Abstract
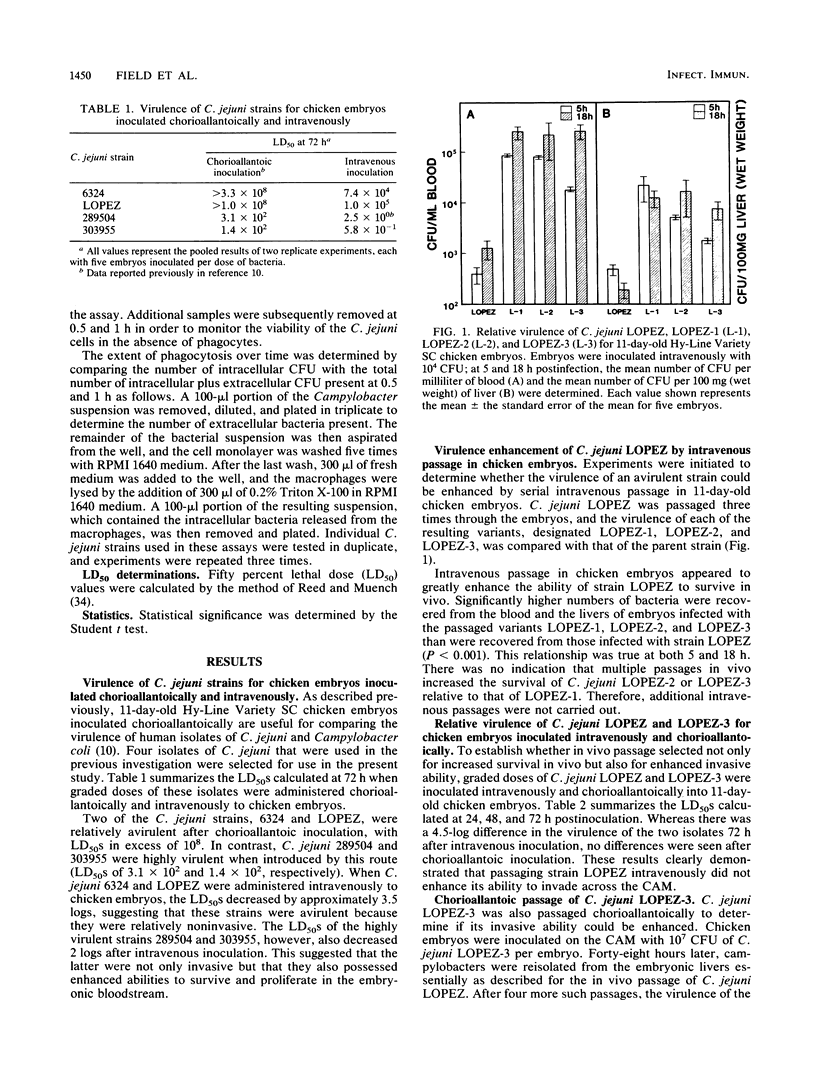
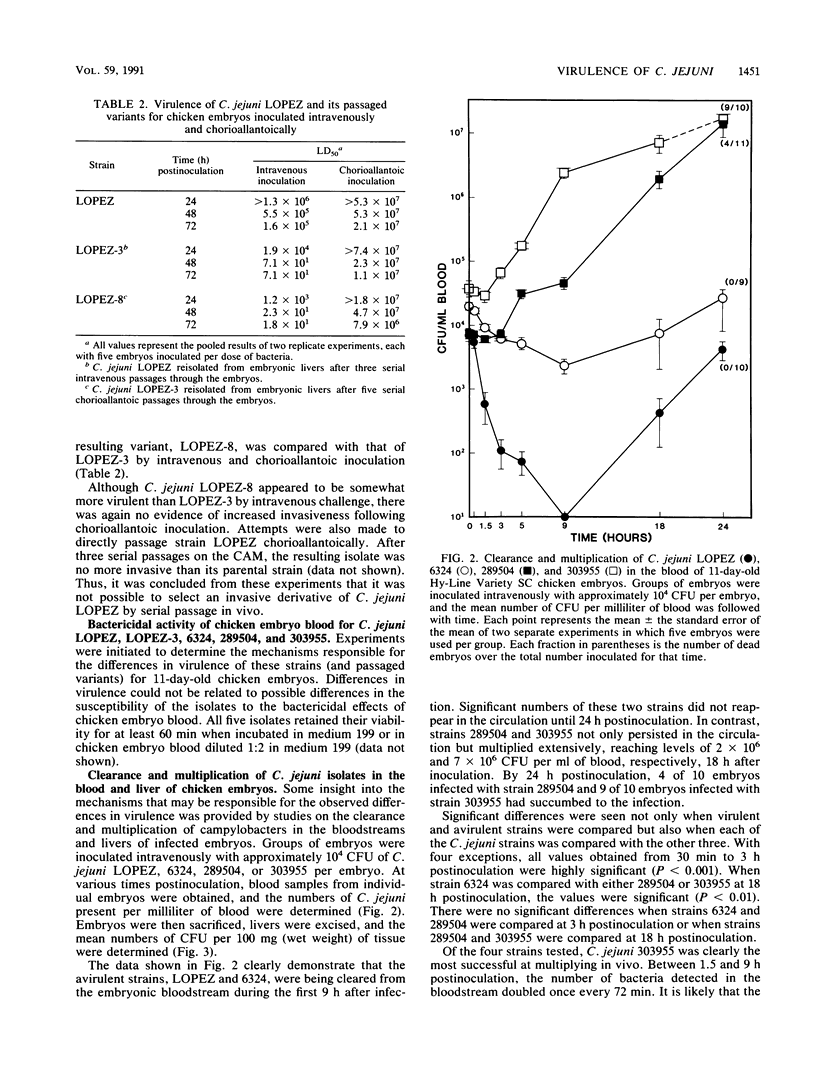
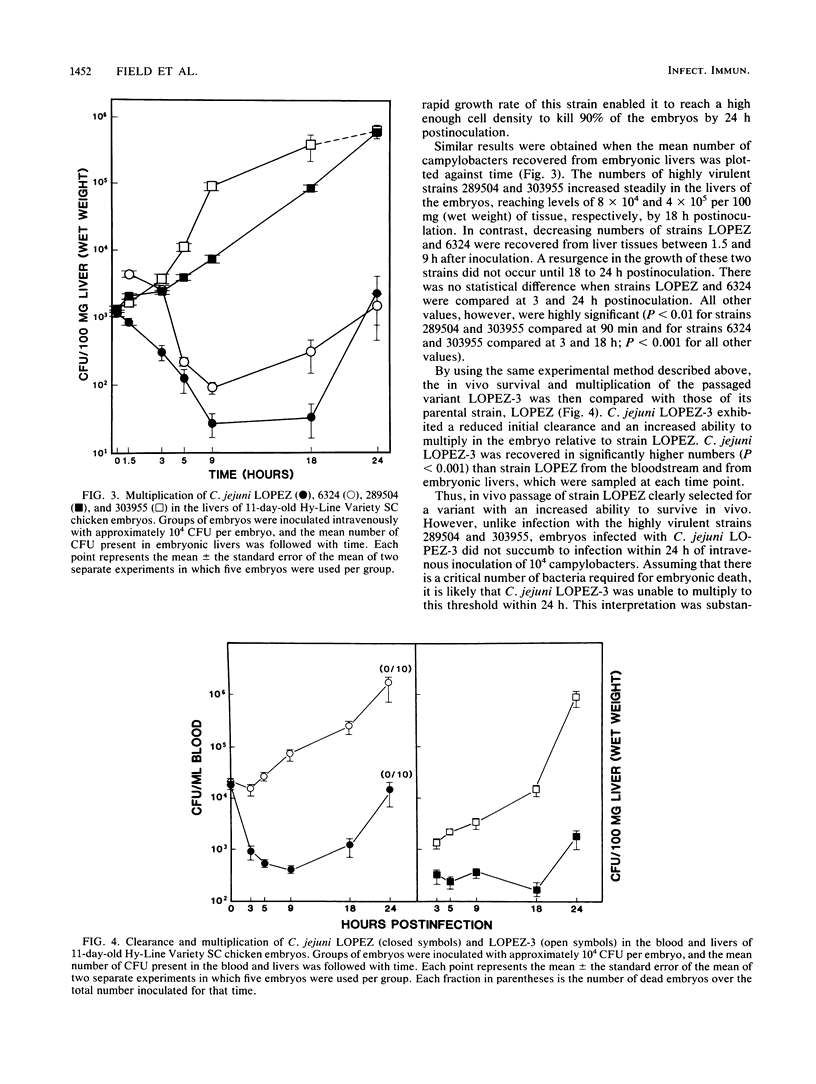
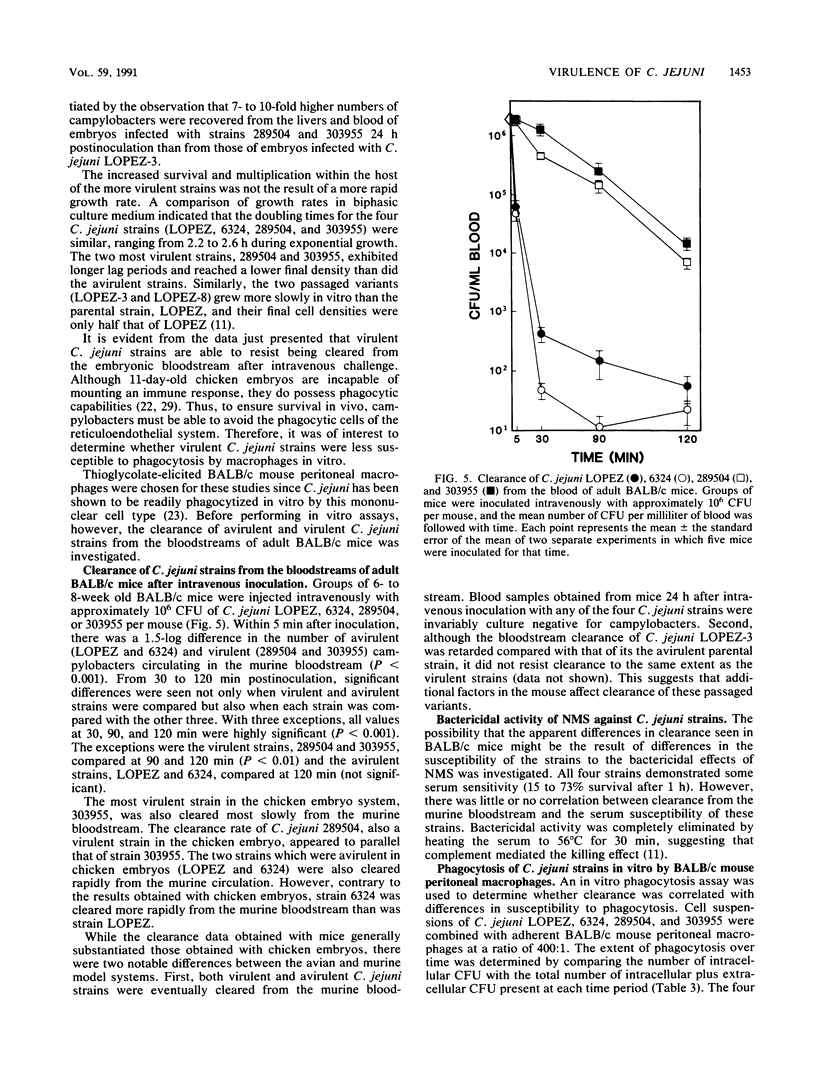
The 11-day-old chicken embryo has been shown to be a useful animal model for comparing the virulence of human isolates of Campylobacter jejuni. Virulence in this system is associated with the ability to invade the chorioallantoic membrane and to survive and proliferate in vivo. In this study, the survival and multiplication of C. jejuni in the embryonic host was investigated. It was possible to enhance the virulence of a relatively avirulent C. jejuni strain by passaging it intravenously through the embryos. The resulting isogenic variants demonstrated enhanced abilities to survive in vivo but were still unable to invade when inoculated onto the chorioallantoic membrane. The bloodstream clearance of C. jejuni was studied, and virulent, but not avirulent, strains persisted and multiplied both in the bloodstream and in embryonic liver. Virulent strains also were cleared significantly more slowly from the bloodstream of adult BALB/c mice after intravenous challenge than were avirulent strains. C. jejuni strains which were cleared slowly in vivo were also ingested slowly in vitro by mouse peritoneal macrophages. Clearance studies in mice pretreated with cobra venom factor demonstrated that opsonization by serum complement was not a prerequisite for clearance of campylobacters from the murine bloodstream.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blaser M. J., Smith P. F., Kohler P. F. Susceptibility of Campylobacter isolates to the bactericidal activity of human serum. J Infect Dis. 1985 Feb;151(2):227–235. doi: 10.1093/infdis/151.2.227. [DOI] [PubMed] [Google Scholar]
