Abstract
DNA polymerases contain active sites that are structurally superimposable and highly conserved in sequence. To assess the significance of this preservation and to determine the mutational burden that active sites can tolerate, we randomly mutated a stretch of 13 amino acids within the polymerase catalytic site (motif A) of Thermus aquaticus DNA polymerase I. After selection, by using genetic complementation, we obtained a library of approximately 8,000 active mutant DNA polymerases, of which 350 were sequenced and analyzed. This is the largest collection of physiologically active polymerase mutants. We find that all residues of motif A, except one (Asp-610), are mutable while preserving wild-type activity. A wide variety of amino acid substitutions were obtained at sites that are evolutionarily maintained, and conservative substitutions predominate at regions that stabilize tertiary structures. Several mutants exhibit unique properties, including DNA polymerase activity higher than the wild-type enzyme or the ability to incorporate ribonucleotide analogs. Bacteria dependent on these mutated polymerases for survival are fit to replicate repetitively. The high mutability of the polymerase active site in vivo and the ability to evolve altered enzymes may be required for survival in environments that demand increased mutagenesis. The inherent substitutability of the polymerase active site must be addressed relative to the constancy of nucleotide sequence found in nature.
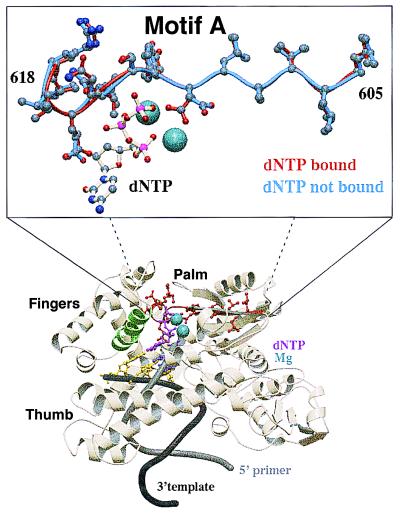
DNA polymerases are responsible for the replication and maintenance of the genome, a role that is central to accurately transmitting genetic information from generation to generation. DNA polymerases have an active site architecture that specifically configures to and incorporates each of the four deoxynucleoside triphosphates, while taking direction from templates with diverse nucleotide sequences (1). In addition, the active site tends to exclude altered nucleotides produced during cellular metabolism. The overall folding pattern of polymerases resembles the human right hand and contains three distinct subdomains (palm, fingers, and thumb; ref. 2; Fig. 1). Whereas the structures of the fingers and thumb subdomains vary greatly between polymerases that differ in size and in cellular functions, the catalytic palm subdomains are all superimposable. A detailed study of polymerase active site within the conserved palm subdomain is crucial to understanding how polymerases function.
Figure 1.
Structure of Taq pol I bound with DNA and incoming dNTP. Evolutionarily conserved motif A (amino acids 605 to 617 highlighted in red) is located within the heart of the polymerase catalytic site. Residues of motif A interact with the incoming dNTP and amino acids in the finger motif during the conformational change step, subsequent to nucleotide binding. Motif A is superimposable in all polymerases with solved structures and begins at a hydrophobic antiparallel β-sheet that proceeds to an α-helix. The orientation of side chains within amino acids of motif A is nearly identical before (in blue) and subsequent to (in red) dNTP binding, with the exception of Asp-610, which rotates around the β carbon while coordinating with the Mg2+–dNTP complex. Motif B (amino acids 656 to 670) is highlighted in green. Coordinate sets 3ktq (ternary complex, closed form) and 4ktq (binary complex) from ref. 4 were obtained from the Protein Data Bank. The coordinates were aligned by using the 1sq_expl command in the software o to generate the inset view.
