Abstract
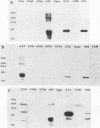
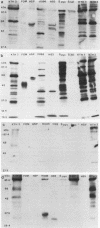
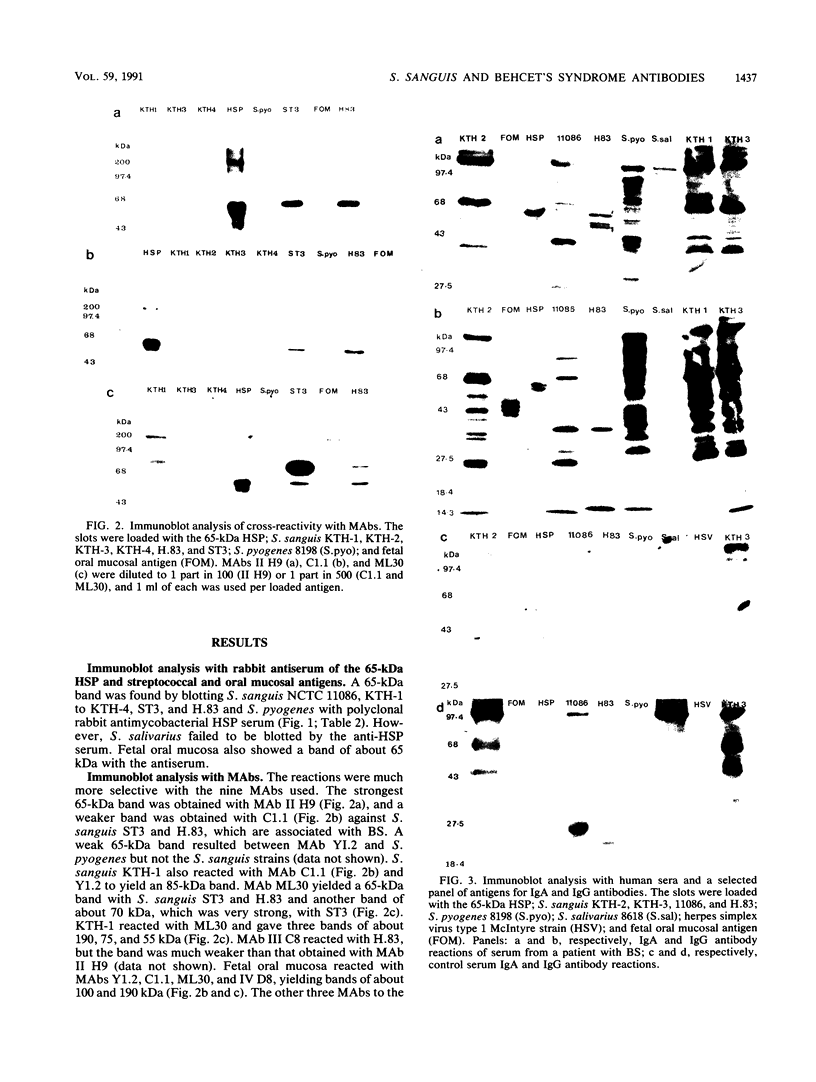
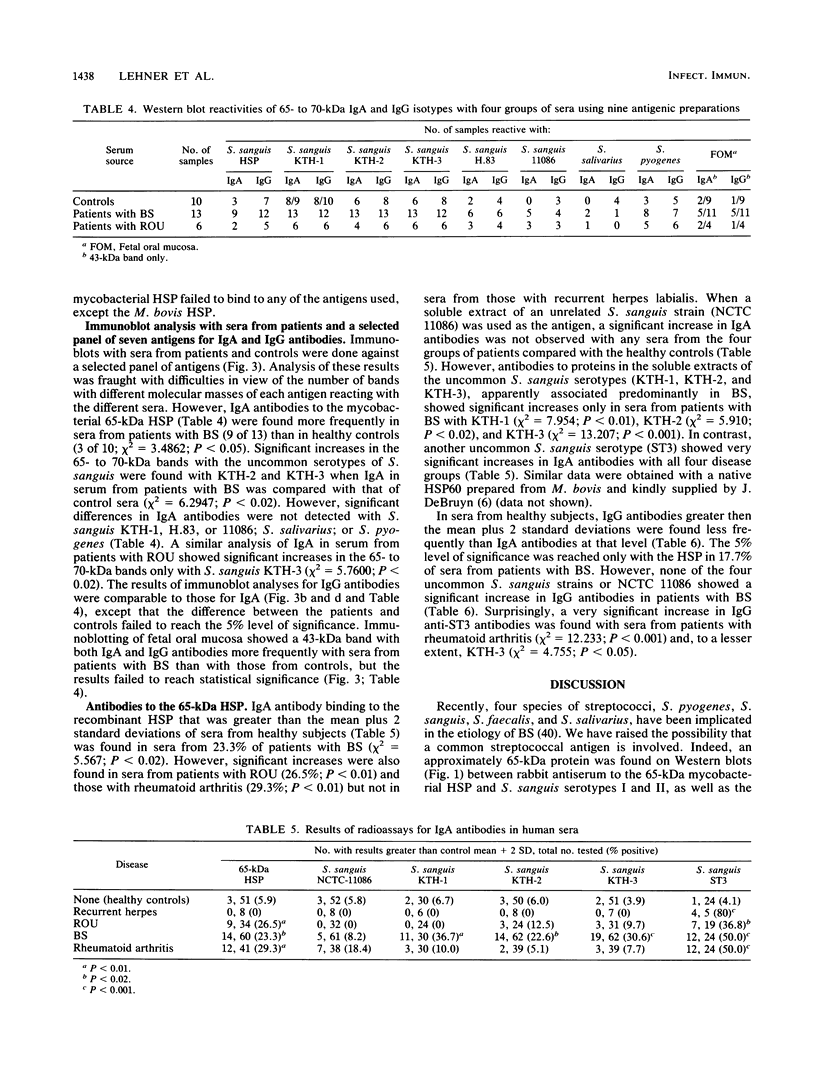
The etiology of Behcet's syndrome (BS) is unknown, but a number of streptococcal species have been implicated. A hypothesis was postulated that a shared antigen, such as a stress protein, might account for some of these findings. Indeed, a rabbit antiserum against a 65-kDa heat shock protein of Mycobacterium tuberculosis revealed a corresponding 65-kDa band with all six Streptococcus sanguis strains examined and S. pyogenes but not with S. salivarius. By applying a panel of nine monoclonal antibodies to the mycobacterial 65-kDa heat shock protein, an approximately 65-kDa antigen was identified in the uncommon serotypes of S. sanguis ST3 and H.83 and one with a different Mr was identified in KTH-1 and S. pyogenes. Monoclonal antibodies Y1.2, C1.1, II H9, and ML30, which reacted with these streptococci, recognize residues 11 to 27, 88 to 123, 107 to 122, and 276 to 297 of the 65-kDa heat shock protein, respectively, suggesting that these residues are conserved among some uncommon serotypes of S. sanguis and S. pyogenes. Immunoblot analyses of sera from patients with BS for immunoglobulin A (IgA) and IgG antibodies revealed bands of 65 to 70 kDa with the mycobacterial heat shock protein, S. sanguis strains, and S. pyogenes, although these reactivities were also found to a lesser extent in controls. A 65- to 70-kDa band was found more frequently with S. sanguis KTH-2 or KTH-3 and IgA in serum from patients with BS than with serum from controls (P less than 0.02). Antibodies in serum were then studied by a radioimmunoassay, and in patients with BS this revealed significantly raised IgA antibodies to the recombinant 65-kDa mycobacterial heat shock protein and to soluble protein extracts of S. sanguis ST3, KTH-1, KTH-2, and KTH-3. Whereas significant anti-65-kDa heat shock protein and anti-S. sanguis ST3 antibodies were also found in sera from patients with rheumatoid arthritis and recurrent oral ulcers, the anti-S. sanguis KTH-1, KTH-2, and KTH-3 antibodies were confined to BS. The results are consistent with the hypothesis that some of the streptococcal antigens are associated with heat shock or stress proteins, which will need to be formally established by isolating heat shock proteins from streptococci.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. C., Barry M. E., Buchanan T. M. Exact definition of species-specific and cross-reactive epitopes of the 65-kilodalton protein of Mycobacterium leprae using synthetic peptides. J Immunol. 1988 Jul 15;141(2):607–613. [PubMed] [Google Scholar]
- Britton W. J., Hellqvist L., Basten A., Raison R. L. Mycobacterium leprae antigens involved in human immune responses. I. Identification of four antigens by monoclonal antibodies. J Immunol. 1985 Dec;135(6):4171–4177. [PubMed] [Google Scholar]