- Board R. G., Fuller R. Non-specific antimicrobial defences of the avian egg, embryo and neonate. Biol Rev Camb Philos Soc. 1974 Feb;49(1):15–49. doi: 10.1111/j.1469-185x.1974.tb01297.x. [DOI] [PubMed] [Google Scholar]
- Brown E. J. Interaction of gram-positive microorganisms with complement. Curr Top Microbiol Immunol. 1985;121:159–187. doi: 10.1007/978-3-642-45604-6_8. [DOI] [PubMed] [Google Scholar]
- Cochrane C. G., Müller-Eberhard H. J., Aikin B. S. Depletion of plasma complement in vivo by a protein of cobra venom: its effect on various immunologic reactions. J Immunol. 1970 Jul;105(1):55–69. [PubMed] [Google Scholar]
- Field L. H., Headley V. L., Payne S. M., Berry L. J. Influence of iron on growth, morphology, outer membrane protein composition, and synthesis of siderophores in Campylobacter jejuni. Infect Immun. 1986 Oct;54(1):126–132. doi: 10.1128/iai.54.1.126-132.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field L. H., Headley V. L., Underwood J. L., Payne S. M., Berry L. J. The chicken embryo as a model for campylobacter invasion: comparative virulence of human isolates of Campylobacter jejuni and Campylobacter coli. Infect Immun. 1986 Oct;54(1):118–125. doi: 10.1128/iai.54.1.118-125.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goossens H., De Boeck M., Butzler J. P. A new selective medium for the isolation of Campylobacter jejuni from human faeces. Eur J Clin Microbiol. 1983 Aug;2(4):389–393. doi: 10.1007/BF02019476. [DOI] [PubMed] [Google Scholar]
