resolution of early experimental syphilis.
Full text
PDF



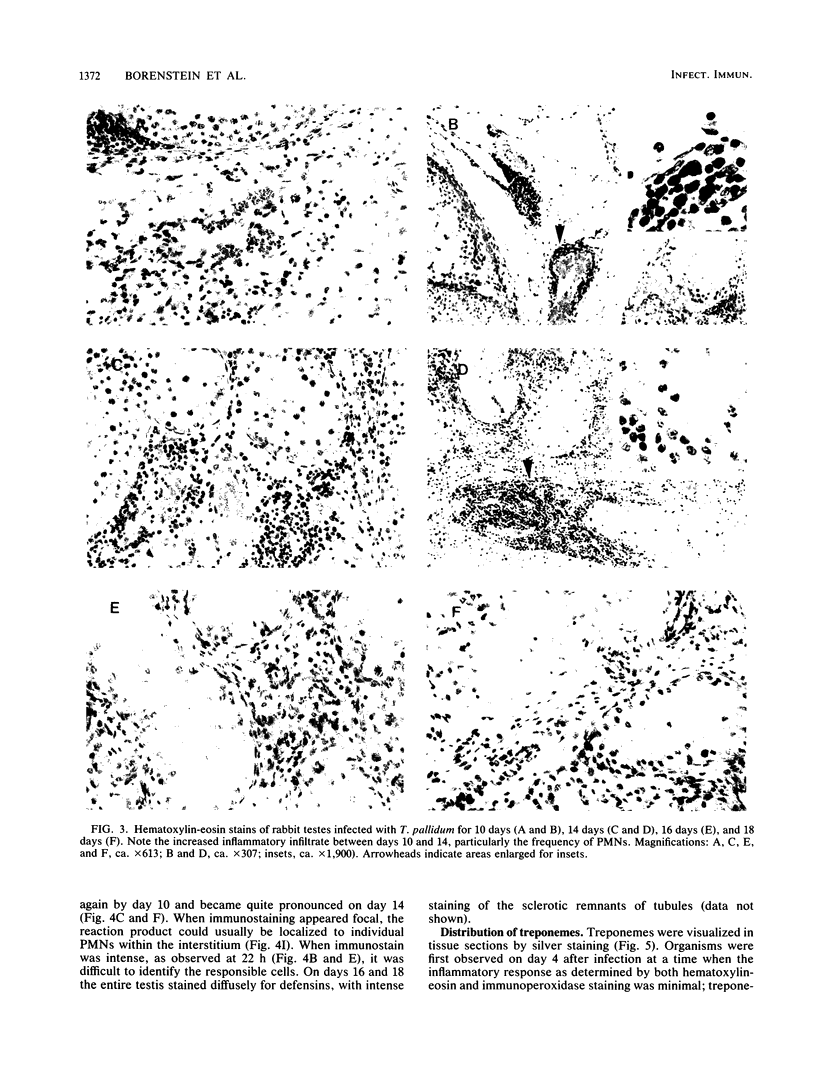





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker-Zander S. A., Fohn M. J., Lukehart S. A. Development of cellular immunity to individual soluble antigens of Treponema pallidum during experimental syphilis. J Immunol. 1988 Dec 15;141(12):4363–4369. [PubMed] [Google Scholar]
- Baughn R. E., Adams C. B., Musher D. M. Circulating immune complexes in experimental syphilis: identification of treponemal antigens and specific antibodies to treponemal antigens in isolated complexes. Infect Immun. 1983 Nov;42(2):585–593. doi: 10.1128/iai.42.2.585-593.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baughn R. E., Tung K. S., Musher D. M. Detection of circulating immune complexes in the sera of rabbits with experimental syphilis: possible role in immunoregulation. Infect Immun. 1980 Aug;29(2):575–582. doi: 10.1128/iai.29.2.575-582.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop N. H., Miller J. N. Humoral immunity in experimental syphilis. II. The relationship of neutralizing factors in immune serum to acquired resistance. J Immunol. 1976 Jul;117(1):197–207. [PubMed] [Google Scholar]
- Borenstein L. A., Selsted M. E., Lehrer R. I., Miller J. N. Antimicrobial activity of rabbit leukocyte defensins against Treponema pallidum subsp. pallidum. Infect Immun. 1991 Apr;59(4):1359–1367. doi: 10.1128/iai.59.4.1359-1367.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockayne A., Penn C. W., Bailey M. J. Surface properties of Treponema pallidum in relation to phagocytosis by human polymorphonuclear leucocytes in vitro. J Gen Microbiol. 1986 Jan;132(1):133–141. doi: 10.1099/00221287-132-1-133. [DOI] [PubMed] [Google Scholar]
- Fleischmann J., Selsted M. E., Lehrer R. I. Opsonic activity of MCP-1 and MCP-2, cationic peptides from rabbit alveolar macrophages. Diagn Microbiol Infect Dis. 1985 May;3(3):233–242. doi: 10.1016/0732-8893(85)90035-5. [DOI] [PubMed] [Google Scholar]
- Ganz T., Sherman M. P., Selsted M. E., Lehrer R. I. Newborn rabbit alveolar macrophages are deficient in two microbicidal cationic peptides, MCP-1 and MCP-2. Am Rev Respir Dis. 1985 Oct;132(4):901–904. doi: 10.1164/arrd.1985.132.4.901. [DOI] [PubMed] [Google Scholar]
- Hanff P. A., Bishop N. H., Miller J. N., Lovett M. A. Humoral immune response in experimental syphilis to polypeptides of Treponema pallidum. J Immunol. 1983 Oct;131(4):1973–1977. [PubMed] [Google Scholar]
- Jones R. H., Nevin T. A., Guest W. J., Logan L. C. Lytic effect of trypsin, lysozyme, and complement on Treponema pallidum. Br J Vener Dis. 1968 Sep;44(3):193–200. doi: 10.1136/sti.44.3.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorizzo J. L., McNeely M. C., Baughn R. E., Cavallo T., Solomon A. R., Smith E. B. Rabbit model of disseminated syphilis: immunoblot and immunohistologic evidence for a role of specific immune complexes in lesion pathogenesis. J Cutan Pathol. 1988 Jun;15(3):150–160. doi: 10.1111/j.1600-0560.1988.tb00535.x. [DOI] [PubMed] [Google Scholar]