All DNA and RNA polymerases share two conserved regions: motif A and motif C. Both are located within the palm subdomain. Structural data show that amino acids within motif A are in position to interact with the incoming dNTP, coordinate with the two divalent metal cofactors, and interact with the fingers subdomain during the conformation change step after dNTP binding (3, 4). In addition, Asp-610 of motif A is in position to stabilize the transition state during the chemical catalysis step, which leads to phosphodiester bond formation (4). A limited number of mutation studies of the active site have shown that substitutions within motif A can lead to altered dNTP recognition and fidelity (5, 6). The primary amino acid sequences of various DNA polymerase active sites are exceptionally conserved, suggesting that motif A evolved slowly. Motif A retains the sequence DYSQIELR in polymerases from organisms separated by many million years of evolution, including Thermus aquaticus, Chlamydia trachomatis, and Escherichia coli. Structurally, motif A is superimposable with a mean deviation of 1 Å among mammalian pol α and prokaryotic pol I family DNA polymerases (7) and begins at an antiparallel β-strand containing predominantly hydrophobic residues and continues to an α-helix (Fig. 1). Taken together, these results indicate that polymerases function by similar catalytic mechanisms and that the active site of polymerases may be immutable to ensure the survival of organisms.
To evaluate the degree of plasticity within the polymerase active site in vivo, we substituted random DNA sequences in T. aquaticus DNA polymerase I (Taq pol I) at the region encoding the 13 amino acids of motif A from Leu-605 to Arg-617, and selected active clones by genetic complementation of E. coli recA718 polA12. This E. coli strain, which contains a temperature-sensitive mutation in the polA gene, which encodes DNA polymerase I, forms colonies at 30°C, but not at 37°C. After selection, we find DNA polymerase catalytic site (motif A) is in fact highly mutable while preserving wild-type (WT) activity. The high mutability of the active site in vivo and the ability to evolve altered enzymes may be required for survival in environments that demand increased mutagenesis.
Methods
Plasmid Construction.
The Taq pol I gene was cloned into low copy (one to three copies per cell) pHSG576 vector containing an E. coli pol I-independent origin of replication, SC101 (8, 9). A silent BisWI site was created in Taq pol I by site-directed mutagenesis (C → A) at position 1758 (pTaq). A nonfunctional stuffer vector (pTaqDUM) was constructed by cloning two hybridized oligonucleotides into pTaq between BisWI and SacII sites; these two restriction sites flank the sequence encoding for motif A. A random library (pTaqLIB) was created by preparing a randomized oligonucleotide with a BisWI site in which nucleotides encoding amino acids Leu-605 to Arg-617 contained 88% WT and 4% each of the other three nucleotides. This oligonucleotide was hybridized with an oligonucleotide primer containing SacII site in equimolar proportions, and T7 DNA polymerase (exo−) was used to copy the template containing the randomized nucleotides and to make duplex DNA. The double-stranded DNA was digested with BisWI and SacII, purified, and inserted into pTaqDUM between BisWI and SacII restriction sites in place of the stuffer fragment. The reconstructed plasmids were transformed into DH5α cells by electroporation, and the cells were incubated in 1 ml of 2× yeast extract and tryptone (YT) at 37°C for 1 h. The number of clones within the library was determined by plating an aliquot onto 2× YT plates containing 30 μg/ml chloramphenicol. The remainder of the transformation mixture was pooled and incubated in 1 liter of 2× YT containing chloramphenicol for 12 h at 37°C. Plasmids were purified (pTaqLIB) by CsCl gradient centrifugation.
Genetic Selection.
