Abstract
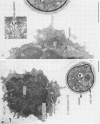
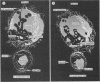
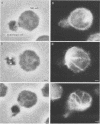
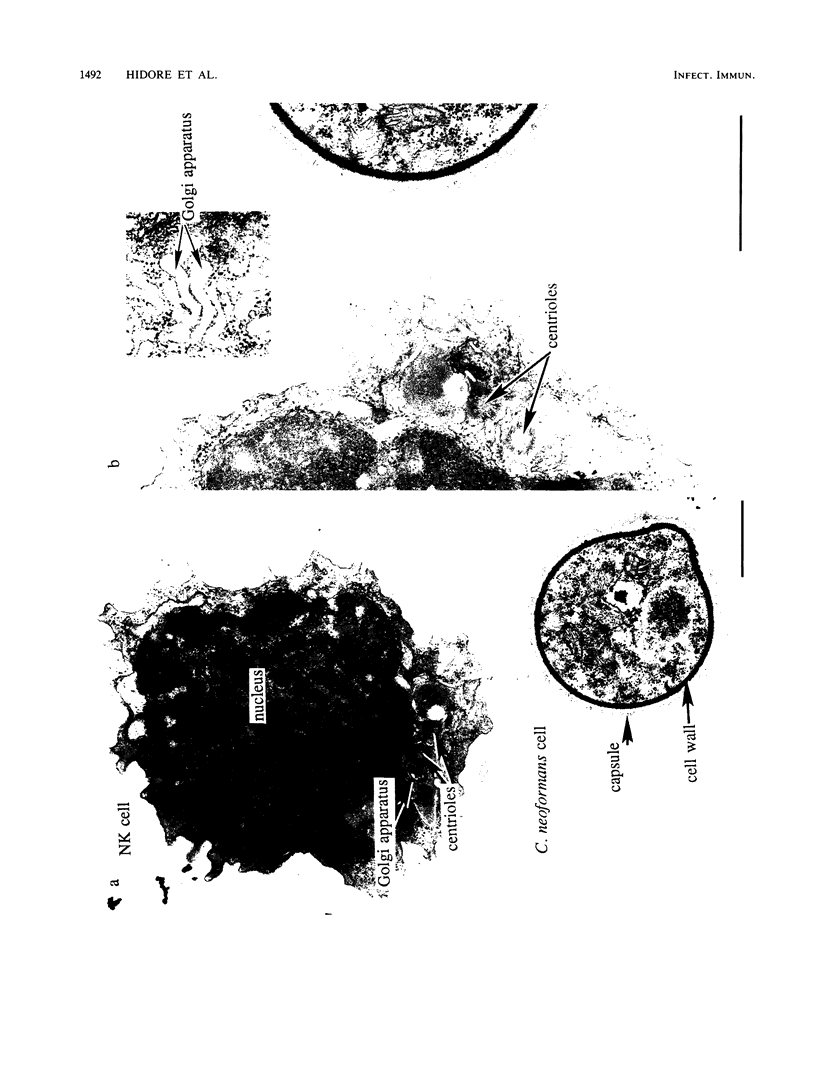
Natural killer (NK) cells bind to and inhibit the growth of the fungal target Cryptococcus neoformans. Since C. neoformans is structurally and chemically distinct from the standard tumor cell target used in the model of NK cell-mediated cytotoxicity, this study was designed to investigate the NK cell response after binding to cryptococci. Transmission electron micrographs and three-dimensional reconstructions of NK cell-cryptococci conjugates demonstrated focusing of the NK cell centrioles and Golgi apparatus toward the cryptococcal attachment site. NK cell cytoskeletal changes after cryptococcal binding were confirmed by immunofluorescence studies in which NK cells were allowed to bind to cryptococci in Mg2(+)-containing, Ca2(+)-free medium. One hour after the addition of Ca2+ to the preformed conjugates, the bound NK cells demonstrated a significant increase in the percentage of microtubule organizing centers focused toward the cryptococcal binding site. Colchicine, a drug that inhibits microtubule assembly, did not affect NK cell-cryptococci binding but abrogated NK cell-mediated cryptococcal growth inhibition, indicating that microtubule assembly, an important prerequisite for the secretory process, is not required for NK cell-cryptococci binding but is essential for inhibition of cryptococcal growth. In addition, the Ca2+ channel-blocking reagents, lidocaine and verapamil, did not affect NK cell-cryptococci binding but blocked the NK cell-mediated anticryptococcal activity, suggesting that a Ca2+ flux is essential for inhibition of cryptococcal growth. Considered together, these data indicate that NK cells respond to binding of a target cell that has a capsule and cell wall, in addition to a cell membrane, in a manner similar to that seen following binding to target cells that are surrounded by only a cell membrane; however, the response of the NK cells to the binding of C. neoformans is slower and possibly less efficient than the response after tumor cell binding.