- Buchanan T. M., Nomaguchi H., Anderson D. C., Young R. A., Gillis T. P., Britton W. J., Ivanyi J., Kolk A. H., Closs O., Bloom B. R. Characterization of antibody-reactive epitopes on the 65-kilodalton protein of Mycobacterium leprae. Infect Immun. 1987 Apr;55(4):1000–1003. doi: 10.1128/iai.55.4.1000-1003.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coates A. R., Hewitt J., Allen B. W., Ivanyi J., Mitchison D. A. Antigenic diversity of Mycobacterium tuberculosis and Mycobacterium bovis detected by means of monoclonal antibodies. Lancet. 1981 Jul 25;2(8239):167–169. doi: 10.1016/s0140-6736(81)90355-x. [DOI] [PubMed] [Google Scholar]
- De Bruyn J., Bosmans R., Turneer M., Weckx M., Nyabenda J., Van Vooren J. P., Falmagne P., Wiker H. G., Harboe M. Purification, partial characterization, and identification of a skin-reactive protein antigen of Mycobacterium bovis BCG. Infect Immun. 1987 Jan;55(1):245–252. doi: 10.1128/iai.55.1.245-252.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeJoy S. Q., Ferguson K. M., Sapp T. M., Zabriskie J. B., Oronsky A. L., Kerwar S. S. Streptococcal cell wall arthritis. Passive transfer of disease with a T cell line and crossreactivity of streptococcal cell wall antigens with Mycobacterium tuberculosis. J Exp Med. 1989 Aug 1;170(2):369–382. doi: 10.1084/jem.170.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denman A. M., Fialkow P. J., Pelton B. K., Salo A. C., Appleford D. J., Gilchrist C. Lymphocyte abnormalities in Behçet's syndrome. Clin Exp Immunol. 1980 Oct;42(1):175–185. [PMC free article] [PubMed] [Google Scholar]
- Dolby A. E. Recurrent aphthous ulceration. Effect of sera and peripheral blood lymphocytes upon oral epithelial tissue culture cells. Immunology. 1969 Nov;17(5):709–714. [PMC free article] [PubMed] [Google Scholar]
- Dubois P. Heat shock proteins and immunity. Res Immunol. 1989 Sep;140(7):653–659. doi: 10.1016/0923-2494(89)90019-9. [DOI] [PubMed] [Google Scholar]
- Dudani A. K., Gupta R. S. Immunological characterization of a human homolog of the 65-kilodalton mycobacterial antigen. Infect Immun. 1989 Sep;57(9):2786–2793. doi: 10.1128/iai.57.9.2786-2793.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eglin R. P., Lehner T., Subak-Sharpe J. H. Detection of RNA complementary to herpes-simplex virus in mononuclear cells from patients with Behçet's syndrome and recurrent oral ulcers. Lancet. 1982 Dec 18;2(8312):1356–1361. doi: 10.1016/s0140-6736(82)91268-5. [DOI] [PubMed] [Google Scholar]
- Emmrich F., Thole J., van Embden J., Kaufmann S. H. A recombinant 64 kilodalton protein of Mycobacterium bovis bacillus Calmette-Guerin specifically stimulates human T4 clones reactive to mycobacterial antigens. J Exp Med. 1986 Apr 1;163(4):1024–1029. doi: 10.1084/jem.163.4.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis T. P., Miller R. A., Young D. B., Khanolkar S. R., Buchanan T. M. Immunochemical characterization of a protein associated with Mycobacterium leprae cell wall. Infect Immun. 1985 Aug;49(2):371–377. doi: 10.1128/iai.49.2.371-377.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada S., Torii M., Kotani S., Masuda N., Ooshima T., Yokogawa K., Kawata S. Lysis of Streptococcus mutans cells with mutanolysin, a lytic enzyme prepared from a culture liquor of Streptomyces globisporus 1829. Arch Oral Biol. 1978;23(7):543–549. doi: 10.1016/0003-9969(78)90268-6. [DOI] [PubMed] [Google Scholar]