In complementation studies, E. coli recA718 polA12 cells were transformed with 0.2 μg each of the plasmids pHSG576, pTaqDUM, pTaq, or pTaqLIB by electroporation, and the cells were allowed to recover in nutrient broth medium for 2 h at 30°C. After recovery, a small fraction of the mixture was plated in duplicate onto nutrient agar plates containing chloramphenicol. One plate was incubated at 30°C and the other at 37°C for 24 h, and the resulting colonies were counted. Only paired samples that contained 200 colonies or fewer at 30°C were analyzed, because dense plating of cells leads to elevated background at 37°C. Complementation experiments with either inactive pHSG576 or pTaqDUM consistently yielded over 100-fold fewer colonies at 37°C relative to 30°C, indicating that the background for our complementation-based selection assay is <1%. Transformation with pTaq consistently yields an equal number of colonies after incubations at 30°C or 37°C, indicating that Taq pol I fully restores the growth-defective phenotype at elevated temperatures. Plasmids harboring WT and mutant Taq pol Is were isolated by miniprep (Promega) after overnight propagation at 37°C in 2× YT, and 200 nt surrounding the randomized region were amplified by PCR and sequenced.
Analysis of Polymerase Activity.
Three hundred fifty random colonies that grew on plates at 37°C were isolated and grown in nutrient broth individually overnight at 30°C. Each culture was grown to an OD595 of 0.3 at 30°C, and Taq pol I expression was induced with 0.5 mM isopropyl β-d-thiogalactoside (IPTG), and the incubations were continued for 4 h. Taq pols were purified by using a modified protocol of refs. 10 and 11, which allows efficient (>50%) purification of Taq pol I while removing endogenous polymerase and nuclease activities. Polymerase activity was assayed by using a 20-μl reaction mixture containing 50 mM KCl, 10 mM Tris⋅HCl (pH 8), 0.1% Triton X-100, 2.5 mM MgCl2, 0.4 mg of activated calf thymus DNA, 10 μM each dNTP, 0.25 mCi of [α-32P]dATP, and 1 μl of WT or mutant Taq pol. Incubations were at 72°C for 5 min, and reactions were stopped with the addition of 100 μl of 0.1 M sodium pyrophosphate, followed by 0.5 ml of 10% trichloroacetic acid (TCA). Polymerase activity was quantified by collecting precipitated radioactive DNA onto glass filter papers, and the amount of radioactivity was measured by scintillation counting. Activities of all selected active clones were confirmed by conducting primer extension reactions at 55°C in the presence of all four dNTPs as described (12). Extension products from these incubations were analyzed by 14% PAGE. The frequency at which 5′-32P-labeled primers are fully extended to the 5′-template terminus correlates well with the activity measured on activated calf thymus DNA.
Sequence Analysis.
Plasmids harboring either selected or unselected Taq pol I were isolated by miniprep (Promega), and a 200-bp fragment containing motif A was sequenced from each isolated plasmid. Natural E. coli isolated from geographically diverse environments were provided by Ron Jones from University of Iowa Hospitals and Clinics. The polA gene was amplified from a single colony of each strain by PCR with Pfu DNA polymerase, and both DNA strands from the 3′ terminus of the gene, including the pol I active site, were sequenced. All sequences were compared with the sequence of a laboratory K-12 strain, which we also determined.
Results and Discussion
Random Mutagenesis and Genetic Complementation.
To determine the contribution of specific active site residues to proper polymerase function, we randomly mutated 13 residues of the most highly conserved region within DNA polymerases, motif A. Our random mutagenesis protocol allows creation of a large population of mutants in which each amino acid can be altered to potentially any of the other 19 (13, 14). When this protocol is coupled with a stringent selection scheme, we can determine the nature of allowable amino acid substitutions in vivo after sequencing selected mutants. We used random sequence mutagenesis to create substitutions within the catalytic site of Taq pol I and selected functional mutants in E. coli recA718 polA12, a strain that contains a temperature-sensitive mutation in the polA gene and can be propagated at 30°C, but not at 37°C. Taq pol I can fully restore the temperature-sensitive phenotype such that E. coli recA718 polA12 harboring Taq pol I exhibits a 100% survival rate at 37°C relative to 30°C. Taq pol I shares 38% amino acid identity with, and has a folding pattern almost identical to, E. coli DNA pol I (4) and likely functions in the same pathways as E. coli pol I. The thermostable nature of this polymerase facilitates efficient purification and subsequent biochemical characterization of all selected mutants. The randomly mutated Taq library contains 200,000 independent clones. After transformation of these clones into E. coli recA718 polA12, 5% formed colonies at 37°C relative to 30°C. After subtracting the background (<1%), we estimate there are 8,000 independent library clones that encode an active Taq pol I. This represents the largest collection of physiologically active polymerase mutants of which we are aware. These results suggest that the polymerase catalytic site can potentially accommodate a surprisingly large number of amino acid substitutions in vivo.