- Lauderdale V., Goldman J. N. Serial ultrathin sectioning demonstrating the intracellularity of T. Pallidum. An electron microscopic study. Br J Vener Dis. 1972 Apr;48(2):87–96. doi: 10.1136/sti.48.2.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan L. C. Rabbit globulin and antiglobulin factors associated with Treponema pallidum growth in rabbits. Br J Vener Dis. 1974 Dec;50(6):421–427. doi: 10.1136/sti.50.6.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukehart S. A. Activation of macrophages by products of lymphocytes from normal and syphilitic rabbits. Infect Immun. 1982 Jul;37(1):64–69. doi: 10.1128/iai.37.1.64-69.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukehart S. A., Baker-Zander S. A., Lloyd R. M., Sell S. Characterization of lymphocyte responsiveness in early experimental syphilis. II. Nature of cellular infiltration and Treponema pallidum distribution in testicular lesions. J Immunol. 1980 Jan;124(1):461–467. [PubMed] [Google Scholar]
- Lukehart S. A., Baker-Zander S. A., Lloyd R. M., Sell S. Characterization of lymphocyte responsiveness in early experimental syphilis. II. Nature of cellular infiltration and Treponema pallidum distribution in testicular lesions. J Immunol. 1980 Jan;124(1):461–467. [PubMed] [Google Scholar]
- Lukehart S. A., Baker-Zander S. A., Sell S. Characterization of the humoral immune response of the rabbit to antigens of Treponema pallidum after experimental infection and therapy. Sex Transm Dis. 1986 Jan-Mar;13(1):9–15. doi: 10.1097/00007435-198601000-00003. [DOI] [PubMed] [Google Scholar]
- Lukehart S. A., Miller J. N. Demonstration of the in vitro phagocytosis of Treponema pallidum by rabbit peritoneal macrophages. J Immunol. 1978 Nov;121(5):2014–2024. [PubMed] [Google Scholar]
- MAGNUSON H. J., THOMAS E. W., OLANSKY S., KAPLAN B. I., DE MELLO L., CUTLER J. C. Inoculation syphilis in human volunteers. Medicine (Baltimore) 1956 Feb;35(1):33–82. doi: 10.1097/00005792-195602000-00002. [DOI] [PubMed] [Google Scholar]
- MILLER J. N. THE APPEARANCE AND PERSISTENCE OF VDRL, RPCF, AND TPI ANTIBODY DURING THE COURSE AND TREATMENT OF EXPERIMENTAL SYPHILIS IN THE RABBIT. J Invest Dermatol. 1964 May;42:367–371. doi: 10.1038/jid.1964.80. [DOI] [PubMed] [Google Scholar]
- MILLER J. N., WHANG S. J., FAZZAN F. P. STUDIES ON IMMUNITY IN EXPERIMENTAL SYPHILIS. I. IMMUNOLOGIC RESPONSE OF RABBITS IMMUNIZED WITH REITER PROTEIN ANTIGEN AND CHALLENGED WITH VIRULENT TREPONEMA PALLIDUM. Br J Vener Dis. 1963 Sep;39:195–198. doi: 10.1136/sti.39.3.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger M., Michalska E. Treponema pallidum opsonophagocytic test. Arch Immunol Ther Exp (Warsz) 1974;22(6):745–758. [PubMed] [Google Scholar]
- Musher D. M., Hague-Park M., Gyorkey F., Anderson D. C., Baughn R. E. The interaction between Treponema pallidum and human polymorphonuclear leukocytes. J Infect Dis. 1983 Jan;147(1):77–86. doi: 10.1093/infdis/147.1.77. [DOI] [PubMed] [Google Scholar]
