Abstract
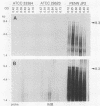
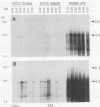
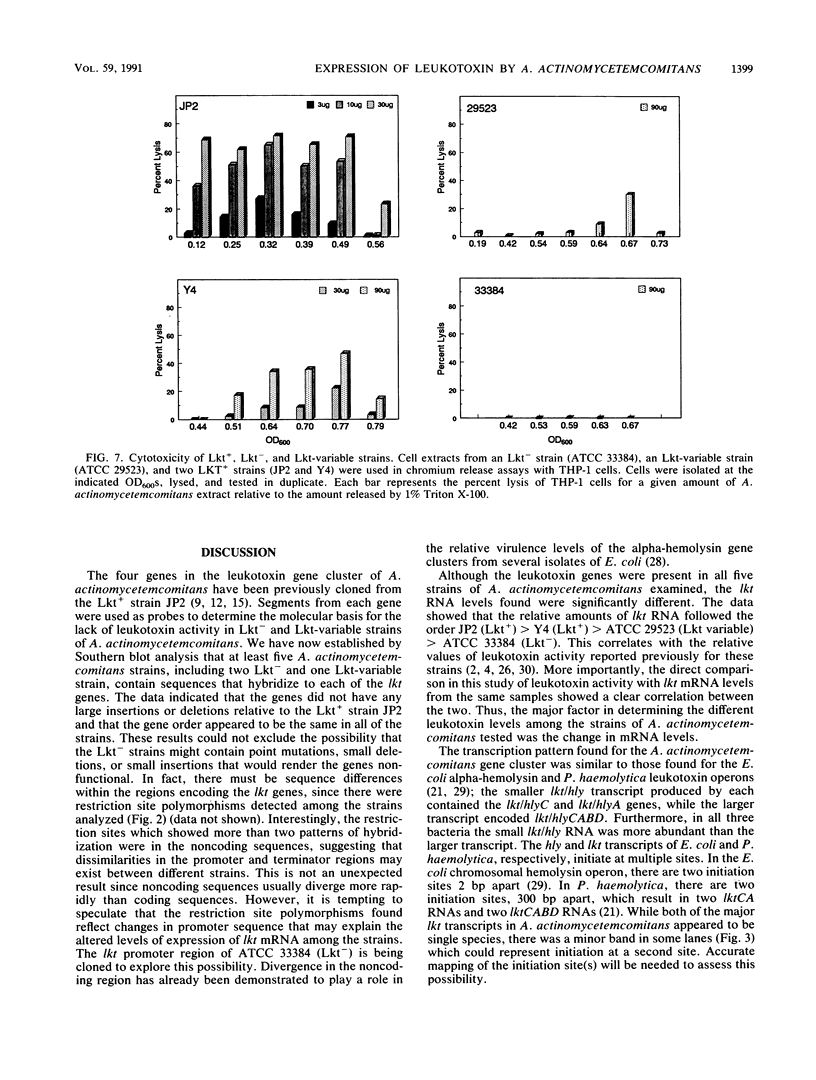
Actinobacillus actinomycetemcomitans is a gram-negative bacterium that has been implicated in the etiology of several forms of periodontitis, especially localized juvenile periodontitis. A potent leukotoxin (Lkt) is produced by most A. actinomycetemcomitans isolates from patients with periodontal disease, but some isolates are leukotoxin nonproducing (Lkt-). The molecular bases for the differences in leukotoxin expression are being explored to clarify the role of leukotoxin in pathogenesis. We have previously cloned the leukotoxin structural gene, lktA, from the leukotoxin-producing (Lkt+) strain JP2 and have shown that it is linked to three other genes, lktB, lktC, and lktD, whose gene products are thought to be required for activation and localization of the leukotoxin. These genes have now been used in Southern blot analysis to demonstrate that Lkt- strains, like Lkt+ strains, contain all four genes of the lkt gene cluster. While restriction fragment length polymorphisms were detected, they did not correlate with toxin phenotype. RNA blot analysis demonstrated that Lkt+ strains produced two transcripts, one 9.3 kb in length and the other 4.3 kb. They encode lktCABD and lktCA. respectively. Lkt- strains contained significantly lower levels of the 4.3-kb transcript with no discernible 9.3-kb message. The leukotoxic activity of the A. actinomycetemcomitans strains, measured by chromium release assays, correlated with the lkt RNA content. Therefore, a major component of leukotoxin regulation is at the level of RNA transcription or stability. Interestingly, the lkt RNAs in JP2 are regulated during growth phase, being greatly reduced in cells approaching stationary phase. Thus, the regulation of lkt RNA can be affected by both genotype and environment.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baehni P. C., Tsai C. C., McArthur W. P., Hammond B. F., Shenker B. J., Taichman N. S. Leukotoxic activity in different strains of the bacterium Actinobacillus actinomycetemcomitans isolated from juvenile periodontitis in man. Arch Oral Biol. 1981;26(8):671–676. doi: 10.1016/0003-9969(81)90164-3. [DOI] [PubMed] [Google Scholar]
- Chang Y. F., Young R., Struck D. K. Cloning and characterization of a hemolysin gene from Actinobacillus (Haemophilus) pleuropneumoniae. DNA. 1989 Nov;8(9):635–647. doi: 10.1089/dna.1.1989.8.635. [DOI] [PubMed] [Google Scholar]
- Ebersole J. L., Kraig E., Bauman G., Spitznagel J. K., Kolodrubetz D. Molecular approaches to leucotoxin as a virulence component in Actinobacillus actinomycetemcomitans. Arch Oral Biol. 1990;35 (Suppl):69S–78S. doi: 10.1016/0003-9969(90)90133-u. [DOI] [PubMed] [Google Scholar]