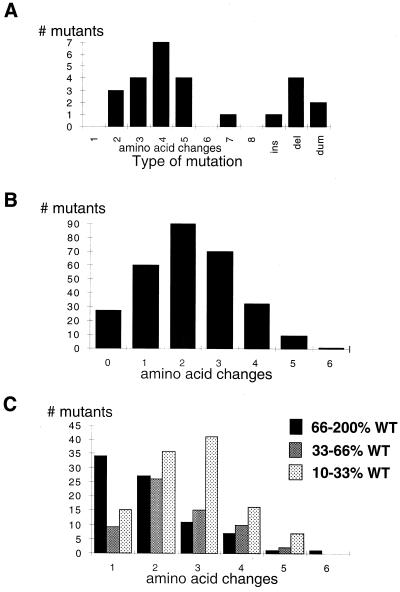
To establish the spectrum of mutations that restored growth of E. coli recA718 polA12, we sequenced the randomized insert from both unselected clones (30°C) and from selected clones (37°C). Analysis of sequences (Fig. 2A) from unselected plasmids, which reflects the distribution of mutants found in the random library before selection, shows that the average number of amino acid substitutions is four. In addition, several clones contained insertions, deletions, and stuffer fragments that arose during library construction. After selection, we randomly picked 350 colonies that grew at 37°C, measured Taq DNA polymerase activity, isolated the plasmids, and sequenced 200 nt encompassing the substituted random sequence. Of the 350 clones, 20 were inactive (<2% DNA polymerase activity relative to WT); 39 clones had low activity at 72°C (2 to 10%); whereas 291 were active (>10 to 200% WT activity). The 291 independent active clones had on average two amino acid changes, ranging from none (27 clones) to 1 clone containing six amino acid changes; no selected active clone contained insertions or deletions (Fig. 2B).
Figure 2.
Distribution of amino acid substitutions. (A) Taq pol I random library contains 200,000 individual clones; sequence analysis shows that the average number of amino acid substitutions within unselected library members is four; in addition, the unselected library contains insertion, deletion, and dummy fragments. (B) There are 8,000 independent clones within the selected, active population; sequence analysis shows that the average number of amino acid changes is two, with no clones containing insertion or deletion errors. (C) Distribution of high, moderate, and low activity mutants relative to number of amino acid changes. Most mutants containing a single amino acid substitution exhibit WT-like DNA polymerase activity. Mutants with moderate or low activity contain on average two or three substitutions, respectively. Mutants with high activity generally produce greater yields during PCR and presumably have higher processivity.
A large number of these selected mutants retain DNA polymerase activity comparable to WT (66–200% WT). Of the 264 active mutants, approximately one-third of the mutants exhibit WT DNA polymerase activity (Fig. 2C). Most mutants containing a single amino acid substitution possess WT activity. Twenty percent (7 of 33) of mutants with four amino acid changes maintain normal activity, and a clone containing six amino acid substitutions also exhibits WT activity. The number of mutants exhibiting moderate activity (33–66% WT) or low activity (10–33% WT) follows a Poisson distribution relative to number of amino acid changes, with a median of two or three amino acid substitutions per clone, respectively. Plasmids (27 clones) that encode WT enzyme at the amino acid sequence contain silent nucleotide substitutions; these enzymes have activities similar to those of WT controls. These data indicate that, even in cases of especially pronounced mutation burden with many amino acids substituted within an evolutionarily conserved motif, a large number of mutants can exhibit high activity.