- Goossens H., Rummens E., Cadranel S., Butzler J. P., Takeda Y. Cytotoxic activity on Chinese hamster ovary cells in culture filtrates of Campylobacter jejuni/coli. Lancet. 1985 Aug 31;2(8453):511–511. doi: 10.1016/s0140-6736(85)90454-4. [DOI] [PubMed] [Google Scholar]
- Horwitz M. A. Phagocytosis of microorganisms. Rev Infect Dis. 1982 Jan-Feb;4(1):104–123. doi: 10.1093/clinids/4.1.104. [DOI] [PubMed] [Google Scholar]
- Johnson W. M., Lior H. Cytotoxic and cytotonic factors produced by Campylobacter jejuni, Campylobacter coli, and Campylobacter laridis. J Clin Microbiol. 1986 Aug;24(2):275–281. doi: 10.1128/jcm.24.2.275-281.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson W. M., Lior H. Toxins produced by Campylobacter jejuni and Campylobacter coli. Lancet. 1984 Jan 28;1(8370):229–230. doi: 10.1016/s0140-6736(84)92155-x. [DOI] [PubMed] [Google Scholar]
- Joiner K. A., Brown E. J., Frank M. M. Complement and bacteria: chemistry and biology in host defense. Annu Rev Immunol. 1984;2:461–491. doi: 10.1146/annurev.iy.02.040184.002333. [DOI] [PubMed] [Google Scholar]
- Kiehlbauch J. A., Albach R. A., Baum L. L., Chang K. P. Phagocytosis of Campylobacter jejuni and its intracellular survival in mononuclear phagocytes. Infect Immun. 1985 May;48(2):446–451. doi: 10.1128/iai.48.2.446-451.