- Hemmingsen S. M., Woolford C., van der Vies S. M., Tilly K., Dennis D. T., Georgopoulos C. P., Hendrix R. W., Ellis R. J. Homologous plant and bacterial proteins chaperone oligomeric protein assembly. Nature. 1988 May 26;333(6171):330–334. doi: 10.1038/333330a0. [DOI] [PubMed] [Google Scholar]
- Holoshitz J., Koning F., Coligan J. E., De Bruyn J., Strober S. Isolation of CD4- CD8- mycobacteria-reactive T lymphocyte clones from rheumatoid arthritis synovial fluid. Nature. 1989 May 18;339(6221):226–229. doi: 10.1038/339226a0. [DOI] [PubMed] [Google Scholar]
- Holoshitz J., Matitiau A., Cohen I. R. Arthritis induced in rats by cloned T lymphocytes responsive to mycobacteria but not to collagen type II. J Clin Invest. 1984 Jan;73(1):211–215. doi: 10.1172/JCI111193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holoshitz J., Naparstek Y., Ben-Nun A., Cohen I. R. Lines of T lymphocytes induce or vaccinate against autoimmune arthritis. Science. 1983 Jan 7;219(4580):56–58. doi: 10.1126/science.6336851. [DOI] [PubMed] [Google Scholar]
- Ivanyi J., Sharp K., Jackett P., Bothamley G. Immunological study of the defined constituents of mycobacteria. Springer Semin Immunopathol. 1988;10(4):279–300. doi: 10.1007/BF02053841. [DOI] [PubMed] [Google Scholar]
- Ivanyi J., Sinha S., Aston R., Cussell D., Keen M., Sengupta U. Definition of species specific and cross-reactive antigenic determinants of Mycobacterium leprae using monoclonal antibodies. Clin Exp Immunol. 1983 Jun;52(3):528–536. [PMC free article] [PubMed] [Google Scholar]
- Jindal S., Dudani A. K., Singh B., Harley C. B., Gupta R. S. Primary structure of a human mitochondrial protein homologous to the bacterial and plant chaperonins and to the 65-kilodalton mycobacterial antigen. Mol Cell Biol. 1989 May;9(5):2279–2283. doi: 10.1128/mcb.9.5.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko F., Takahashi Y., Muramatsu Y., Miura Y. Immunological studies on aphthous ulcer and erythema nodosum-like eruptions in Behcet's disease. Br J Dermatol. 1985 Sep;113(3):303–312. doi: 10.1111/j.1365-2133.1985.tb02082.x. [DOI] [PubMed] [Google Scholar]
- Kaufmann S. H., Väth U., Thole J. E., Van Embden J. D., Emmrich F. Enumeration of T cells reactive with Mycobacterium tuberculosis organisms and specific for the recombinant mycobacterial 64-kDa protein. Eur J Immunol. 1987 Mar;17(3):351–357. doi: 10.1002/eji.1830170308. [DOI] [PubMed] [Google Scholar]
- LEHNER T. RECURRENT APHTHOUS ULCERATION AND AUTOIMMUNITY. Lancet. 1964 Nov 28;2(7370):1154–1155. doi: 10.1016/s0140-6736(64)92673-x. [DOI] [PubMed] [Google Scholar]
- Lamb J. R., Bal V., Mendez-Samperio P., Mehlert A., So A., Rothbard J., Jindal S., Young R. A., Young D. B. Stress proteins may provide a link between the immune response to infection and autoimmunity. Int Immunol. 1989;1(2):191–196. doi: 10.1093/intimm/1.2.191. [DOI] [PubMed] [Google Scholar]