Nature of Allowable Substitutions.
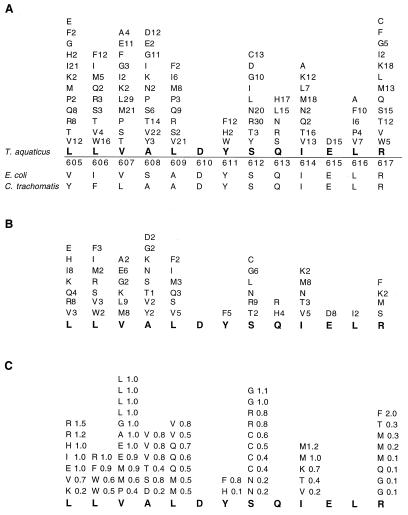
Sequence analysis of all 291 selected active clones (10–200% WT activity; Fig. 3A), including the 87 most active clones (>66–200% WT activity; Fig. 3B), showed that most motif A residues tolerate a wide spectrum of substitutions (Leu-605, Leu-606, Val-607, Ala-608, Leu-609, Ser-612, Ile-614, and Arg-617), some residues tolerate predominantly conservative substitutions (Tyr-611, Gln-613, Glu-615, and Leu-616), and only one residue is immutable (Asp-610). Of the highly mutable residues, Ser-612, which is present in nearly all eukaryotic and prokaryotic DNA polymerases sequenced, tolerates substitutions that are diverse in size and hydrophilicity while often preserving WT-like activity. Consistent with these mutability data, structural analysis shows that Ser-612 projects away from the catalytic site and does not appear to maintain significant interactions. Of the other highly mutable amino acids, hydrophobic residues Leu-605 to Leu-609 form a strand of the structurally conserved antiparallel β sheet that accommodates the triphosphate portion of the incoming dNTP. That these hydrophobic residues can be replaced indicates none are essential for WT activity; in fact, a mutant with six substitutions (Leu-605 → Arg, Leu-606 → Met, Val-607 → Lys, Ala-608 → Ser, Leu-609 → Ile, and Ser-612 → Arg) exhibits WT DNA polymerase activity.
Figure 3.
High mutability of motif A. To test the importance of this conservation, Residues L605 to R617 (D610YSQIELR617) were randomly mutated such that each contiguous amino acid can be replaced by potentially any of the other 19. (A) The degree of mutability of each amino acid within motif A from all active clones (>10% to 200% activity relative to WT) complementing an E. coli DNA polymerase I temperature-sensitive strain. Amino acid substitutions at the locus are listed, along with the number of times each substitution is observed. (B) Mutations in clones exhibiting high activity (66% to 200% WT). (C) Mutations in clones containing a single amino acid substitution followed by activity relative to WT. Clones containing the same amino acid substitution contain unique silent changes at other loci; thus, they represent independent sequences.
Those residues that tolerate predominantly conservative changes (Tyr-611, Gln-613, Glu-615, and Leu-616) are believed to function in dNTP binding and/or for protein stability (4). Tyr-611 projects into a large hydrophobic pocket, anchors motif A, and is replaced only by a planar ringed amino acid. Gln-613 projects out into a small pocket near the finger motif, limits the extent of the conformational change of the finger motif during the dNTP binding step (Fig. 1), and is replaced only by amino acids that can fit into this pocket. Glu-615 hydrogen bonds with Tyr-671 (a residue located in helix O within the finger motif and stacks with the base portion of the incoming dNTP) and can be replaced only by Asp, thus retaining the hydrogen bonding potential. Leu-616 interacts with the finger motif during the dNTP binding step and is predominantly replaced by other hydrophobic amino acids. The only immutable residue is Asp-610, which even in the context of other mutations (Fig. 3 A and B), cannot be replaced even by glutamic acid. Asp-610 functions to coordinate the metal-mediated catalysis reaction, leading to the incorporation of the incoming nucleotide.
