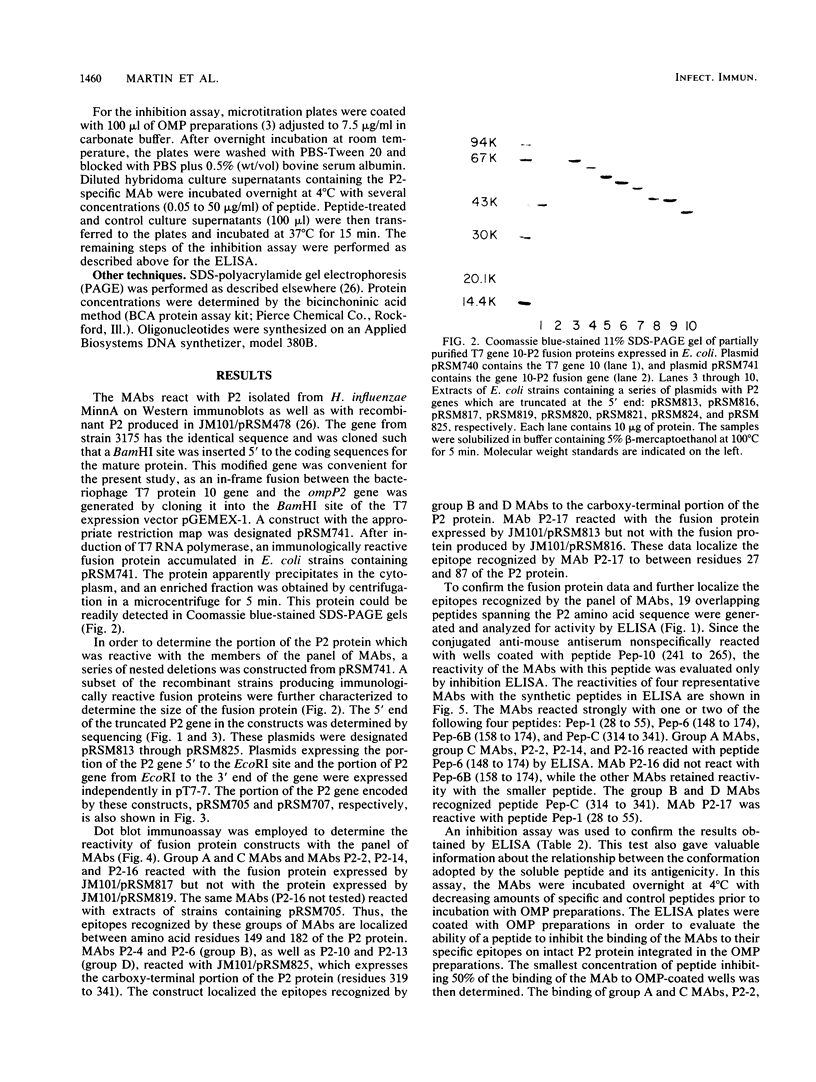
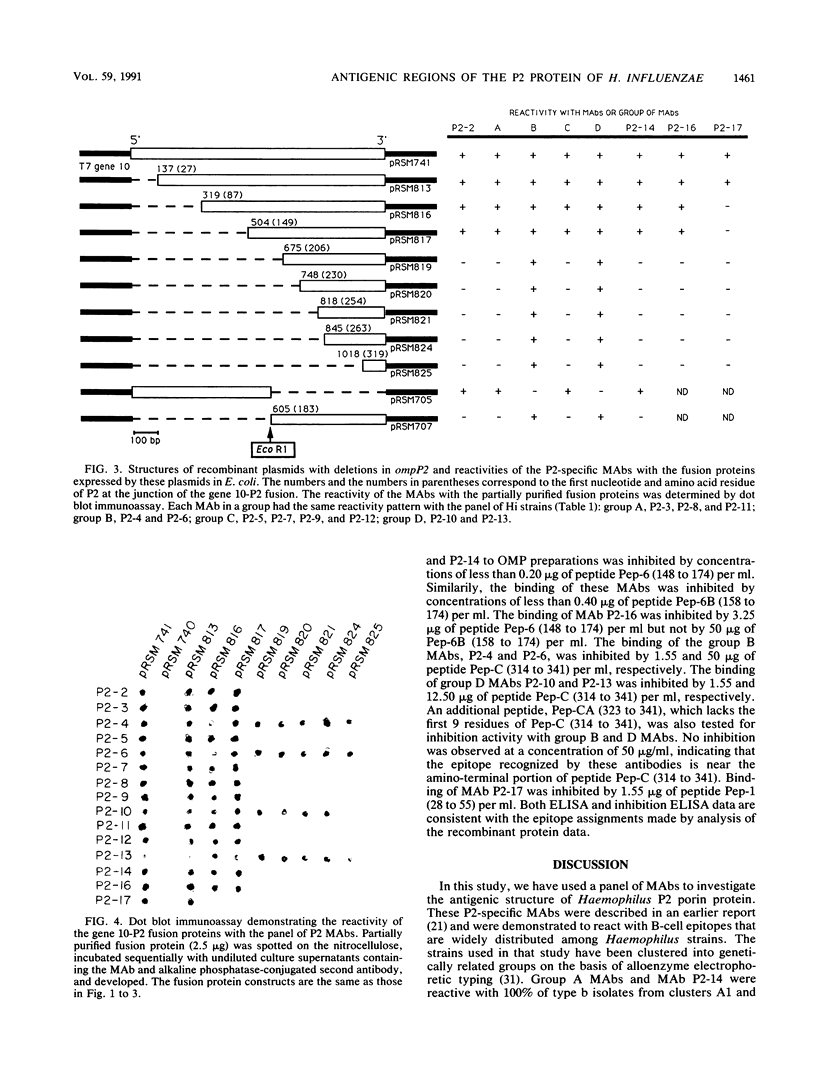
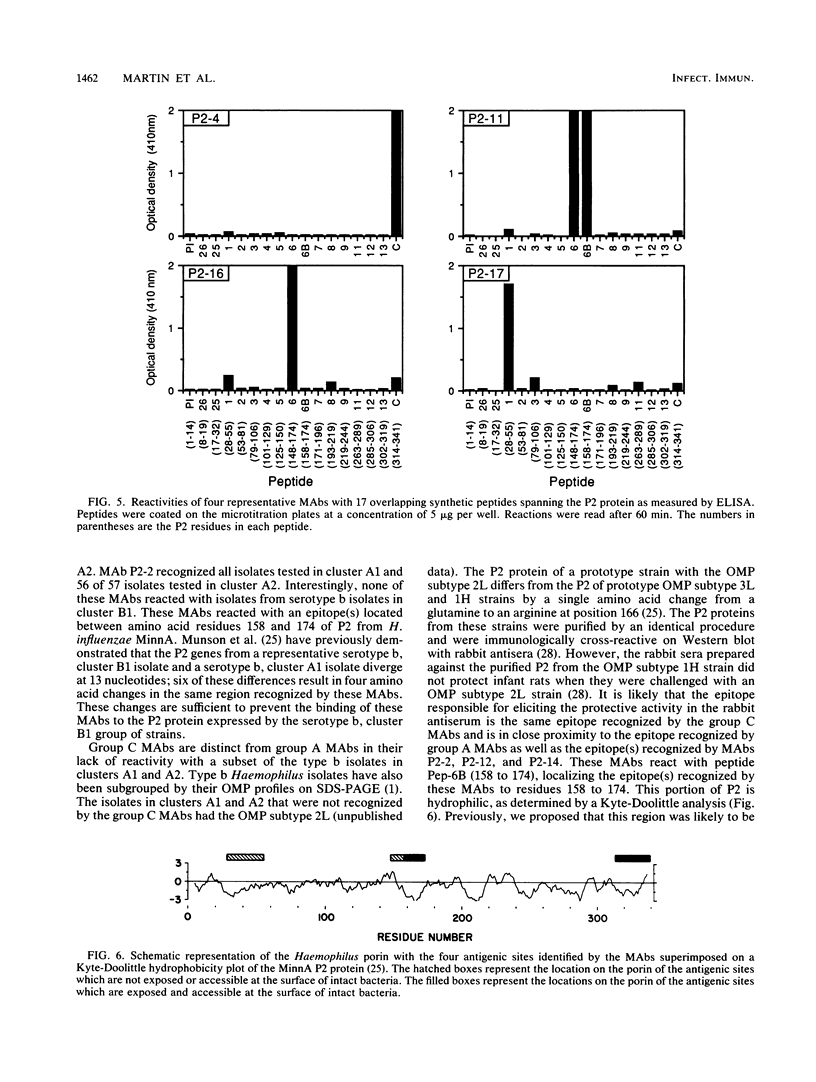
Abstract
The P2 protein of Haemophilus influenzae type b has a porin activity and is the most abundant protein in the outer membrane. We have employed fusion protein constructs and synthetic peptides along with monoclonal antibodies to map B-cell epitopes in this protein. A linear, surface-exposed epitope was identified between residues 158 and 174. A second surface-exposed epitope was identified near the carboxy-terminal end of the protein (residues 319 to 341). Two additional B-cell epitopes were identified. One was localized between residues 28 and 55, whereas the other was located between residues 148 and 174. These epitopes were not present on the surface of intact H. influenzae cells. Thus, four distinct immunogenic and antigenic regions on the P2 protein have been identified.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barenkamp S. J., Munson R. S., Jr, Granoff D. M. Subtyping isolates of Haemophilus influenzae type b by outer-membrane protein profiles. J Infect Dis. 1981 May;143(5):668–676. doi: 10.1093/infdis/143.5.668. [DOI] [PubMed] [Google Scholar]
- Barnes W. M. Sequencing DNA with dideoxyribonucleotides as chain terminators: hints and strategies for big projects. Methods Enzymol. 1987;152:538–556. doi: 10.1016/0076-6879(87)52060-2. [DOI] [PubMed] [Google Scholar]
- Brodeur B. R., Larose Y., Tsang P., Hamel J., Ashton F., Ryan A. Protection against infection with Neisseria meningitidis group B serotype 2b by passive immunization with serotype-specific monoclonal antibody. Infect Immun. 1985 Nov;50(2):510–516. doi: 10.1128/iai.50.2.510-516.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deich R. A., Anilionis A., Fulginiti J., Metcalf B. J., Quataert S., Quinn-Dey T., Zlotnick G. W., Green B. A. Antigenic conservation of the 15,000-dalton outer membrane lipoprotein PCP of Haemophilus influenzae and biologic activity of anti-PCP antisera. Infect Immun. 1990 Oct;58(10):3388–3393. doi: 10.1128/iai.58.10.3388-3393.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desaymard C., Débarbouillé M., Jolit M., Schwartz M. Mutations affecting antigenic determinants of an outer membrane protein of Escherichia coli. EMBO J. 1986 Jun;5(6):1383–1388. doi: 10.1002/j.1460-2075.1986.tb04371.