- NELSON R. A., Jr, DIESENDRUCK J. A., ZHEUTLIN H. E. C., STACK P. S., BARNETT M. Studies on treponemal immobilizing antibodies in syphilis. I. Techniques of measurement and factors influencing immobilization. J Immunol. 1951 Jun;66(6):667–685. [PubMed] [Google Scholar]
- Ovcinnikov N. M., Delektorskij V. V. Electron microscopy of phagocytosis in syphilis and yaws. Br J Vener Dis. 1972 Aug;48(4):227–248. doi: 10.1136/sti.48.4.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavia C. S., Folds J. D., Baseman J. B. Development of of macrophage migration inhibition in rabbits infected with virulent Treponema pallidum. Infect Immun. 1977 Sep;17(3):651–654. doi: 10.1128/iai.17.3.651-654.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichlin M. Use of glutaraldehyde as a coupling agent for proteins and peptides. Methods Enzymol. 1980;70(A):159–165. doi: 10.1016/s0076-6879(80)70047-2. [DOI] [PubMed] [Google Scholar]
- Sell S., Baker-Zander S., Powell H. C. Experimental syphilitic orchitis in rabbits: ultrastructural appearance of Treponema pallidum during phagocytosis and dissolution by macrophages in vivo. Lab Invest. 1982 Apr;46(4):355–364. [PubMed] [Google Scholar]
- Sell S., Gamboa D., Baker-Zander S. A., Lukehart S. A., Miller J. N. Host response to Treponema pallidum in intradermally-infected rabbits: evidence for persistence of infection at local and distant sites. J Invest Dermatol. 1980 Dec;75(6):470–475. doi: 10.1111/1523-1747.ep12524230. [DOI] [PubMed] [Google Scholar]
- Sell S., Salman J., Norris S. J. Reinfection of chancre-immune rabbits with Treponema pallidum. I. Light and immunofluorescence studies. Am J Pathol. 1985 Feb;118(2):248–255. [PMC free article] [PubMed] [Google Scholar]
- Selsted M. E., Szklarek D., Lehrer R. I. Purification and antibacterial activity of antimicrobial peptides of rabbit granulocytes. Infect Immun. 1984 Jul;45(1):150–154. doi: 10.1128/iai.45.1.150-154.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner B., Wong G. H., Graves S. Susceptibility of Treponema pallidum to the toxic products of oxygen reduction and the non-treponemal nature of its catalase. Br J Vener Dis. 1984 Feb;60(1):14–22. doi: 10.1136/sti.60.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Territo M. C., Ganz T., Selsted M. E., Lehrer R. Monocyte-chemotactic activity of defensins from human neutrophils. J Clin Invest. 1989 Dec;84(6):2017–2020. doi: 10.1172/JCI114394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicher V., Blakowski S., Wicher K. Nitroblue tetrazolium test in experimental syphilis. Br J Vener Dis. 1977 Oct;53(5):292–294. doi: 10.1136/sti.53.5.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicher V., Wicher K. Cell response in rabbits infected with T. pallidum as measured by the leucocyte migration inhibition test. Br J Vener Dis. 1975 Aug;51(4):240–245. doi: 10.1136/sti.51.4.240. [DOI] [PMC free article] [PubMed] [Google Scholar]