Analysis of 60 mutants with a single amino acid change yields a similar distribution of mutability and allows us to determine the effect on activity conferred by specific amino acid substitutions (Fig. 3C). Residues of the antiparallel β-strand can accommodate numerous single amino acid substitutions without significantly compromising activity. Some of these substitutions, including Leu-605 → Arg, confer greater polymerase activity relative to WT Taq pol I; all selected Leu-605 → Arg mutants occurring in context of multiple mutations also exhibit high activity (Fig. 3 A and B). The single substitution Arg-617 → Phe confers twice the activity of WT Taq Pol I, although other substitutions at this locus lower Taq pol I activity.
Evolution of the Polymerase Active Site.
Our genetic selection protocol allows isolation of mutant polymerases that retain a high DNA polymerase activity. Bacteria dependent on these approximately 300 mutant active polymerases can be grown under logarithmic growth conditions in liquid broth at 37°C (before plasmid isolation and protein purification) or as colonies in solid agar at 37°C (>50 generations) without significant variations in growth kinetics. Thus, bacteria dependent on mutant enzymes for survival are fit to replicate repetitively. In addition, most enzymes containing substitutions within motif A exhibit WT-like biochemical properties; however, a subset of enzymes (approximately 10%) exhibit diverse base pairing fidelity or altered dNTP/rNTP specificity relative to the WT enzyme (unpublished data). This inherent plasticity of the active site amino acid sequence is in contrast with the constancy of motif A found in nature. GenBank blast search and sequence alignment of polA genes from 34 individual prokaryotes and eubacteria showed that 27 organisms have maintained the DYSQIELR motif A sequence. The few naturally occurring amino acid substitutions within motif A include Leu → Met in species within the Mycobacterium genus, Tyr → Phe in Aquifex aeolicus, Ser → Val in Rhodothermus obamensis, Arg → Ala in Borrelia burgdorferi, and Ser → Thr and Arg → Val in Treponema pallidum. These data indicate that the polA gene, and specifically the active site, evolves very slowly.
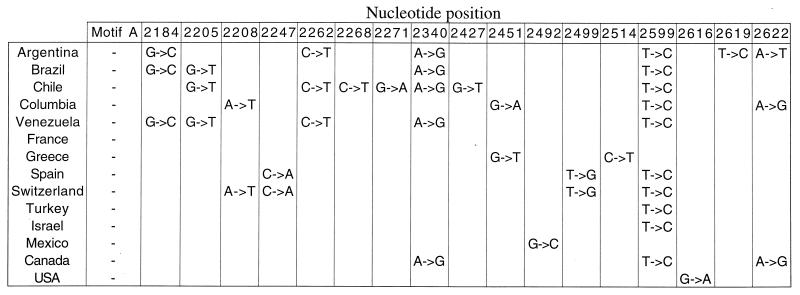
To further characterize the evolution of the polA gene, we collected strains of E. coli from geographically diverse regions and sequenced a portion of the polA gene. We assume that this geographical diversity can be equated with multiple rounds of division. The inherent mutation rate of E. coli (10−5 per nucleotide per division in mutators to 10−9 per nucleotide per division in nonmutators; ref. 15) predicts that, after over 108 years of E. coli and archaeal evolution (16), their polA genes could potentially contain silent or neutral mutations at each codon position. However, analysis of E. coli strains isolated from geographically distinct locations shows that each has a nearly identical amino acid and nucleotide sequence (>99% identity); nucleotides encoding motif A amino acids are 100% identical in all of these distinct E. coli strains (Fig. 4). Most mutations cluster in geographically distinct loci, suggesting that these alterations occurred in an early founder population and have been maintained with time. Whereas these data show that some mutations do occur in the polA gene, the degree of mutability is much less than that expected from our random mutagenesis study and from that predicted by the normal mutation rate of E. coli. Consistent with the E. coli sequence data, two geographically distinct strains of Rickettsia prowazekii (strains B and Madrid E) have identical amino acid and nucleotide sequences for the entire polA gene (Fig. 5 and data not shown). Although these data show high sequence identity in the polA gene of diverse E. coli, they do not allow us to determine whether or not motif A nucleotide sequence is uniquely conserved.