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A., O'Rourke J. L., Barrington P. J., Trust T. J. Mucus colonization as a determinant of pathogenicity in intestinal infection by Campylobacter jejuni: a mouse cecal model. Infect Immun. 1986 Feb;51(2):536–546. doi: 10.1128/iai.51.2.536-546.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang-Takasaki C. J., Saxén H., Mäkelä P. H., Leive L. Complement activation by polysaccharide of lipopolysaccharide: an important virulence determinant of salmonellae. Infect Immun. 1983 Aug;41(2):563–569. doi: 10.1128/iai.41.2.563-569.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCardell B. A., Madden J. M., Lee E. C. Production of cholera-like toxin by Campylobacter jejuni/coli. Lancet. 1984 Feb 25;1(8374):448–449. doi: 10.1016/s0140-6736(84)91772-0. [DOI] [PubMed] [Google Scholar]
- McCardell B. A., Madden J. M., Stanfield J. T. Production of cytotoxins by Campylobacter. Lancet. 1986 May 3;1(8488):1031–1031. doi: 10.1016/s0140-6736(86)91297-3. [DOI] [PubMed] [Google Scholar]
- McSweegan E., Walker R. I. Identification and characterization of two Campylobacter jejuni adhesins for cellular and mucous substrates. Infect Immun. 1986 Jul;53(1):141–148. doi: 10.1128/iai.53.1.141-148.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizejewski G. J., Ramm G. M. Phagocytosis as related to the reticuloendothelial system (RES) in the developing chick. Growth. 1969 Mar;33(1):47–56. [PubMed] [Google Scholar]
- Mullink H., Von Blomberg M., Wilders M. M., Drexhage H. A., Alons C. L. A simple cytochemical method for distinguishing EAC rosettes formed by lymphocytes and monocytes. J Immunol Methods. 1979;29(2):133–137. doi: 10.1016/0022-1759(79)90062-0. [DOI] [PubMed] [Google Scholar]