Full text
PDF


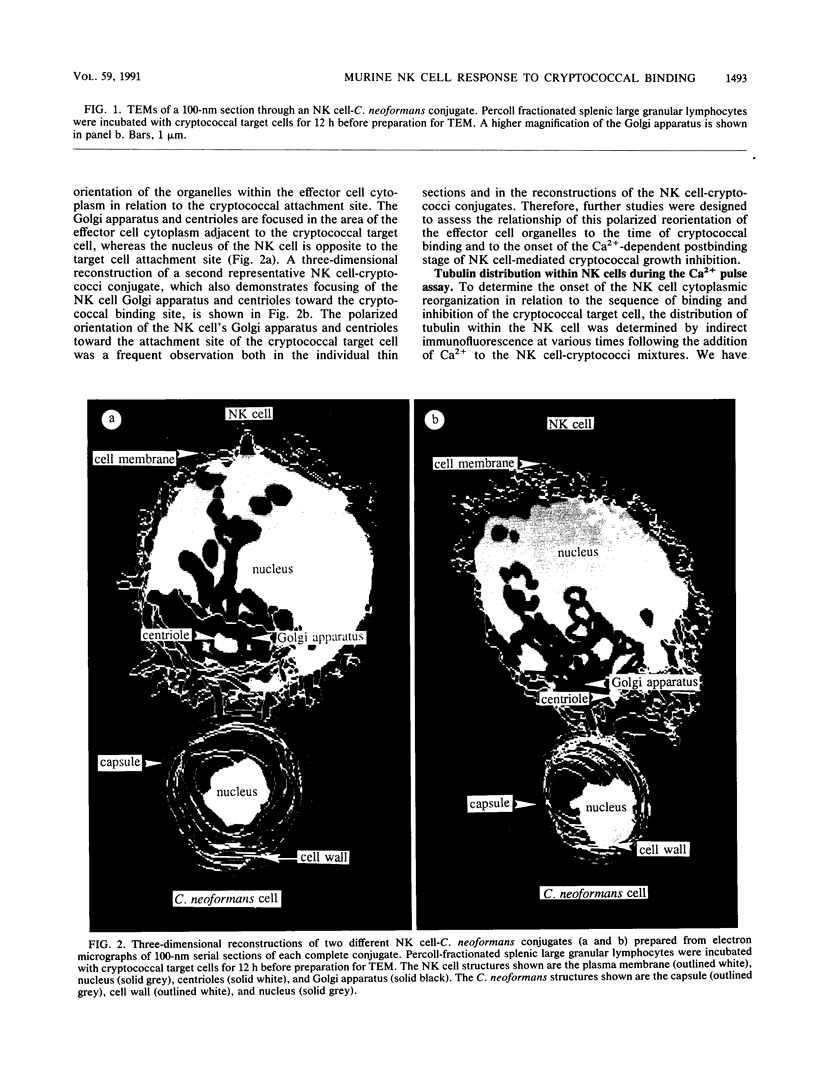

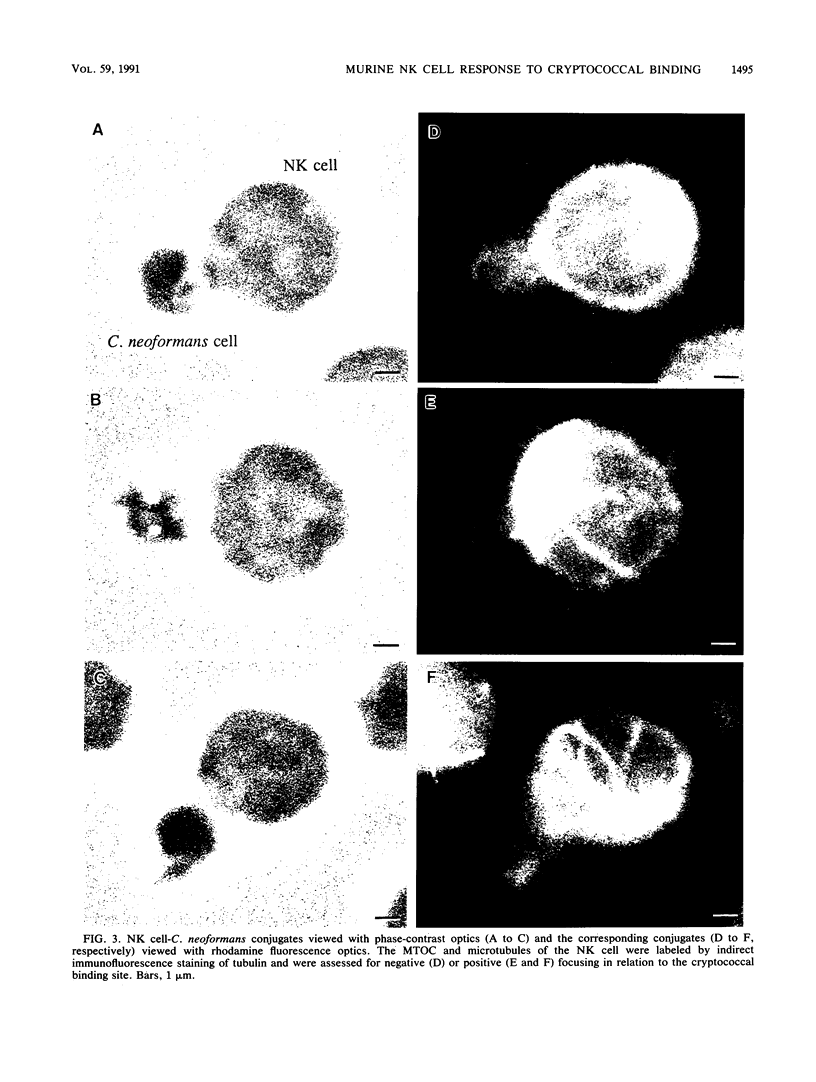
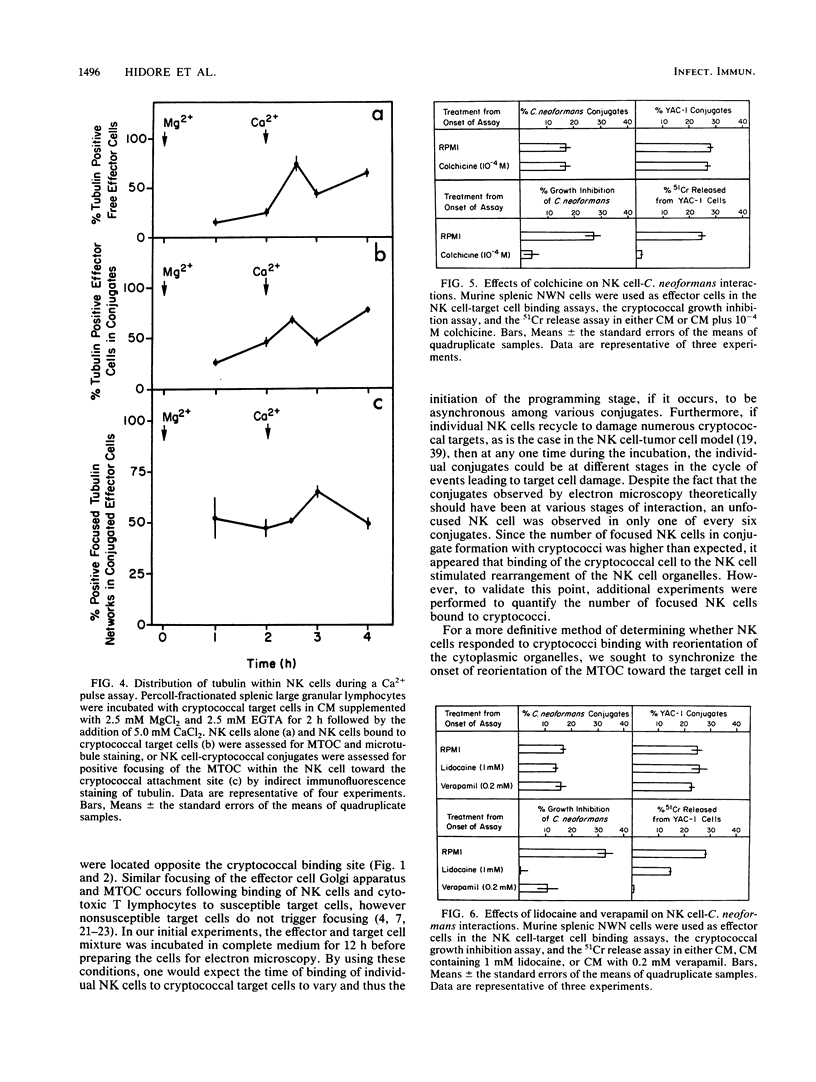



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown D. L., Reuhl K. R., Bormann S., Little J. E. Effects of methyl mercury on the microtubule system of mouse lymphocytes. Toxicol Appl Pharmacol. 1988 Jun 15;94(1):66–75. doi: 10.1016/0041-008x(88)90337-7. [DOI] [PubMed] [Google Scholar]
- Carpen O., Virtanen I., Saksela E. Ultrastructure of human natural killer cells: nature of the cytolytic contacts in relation to cellular secretion. J Immunol. 1982 Jun;128(6):2691–2697. [PubMed] [Google Scholar]
- Carpén O., Virtanen I., Lehto V. P., Saksela E. Polarization of NK cell cytoskeleton upon conjugation with sensitive target cells. J Immunol. 1983 Dec;131(6):2695–2698. [PubMed] [Google Scholar]
- Carpén O., Virtanen I., Saksela E. The cytotoxic activity of human natural killer cells requires an intact secretory apparatus. Cell Immunol. 1981 Feb;58(1):97–106. doi: 10.1016/0008-8749(81)90152-0. [DOI] [PubMed] [Google Scholar]
- Dennert G., Kupfer A., Anderson C. G., Singer S. J. Reorientation of the Golgi apparatus and the microtubule organizing center: is it a means to polarize cell-mediated cytotoxicity? Adv Exp Med Biol. 1985;184:83–97. doi: 10.1007/978-1-4684-8326-0_7. [DOI] [PubMed] [Google Scholar]
- Galey F. R., Nilsson S. E. A new method for transferring sections from the liquid surface of the trough through staining solutions to the supporting film of a grid. J Ultrastruct Res. 1966 Feb;14(3):405–410. doi: 10.1016/s0022-5320(66)80057-6. [DOI] [PubMed] [Google Scholar]
- Geiger B., Rosen D., Berke G. Spatial relationships of microtubule-organizing centers and the contact area of cytotoxic T lymphocytes and target cells. J Cell Biol. 1982 Oct;95(1):137–143. doi: 10.1083/jcb.95.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herberman R. B. Natural killer cells. Annu Rev Med. 1986;37:347–352. doi: 10.1146/annurev.me.37.020186.002023. [DOI] [PubMed] [Google Scholar]