Figure 4.
Sequence of the polA gene from natural E. coli isolates. The sequence of both strands of the polA gene from nucleotide 2113 to 2642 encoding residues of motif A and the C terminus portion of DNA pol I was determined. The base change and position is shown for each isolate; nucleotides encoding motif A show no change. Position 2599 contains a cytosine in most isolates, and thus could represent the global WT polA sequence. An isolate from France has the identical nucleotide sequence as the K-12 strain.
Figure 5.
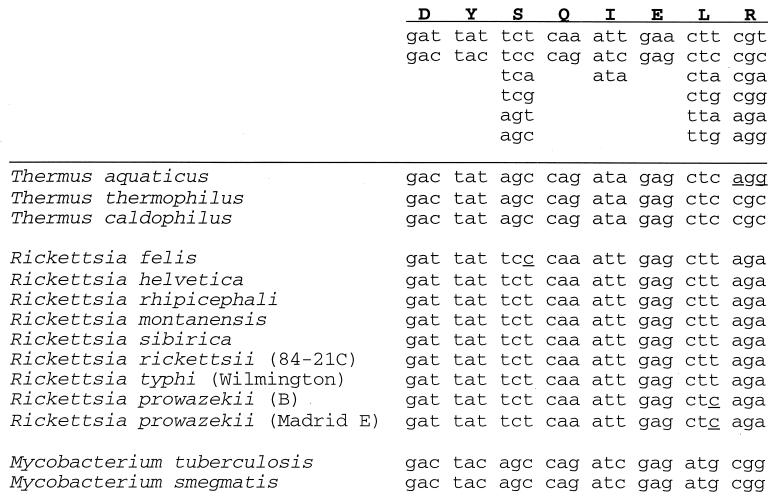
Nucleotide sequence conservation of the pol I catalytic site within individual genus. Most prokaryote and eubacteria polA genes sequenced retain the DYSQIELR motif A sequence; however, a few organisms, including those in the Mycobacterium genus, contain amino acid substitutions (e.g., Leu → Met). To further characterize the apparently slow evolution of the polymerase active site, nucleotide sequences obtained from GenBank blast searches were compared. These results show nearly 0% base sequence change of motif A residues within each genus. Outside of motif A, 10–50% of identical amino acids at specific loci are encoded by alternate codons. Universal codons from the DNA (+) strand encoding for motif A amino acids are shown on Top.
To determine the level of nucleotide conservation within the active sites of polymerases from other organisms, we aligned both the amino acid and nucleotide sequence of polA genes from diverse prokaryote and eubacteria species (Fig. 5). Only if both sequences contained an identical amino acid at a locus, was the nucleotide codon then compared. Sequence alignments of related species within the same genus (e.g., Rickettsia genus, Mycobacterium genus, or Thermus genus), which branched many million years ago, show nearly complete nucleotide conservation of motif A within each genus (Fig. 5). Comparison of polA sequences from individual species within the Rickettsia genus showed that the greatest degree of nucleotide sequence identity (as judged by the presence of synonymous codons) occurs within the DNA polymerase and 5′ nuclease catalytic sites. For example, 24 amino acid residues within and flanking motif A are encoded by the identical codons in at least seven Rickettsia species. The frequency for this level of conservation for any one codon position within the entire polA gene of Rickettsia is 0.8. Thus, the probability of 24 contiguous amino acids being encoded by the same codon by random chance is <0.005 (= 0.824). The sequence homogeneity of the active site is unlikely due to codon selection bias through the use of a specific set of tRNAs, because multiple codons encoding the same amino acids are used randomly elsewhere in the polA gene within individual species of the Rickettsia, Mycobacterium, Thermus, and Escherichia genera.