- Felmlee T., Pellett S., Welch R. A. Nucleotide sequence of an Escherichia coli chromosomal hemolysin. J Bacteriol. 1985 Jul;163(1):94–105. doi: 10.1128/jb.163.1.94-105.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser P., Sakamoto H., Bellalou J., Ullmann A., Danchin A. Secretion of cyclolysin, the calmodulin-sensitive adenylate cyclase-haemolysin bifunctional protein of Bordetella pertussis. EMBO J. 1988 Dec 1;7(12):3997–4004. doi: 10.1002/j.1460-2075.1988.tb03288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthmiller J. M., Kolodrubetz D., Cagle M. P., Kraig E. Sequence of the lktB gene from Actinobacillus actinomycetemcomitans. Nucleic Acids Res. 1990 Sep 11;18(17):5291–5291. doi: 10.1093/nar/18.17.5291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthmiller J. M., Kraig E., Cagle M. P., Kolodrubetz D. Sequence of the lktD gene from Actinobacillus actinomycetemcomitans. Nucleic Acids Res. 1990 Sep 11;18(17):5292–5292. doi: 10.1093/nar/18.17.5292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Highlander S. K., Chidambaram M., Engler M. J., Weinstock G. M. DNA sequence of the Pasteurella haemolytica leukotoxin gene cluster. DNA. 1989 Jan-Feb;8(1):15–28. doi: 10.1089/dna.1.1989.8.15. [DOI] [PubMed] [Google Scholar]
- Kolodrubetz D., Dailey T., Ebersole J., Kraig E. Cloning and expression of the leukotoxin gene from Actinobacillus actinomycetemcomitans. Infect Immun. 1989 May;57(5):1465–1469. doi: 10.1128/iai.57.5.1465-1469.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koronakis V., Cross M., Hughes C. Expression of the E.coli hemolysin secretion gene hlyB involves transcript anti-termination within the hly operon. Nucleic Acids Res. 1988 Jun 10;16(11):4789–4800. doi: 10.1093/nar/16.11.4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraig E., Dailey T., Kolodrubetz D. Nucleotide sequence of the leukotoxin gene from Actinobacillus actinomycetemcomitans: homology to the alpha-hemolysin/leukotoxin gene family. Infect Immun. 1990 Apr;58(4):920–929. doi: 10.1128/iai.58.4.920-929.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lally E. T., Kieba I. R., Demuth D. R., Rosenbloom J., Golub E. E., Taichman N. S., Gibson C. W. Identification and expression of the Actinobacillus actinomycetemcomitans leukotoxin gene. Biochem Biophys Res Commun. 1989 Feb 28;159(1):256–262. doi: 10.1016/0006-291x(89)92431-5. [DOI] [PubMed] [Google Scholar]
- Létoffé S., Delepelaire P., Wandersman C. Protease secretion by Erwinia chrysanthemi: the specific secretion functions are analogous to those of Escherichia coli alpha-haemolysin. EMBO J. 1990 May;9(5):1375–1382. doi: 10.1002/j.1460-2075.1990.tb08252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackman N., Nicaud J. M., Gray L., Holland I. B. Secretion of haemolysin by Escherichia coli. Curr Top Microbiol Immunol. 1986;125:159–181. doi: 10.1007/978-3-642-71251-7_10. [DOI] [PubMed] [Google Scholar]
- Nicaud J. M., Mackman N., Gray L., Holland I. B. Regulation of haemolysin synthesis in E. coli determined by HLY genes of human origin. Mol Gen Genet. 1985;199(1):111–116. doi: 10.1007/BF00327519. [DOI] [PubMed] [Google Scholar]