- Herberman R. B., Reynolds C. W., Ortaldo J. R. Mechanism of cytotoxicity by natural killer (NK) cells. Annu Rev Immunol. 1986;4:651–680. doi: 10.1146/annurev.iy.04.040186.003251. [DOI] [PubMed] [Google Scholar]
- Hidore M. R., Murphy J. W. Correlation of natural killer cell activity and clearance of Cryptococcus neoformans from mice after adoptive transfer of splenic nylon wool-nonadherent cells. Infect Immun. 1986 Feb;51(2):547–555. doi: 10.1128/iai.51.2.547-555.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidore M. R., Murphy J. W. Murine natural killer cell interactions with a fungal target, Cryptococcus neoformans. Infect Immun. 1989 Jul;57(7):1990–1997. doi: 10.1128/iai.57.7.1990-1997.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidore M. R., Murphy J. W. Natural cellular resistance of beige mice against Cryptococcus neoformans. J Immunol. 1986 Dec 1;137(11):3624–3631. [PubMed] [Google Scholar]
- Hidore M. R., Nabavi N., Reynolds C. W., Henkart P. A., Murphy J. W. Cytoplasmic components of natural killer cells limit the growth of Cryptococcus neoformans. J Leukoc Biol. 1990 Jul;48(1):15–26. doi: 10.1002/jlb.48.1.15. [DOI] [PubMed] [Google Scholar]
- Hiserodt J. C., Britvan L. J., Targan S. R. Characterization of the cytolytic reaction mechanism of the human natural killer (NK) lymphocyte: resolution into binding, programming, and killer cell-independent steps. J Immunol. 1982 Oct;129(4):1782–1787. [PubMed] [Google Scholar]
- Hiserodt J. C., Britvan L. J., Targan S. R. Differential effects of various pharmacological agents on the cytolytic reaction mechanism of the human natural killer lymphocyte: further resolution of programming for lysis and KCIL into discrete stages. J Immunol. 1982 Nov;129(5):2266–2270. [PubMed] [Google Scholar]
- Huwyler T., Hirt A., Felix D., Morell A. Effect of cations and cation channel blockers on human natural killer cells. Int J Immunopharmacol. 1985;7(4):573–576. doi: 10.1016/0192-0561(85)90079-7. [DOI] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Katz P., Zaytoun A. M., Lee J. H., Jr Mechanisms of human cell-mediated cytotoxicity. III. Dependence of natural killing on microtubule and microfilament integrity. J Immunol. 1982 Dec;129(6):2816–2825. [PubMed] [Google Scholar]