- Newell D. G., McBride H., Dolby J. M. Investigations on the role of flagella in the colonization of infant mice with Campylobacter jejuni and attachment of Campylobacter jejuni to human epithelial cell lines. J Hyg (Lond) 1985 Oct;95(2):217–227. doi: 10.1017/s0022172400062653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepys M. B. Studies in vivo of cobra factor and murine C3. Immunology. 1975 Feb;28(2):369–377. [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Palacios G. M., Torres J., Torres N. I., Escamilla E., Ruiz-Palacios B. R., Tamayo J. Cholera-like enterotoxin produced by Campylobacter jejuni. Characterisation and clinical significance. Lancet. 1983 Jul 30;2(8344):250–253. doi: 10.1016/s0140-6736(83)90234-9. [DOI] [PubMed] [Google Scholar]
- SPIEGELBERG H. L., MIESCHER P. A., BENACERRAF B. STUDIES ON THE ROLE OF COMPLEMENT IN THE IMMUNE CLEARANCE OF ESCHERICHIA COLI AND RAT ERYTHROCYTES BY THE RETICULOENDOTHELIAL SYSTEM IN MICE. J Immunol. 1963 May;90:751–759. [PubMed] [Google Scholar]
- Walker R. I., Caldwell M. B., Lee E. C., Guerry P., Trust T. J., Ruiz-Palacios G. M. Pathophysiology of Campylobacter enteritis. Microbiol Rev. 1986 Mar;50(1):81–94. doi: 10.1128/mr.50.1.81-94.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeen W. P., Puthucheary S. D., Pang T. Demonstration of a cytotoxin from Campylobacter jejuni. J Clin Pathol. 1983 Nov;36(11):1237–1240. doi: 10.1136/jcp.36.11.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]