- Strathdee C. A., Lo R. Y. Cloning, nucleotide sequence, and characterization of genes encoding the secretion function of the Pasteurella haemolytica leukotoxin determinant. J Bacteriol. 1989 Feb;171(2):916–928. doi: 10.1128/jb.171.2.916-928.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathdee C. A., Lo R. Y. Extensive homology between the leukotoxin of Pasteurella haemolytica A1 and the alpha-hemolysin of Escherichia coli. Infect Immun. 1987 Dec;55(12):3233–3236. doi: 10.1128/iai.55.12.3233-3236.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathdee C. A., Lo R. Y. Regulation of expression of the Pasteurella haemolytica leukotoxin determinant. J Bacteriol. 1989 Nov;171(11):5955–5962. doi: 10.1128/jb.171.11.5955-5962.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taichman N. S., Dean R. T., Sanderson C. J. Biochemical and morphological characterization of the killing of human monocytes by a leukotoxin derived from Actinobacillus actinomycetemcomitans. Infect Immun. 1980 Apr;28(1):258–268. doi: 10.1128/iai.28.1.258-268.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taichman N. S., Wilton J. M. Leukotoxicity of an extract from Actinobacillus actinomycetemcomitans for human gingival polymorphonuclear leukocytes. Inflammation. 1981 Mar;5(1):1–12. doi: 10.1007/BF00910774. [DOI] [PubMed] [Google Scholar]
- Tsai C. C., McArthur W. P., Baehni P. C., Hammond B. F., Taichman N. S. Extraction and partial characterization of a leukotoxin from a plaque-derived Gram-negative microorganism. Infect Immun. 1979 Jul;25(1):427–439. doi: 10.1128/iai.25.1.427-439.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. C., Shenker B. J., DiRienzo J. M., Malamud D., Taichman N. S. Extraction and isolation of a leukotoxin from Actinobacillus actinomycetemcomitans with polymyxin B. Infect Immun. 1984 Feb;43(2):700–705. doi: 10.1128/iai.43.2.700-705.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandersman C., Delepelaire P. TolC, an Escherichia coli outer membrane protein required for hemolysin secretion. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4776–4780. doi: 10.1073/pnas.87.12.4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch R. A., Falkow S. Characterization of Escherichia coli hemolysins conferring quantitative differences in virulence. Infect Immun. 1984 Jan;43(1):156–160. doi: 10.1128/iai.43.1.156-160.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch R. A., Pellett S. Transcriptional organization of the Escherichia coli hemolysin genes. J Bacteriol. 1988 Apr;170(4):1622–1630. doi: 10.1128/jb.170.4.1622-1630.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambon J. J. Actinobacillus actinomycetemcomitans in human periodontal disease. J Clin Periodontol. 1985 Jan;12(1):1–20. doi: 10.1111/j.1600-051x.1985.tb01348.x. [DOI] [PubMed] [Google Scholar]
- Zambon J. J., DeLuca C., Slots J., Genco R. J. Studies of leukotoxin from Actinobacillus actinomycetemcomitans using the promyelocytic HL-60 cell line. Infect Immun. 1983 Apr;40(1):205–212. doi: 10.1128/iai.40.1.205-212.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambon J. J., Slots J., Genco R. J. Serology of oral Actinobacillus actinomycetemcomitans and serotype distribution in human periodontal disease. Infect Immun. 1983 Jul;41(1):19–27. doi: 10.1128/iai.41.1.19-27.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]