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring K., Charbit A., Brissaud E., Hofnung M. Bacteriophage lambda receptor site on the Escherichia coli K-12 LamB protein. J Bacteriol. 1987 May;169(5):2103–2106. doi: 10.1128/jb.169.5.2103-2106.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granoff D. M., Munson R. S., Jr Prospects for prevention of Haemophilus influenzae type b disease by immunization. J Infect Dis. 1986 Mar;153(3):448–461. doi: 10.1093/infdis/153.3.448. [DOI] [PubMed] [Google Scholar]
- Green B. A., Metcalf B. J., Quinn-Dey T., Kirkley D. H., Quataert S. A., Deich R. A. A recombinant non-fatty acylated form of the Hi-PAL (P6) protein of Haemophilus influenzae elicits biologically active antibody against both nontypeable and type b H. influenzae. Infect Immun. 1990 Oct;58(10):3272–3278. doi: 10.1128/iai.58.10.3272-3278.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green B. A., Quinn-Dey T., Zlotnick G. W. Biologic activities of antibody to a peptidoglycan-associated lipoprotein of Haemophilus influenzae against multiple clinical isolates of H. influenzae type b. Infect Immun. 1987 Dec;55(12):2878–2883. doi: 10.1128/iai.55.12.2878-2883.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamel J., Brodeur B. R., Larose Y., Tsang P. S., Belmaaza A., Montplaisir S. A monoclonal antibody directed against a serotype-specific, outer-membrane protein of Haemophilus influenzae type b. J Med Microbiol. 1987 Mar;23(2):163–170. doi: 10.1099/00222615-23-2-163. [DOI] [PubMed] [Google Scholar]
- Hansen E. J., Hasemann C., Clausell A., Capra J. D., Orth K., Moomaw C. R., Slaughter C. A., Latimer J. L., Miller E. E. Primary structure of the porin protein of Haemophilus influenzae type b determined by nucleotide sequence analysis. Infect Immun. 1989 Apr;57(4):1100–1107. doi: 10.1128/iai.57.4.1100-1107.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen E. J., Pelzel S. E., Orth K., Moomaw C. R., Radolf J. D., Slaughter C. A. Structural and antigenic conservation of the P2 porin protein among strains of Haemophilus influenzae type b. Infect Immun. 1989 Nov;57(11):3270–3275. doi: 10.1128/iai.57.11.3270-3275.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckels J. E., Virji M., Tinsley C. R. Vaccination against gonorrhoea: the potential protective effect of immunization with a synthetic peptide containing a conserved epitope of gonococcal outer membrane protein IB. Vaccine. 1990 Jun;8(3):225–230. doi: 10.1016/0264-410x(90)90050-v. [DOI] [PubMed] [Google Scholar]