- Lamb J. R., Ivanyi J., Rees A., Young R. A., Young D. B. The identification of T cell epitopes in Mycobacterium tuberculosis using human T lymphocyte clones. Lepr Rev. 1986 Dec;57 (Suppl 2):131–137. [PubMed] [Google Scholar]
- Lehner T. Autoimmunity in oral diseases, with special reference to recurrent oral ulceration. Proc R Soc Med. 1968 May;61(5):515–524. doi: 10.1177/003591576806100543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehner T. Behçet's syndrome and autoimmunity. Br Med J. 1967 Feb 25;1(5538):465–467. doi: 10.1136/bmj.1.5538.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levis W. R., Meeker H. C., Schuller-Levis G. B., Gillis T. P., Marino L. J., Jr, Zabriskie J. Serodiagnosis of leprosy: relationships between antibodies to Mycobacterium leprae phenolic glycolipid I and protein antigens. J Clin Microbiol. 1986 Dec;24(6):917–921. doi: 10.1128/jcm.24.6.917-921.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist S., Craig E. A. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- Mehra V., Sweetser D., Young R. A. Efficient mapping of protein antigenic determinants. Proc Natl Acad Sci U S A. 1986 Sep;83(18):7013–7017. doi: 10.1073/pnas.83.18.7013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima Y. Recent research into Behçet's disease in Japan. Int J Tissue React. 1988;10(2):59–65. [PubMed] [Google Scholar]
- Oshima Y., Shimizu T., Yokohari R., Matsumoto T., Kano K., Kagami T., Nagaya H. Clinical Studies on Behçet's Syndrome. Ann Rheum Dis. 1963 Jan;22(1):36–45. doi: 10.1136/ard.22.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polla B. S. A role for heat shock proteins in inflammation? Immunol Today. 1988 May;9(5):134–137. doi: 10.1016/0167-5699(88)91199-1. [DOI] [PubMed] [Google Scholar]
- Rogers R. S., 3rd, Sams W. M., Jr, Shorter R. G. Lymphocytotoxicity in recurrent aphthous stomatitis. Lymphocytotoxicity for oral epithelial cells in recurrent aphthous stomatitis and Bechet syndrome. Arch Dermatol. 1974 Mar;109(3):361–363. [PubMed] [Google Scholar]
- Shinnick T. M., Sweetser D., Thole J., van Embden J., Young R. A. The etiologic agents of leprosy and tuberculosis share an immunoreactive protein antigen with the vaccine strain Mycobacterium bovis BCG. Infect Immun. 1987 Aug;55(8):1932–1935. doi: 10.1128/iai.55.8.1932-1935.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinnick T. M., Vodkin M. H., Williams J. C. The Mycobacterium tuberculosis 65-kilodalton antigen is a heat shock protein which corresponds to common antigen and to the Escherichia coli GroEL protein. Infect Immun. 1988 Feb;56(2):446–451. doi: 10.1128/iai.56.2.446-451.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skin hypersensitivity to streptococcal antigens and the induction of systemic symptoms by the antigens in Behçet's disease--a multicenter study. The Behcet's Disease Research Committee of Japan. J Rheumatol. 1989 Apr;16(4):506–511. [PubMed] [Google Scholar]