- Kiessling R., Klein E., Wigzell H. "Natural" killer cells in the mouse. I. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Specificity and distribution according to genotype. Eur J Immunol. 1975 Feb;5(2):112–117. doi: 10.1002/eji.1830050208. [DOI] [PubMed] [Google Scholar]
- Kupfer A., Dennert G., Singer S. J. Polarization of the Golgi apparatus and the microtubule-organizing center within cloned natural killer cells bound to their targets. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7224–7228. doi: 10.1073/pnas.80.23.7224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupfer A., Dennert G., Singer S. J. The reorientation of the Golgi apparatus and the microtubule-organizing center in the cytotoxic effector cell is a prerequisite in the lysis of bound target cells. J Mol Cell Immunol. 1985;2(1):37–49. [PubMed] [Google Scholar]
- Kupfer A., Singer S. J. Cell biology of cytotoxic and helper T cell functions: immunofluorescence microscopic studies of single cells and cell couples. Annu Rev Immunol. 1989;7:309–337. doi: 10.1146/annurev.iy.07.040189.001521. [DOI] [PubMed] [Google Scholar]
- Luini W., Boraschi D., Alberti S., Aleotti A., Tagliabue A. Morphological characterization of a cell population responsible for natural killer activity. Immunology. 1981 Aug;43(4):663–668. [PMC free article] [PubMed] [Google Scholar]
- Millard R. W., Lathrop D. A., Grupp G., Ashraf M., Grupp I. L., Schwartz A. Differential cardiovascular effects of calcium channel blocking agents: potential mechanisms. Am J Cardiol. 1982 Feb 18;49(3):499–506. doi: 10.1016/s0002-9149(82)80002-7. [DOI] [PubMed] [Google Scholar]
- Murphy J. W., Cozad G. C. Immunological unresponsiveness induced by cryptococcal capsular polysaccharide assayed by the hemolytic plaque technique. Infect Immun. 1972 Jun;5(6):896–901. doi: 10.1128/iai.5.6.896-901.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy J. W., McDaniel D. O. In vitro reactivity of natural killer (NK) cells against Cryptococcus neoformans. J Immunol. 1982 Apr;128(4):1577–1583. [PubMed] [Google Scholar]