- Jap B. K., Downing K. H., Walian P. J. Structure of PhoE porin in projection at 3.5 A resolution. J Struct Biol. 1990 Mar;103(1):57–63. doi: 10.1016/1047-8477(90)90086-r. [DOI] [PubMed] [Google Scholar]
- Kimura A., Gulig P. A., McCracken G. H., Jr, Loftus T. A., Hansen E. J. A minor high-molecular-weight outer membrane protein of Haemophilus influenzae type b is a protective antigen. Infect Immun. 1985 Jan;47(1):253–259. doi: 10.1128/iai.47.1.253-259.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebba P. E., Benson S. A., Bala S., Abdullah T., Reid J., Singh S. P., Nikaido H. Determinants of OmpF porin antigenicity and structure. J Biol Chem. 1990 Apr 25;265(12):6800–6810. [PubMed] [Google Scholar]
- Klingman K. L., Jansen E. M., Murphy T. F. Nearest neighbor analysis of outer membrane proteins of nontypeable Haemophilus influenzae. Infect Immun. 1988 Dec;56(12):3058–3063. doi: 10.1128/iai.56.12.3058-3063.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepault J., Dargent B., Tichelaar W., Rosenbusch J. P., Leonard K., Pattus F. Three-dimensional reconstruction of maltoporin from electron microscopy and image processing. EMBO J. 1988 Jan;7(1):261–268. doi: 10.1002/j.1460-2075.1988.tb02808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb M. R. Protection of infant rats from Haemophilus influenzae type b infection by antiserum to purified outer membrane protein a. Infect Immun. 1987 Nov;55(11):2612–2618. doi: 10.1128/iai.55.11.2612-2618.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb M. R., Smith D. H. Outer membrane protein composition in disease isolates of Haemophilus influenzae: pathogenic and epidemiological implications. Infect Immun. 1980 Dec;30(3):709–717. doi: 10.1128/iai.30.3.709-717.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin D., Hamel J., Brodeur B. R., Musser J. M. Antigenic relationships among the porin proteins of encapsulated Haemophilus influenzae clones. J Clin Microbiol. 1990 Aug;28(8):1720–1724. doi: 10.1128/jcm.28.8.1720-1724.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffatt B. A., Studier F. W. T7 lysozyme inhibits transcription by T7 RNA polymerase. Cell. 1987 Apr 24;49(2):221–227. doi: 10.1016/0092-8674(87)90563-0. [DOI] [PubMed] [Google Scholar]