- Smith R., Lehner T. A radioimmunoassay for serum and gingival crevicular fluid antibodies to a purified protein of Streptococcus mutans. Clin Exp Immunol. 1981 Feb;43(2):417–424. [PMC free article] [PubMed] [Google Scholar]
- Thole J. E., Dauwerse H. G., Das P. K., Groothuis D. G., Schouls L. M., van Embden J. D. Cloning of Mycobacterium bovis BCG DNA and expression of antigens in Escherichia coli. Infect Immun. 1985 Dec;50(3):800–806. doi: 10.1128/iai.50.3.800-806.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thole J. E., Hindersson P., de Bruyn J., Cremers F., van der Zee J., de Cock H., Tommassen J., van Eden W., van Embden J. D. Antigenic relatedness of a strongly immunogenic 65 kDA mycobacterial protein antigen with a similarly sized ubiquitous bacterial common antigen. Microb Pathog. 1988 Jan;4(1):71–83. doi: 10.1016/0882-4010(88)90049-6. [DOI] [PubMed] [Google Scholar]
- Thole J. E., Keulen W. J., De Bruyn J., Kolk A. H., Groothuis D. G., Berwald L. G., Tiesjema R. H., van Embden J. D. Characterization, sequence determination, and immunogenicity of a 64-kilodalton protein of Mycobacterium bovis BCG expressed in escherichia coli K-12. Infect Immun. 1987 Jun;55(6):1466–1475. doi: 10.1128/iai.55.6.1466-1475.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thole J. E., van Schooten W. C., Keulen W. J., Hermans P. W., Janson A. A., de Vries R. R., Kolk A. H., van Embden J. D. Use of recombinant antigens expressed in Escherichia coli K-12 to map B-cell and T-cell epitopes on the immunodominant 65-kilodalton protein of Mycobacterium bovis BCG. Infect Immun. 1988 Jun;56(6):1633–1640. doi: 10.1128/iai.56.6.1633-1640.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsoulfa G., Rook G. A., Van-Embden J. D., Young D. B., Mehlert A., Isenberg D. A., Hay F. C., Lydyard P. M. Raised serum IgG and IgA antibodies to mycobacterial antigens in rheumatoid arthritis. Ann Rheum Dis. 1989 Feb;48(2):118–123. doi: 10.1136/ard.48.2.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winfield J. B. Stress proteins, arthritis, and autoimmunity. Arthritis Rheum. 1989 Dec;32(12):1497–1504. doi: 10.1002/anr.1780321202. [DOI] [PubMed] [Google Scholar]
- Young D., Lathigra R., Hendrix R., Sweetser D., Young R. A. Stress proteins are immune targets in leprosy and tuberculosis. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4267–4270. doi: 10.1073/pnas.85.12.4267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Eden W., Thole J. E., van der Zee R., Noordzij A., van Embden J. D., Hensen E. J., Cohen I. R. Cloning of the mycobacterial epitope recognized by T lymphocytes in adjuvant arthritis. Nature. 1988 Jan 14;331(6152):171–173. doi: 10.1038/331171a0. [DOI] [PubMed] [Google Scholar]
- van den Broek M. F., Hogervorst E. J., Van Bruggen M. C., Van Eden W., van der Zee R., van den Berg W. B. Protection against streptococcal cell wall-induced arthritis by pretreatment with the 65-kD mycobacterial heat shock protein. J Exp Med. 1989 Aug 1;170(2):449–466. doi: 10.1084/jem.170.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]