- Nabavi N., Murphy J. W. In vitro binding of natural killer cells to Cryptococcus neoformans targets. Infect Immun. 1985 Oct;50(1):50–57. doi: 10.1128/iai.50.1.50-57.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podack E. R. Granule-mediated cytolysis of target cells. Curr Top Microbiol Immunol. 1989;140:1–9. doi: 10.1007/978-3-642-73911-8_1. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roder J. C., Kiessling R., Biberfeld P., Andersson B. Target-effector interaction in the natural killer (NK) cell system. II. The isolation of NK cells and studies on the mechanism of killing. J Immunol. 1978 Dec;121(6):2509–2517. [PubMed] [Google Scholar]
- Rogers K. A., Khoshbaf M. A., Brown D. L. Relationship of microtubule organization in lymphocyte to the capping of immunoglobulin. Eur J Cell Biol. 1981 Apr;24(1):1–8. [PubMed] [Google Scholar]
- Ryser J. E., Rungger-Brändle E., Chaponnier C., Gabbiani G., Vassalli P. The area of attachment of cytotoxic T lymphocytes to their target cells shows high motility and polarization of actin, but not myosin. J Immunol. 1982 Mar;128(3):1159–1162. [PubMed] [Google Scholar]
- Sirianni M. C., Soddu S., Malorni W., Arancia G., Aiuti F., Soddus S. Mechanism of defective natural killer cell activity in patients with AIDS is associated with defective distribution of tubulin. J Immunol. 1988 Apr 15;140(8):2565–2568. [PubMed] [Google Scholar]
- Solovera J. J., Alvarez-Mon M., Casas J., Carballido J., Durantez A. Inhibition of human natural killer (NK) activity by calcium channel modulators and a calmodulin antagonist. J Immunol. 1987 Aug 1;139(3):876–880. [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Ullberg M., Jondal M. Recycling and target binding capacity of human natural killer cells. J Exp Med. 1981 Mar 1;153(3):615–628. doi: 10.1084/jem.153.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]