- Molla A., Charbit A., Le Guern A., Ryter A., Hofnung M. Antibodies against synthetic peptides and the topology of LamB, an outer membrane protein from Escherichia coli K12. Biochemistry. 1989 Oct 3;28(20):8234–8241. doi: 10.1021/bi00446a040. [DOI] [PubMed] [Google Scholar]
- Moxon E. R., Vaughn K. A. The type b capsular polysaccharide as a virulence determinant of Haemophilus influenzae: studies using clinical isolates and laboratory transformants. J Infect Dis. 1981 Apr;143(4):517–524. doi: 10.1093/infdis/143.4.517. [DOI] [PubMed] [Google Scholar]
- Munson R. S., Jr, Granoff D. M. Purification and partial characterization of outer membrane proteins P5 and P6 from Haemophilus influenzae type b. Infect Immun. 1985 Sep;49(3):544–549. doi: 10.1128/iai.49.3.544-549.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munson R. S., Jr, Shenep J. L., Barenkamp S. J., Granoff D. M. Purification and comparison of outer membrane protein P2 from Haemophilus influenzae type b isolates. J Clin Invest. 1983 Aug;72(2):677–684. doi: 10.1172/JCI111017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munson R., Jr, Bailey C., Grass S. Diversity of the outer membrane protein P2 gene from major clones of Haemophilus influenzae type b. Mol Microbiol. 1989 Dec;3(12):1797–1803. doi: 10.1111/j.1365-2958.1989.tb00165.x. [DOI] [PubMed] [Google Scholar]
- Munson R., Jr, Tolan R. W., Jr Molecular cloning, expression, and primary sequence of outer membrane protein P2 of Haemophilus influenzae type b. Infect Immun. 1989 Jan;57(1):88–94. doi: 10.1128/iai.57.1.88-94.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy T. F., Bartos L. C. Human bactericidal antibody response to outer membrane protein P2 of nontypeable Haemophilus influenzae. Infect Immun. 1988 Oct;56(10):2673–2679. doi: 10.1128/iai.56.10.2673-2679.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy T. F., Bartos L. C. Purification and analysis with monoclonal antibodies of P2, the major outer membrane protein of nontypable Haemophilus influenzae. Infect Immun. 1988 May;56(5):1084–1089. doi: 10.1128/iai.56.5.1084-1089.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musser J. M., Kroll J. S., Granoff D. M., Moxon E. R., Brodeur B. R., Campos J., Dabernat H., Frederiksen W., Hamel J., Hammond G. Global genetic structure and molecular epidemiology of encapsulated Haemophilus influenzae. Rev Infect Dis. 1990 Jan-Feb;12(1):75–111. doi: 10.1093/clinids/12.1.75. [DOI] [PubMed] [Google Scholar]
- Sass H. J., Büldt G., Beckmann E., Zemlin F., van Heel M., Zeitler E., Rosenbusch J. P., Dorset D. L., Massalski A. Densely packed beta-structure at the protein-lipid interface of porin is revealed by high-resolution cryo-electron microscopy. J Mol Biol. 1989 Sep 5;209(1):171–175. doi: 10.1016/0022-2836(89)90180-0. [DOI] [PubMed] [Google Scholar]
- Schenkman S., Couture E., Schwartz M. Monoclonal antibodies reveal lamB antigenic determinants on both faces of the Escherichia coli outer membrane. J Bacteriol. 1983 Sep;155(3):1382–1392. doi: 10.1128/jb.155.3.1382-1392.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W., Moffatt B. A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986 May 5;189(1):113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1074–1078. doi: 10.1073/pnas.82.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turk D. C. The pathogenicity of Haemophilus influenzae. J Med Microbiol. 1984 Aug;18(1):1–16. doi: 10.1099/00222615-18-1-1. [DOI] [PubMed] [Google Scholar]
- Vachon V., Kristjanson D. N., Coulton J. W. Outer membrane porin protein of Haemophilus influenzae type b: pore size and subunit structure. Can J Microbiol. 1988 Feb;34(2):134–140. doi: 10.1139/m88-027. [DOI] [PubMed] [Google Scholar]
- Vachon V., Lyew D. J., Coulton J. W. Transmembrane permeability channels across the outer membrane of Haemophilus influenzae type b. J Bacteriol. 1985 Jun;162(3):918–924. doi: 10.1128/jb.162.3.918-924.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller P. F., Smith A. L., Anderson P., Smith D. H. The role of encapsulation and host age in the clearance of Haemophilus influenzae bacteremia. J Infect Dis. 1977 Jan;135(1):34–41. doi: 10.1093/infdis/135.1.34. [DOI] [PubMed] [Google Scholar]
- van der Ley P., Struyvé M., Tommassen J. Topology of outer membrane pore protein PhoE of Escherichia coli. Identification of cell surface-exposed amino acids with the aid of monoclonal antibodies. J Biol Chem. 1986 Sep 15;261(26):12222–12225. [PubMed] [Google Scholar]