Rapid Evolution in Laboratory vs. Slow Evolution in Nature.
We find, after random sequence mutagenesis and selection by genetic complementation, that amino acids of the polymerase catalytic site are highly mutable. This high plasticity is a property not only of the thermostable Taq pol I, because regions just outside of the catalytic site of other polymerases, including mammalian DNA pol β (17) and HIV-1 reverse transcriptase (18) and other proteins (e.g., β-lactamase, herpes simplex virus thymidine kinase, human thymidylate synthetase, and human alkyl transferase) from diverse organisms, are also highly mutable (13, 19–21). In each case, this inherently high plasticity facilitated isolation of highly active enzymes with altered substrate specificity.
Preservation of a plastic, mutable active site could facilitate the generation of beneficial mutants under specific selective forces, such as mutant polymerases with enhanced activity or those unable to incorporate harmful nucleotide analogs during chemotherapy. In addition, the plastic nature of active sites may allow proteins to tolerate high mutation burdens. It has been demonstrated that as few as three successive selection steps yielded a population of E. coli cells that mutated at elevated rates (22), and 1–5% of pathogenic E. coli and Salmonella enterica are mutators (23). These data predict that, after 100 million years of dividing naturally at >100 generations per year (24) and at a mutation rate of at least 10−9 per base per generation (15), geographically isolated prokaryotic organisms should have had a chance to mutate at each nucleotide position and exhibit markedly heterogeneous genotypes. The sequence divergence would be even greater for progeny of cells that existed as transient mutators and divided at a mutation rate up to 10−5 per base per generation (22, 25). However, we find that strains of identical species isolated from geographically diverse areas have nearly the same nucleotide sequence within the polA gene, and species within the same genus have nearly identical active site nucleotide sequence, suggesting that the polA gene evolves at a very slow rate. Other groups have also suggested that the entire prokaryotic and archaeal genomes evolve at rates significantly slower than that predicted by laboratory mutation rates (26).
Thus, we are presented with two surprising and seemingly contradictory findings: DNA pol I is highly plastic and allows multiple amino acid substitutions within its catalytic site, yet the polA gene is highly stable in nucleotide sequence within species. There are at least three possible mechanisms that could account for this dichotomy. (i) Taq pol I contains a highly plastic active site, although other polymerases and enzymes are not so plastic. We doubt this hypothesis is the case, because we have shown that other polymerases and enzymes are also highly mutable (9, 13, 17–21). (ii) Subtle selection pressures maintain the WT active site. Although E. coli dependent on these mutant enzymes are fit to replicate repetitively (>50 generations), it is difficult to fully rule out the effects of long-term selection. However, these pressures must be stringent to maintain WT nucleotide sequences, while allowing almost no third codon position changes after prolonged evolution. At the very least, the data suggest that there are aspects of natural evolution (relative to evolution in the laboratory) that we do not yet comprehend. (iii) Genetic transfer mechanisms maintain homogeneous sequences. Specifically, we propose that the inherent plasticity of proteins we describe here enables tolerance of the high mutation burden during adverse conditions characterized by selection of mutators (22) and facilitates the generation of beneficial mutations with a selective advantage; after successful survival through periods of adverse conditions, WT sequence (one that is fit and the most prevalent) can be generated through genetic transfer. Selection for a fit sequence could potentially be the driving force for genetic transfer. This model is consistent with at least 50-fold higher rate of lateral transfer relative to the mutation rate observed in natural E. coli isolates (27). This model suggests that genetic transfer can function to maintain sequence homogeneity and could account for the dichotomy between inherent amino acid substitutability within diverse enzymes and the constancy of the nucleotide sequence found in nature.
Acknowledgments
We thank Dr. Ellie Adman for Taq pol I structure illustrations and discussions, Drs. Brad Preston and Motoshi Suzuki for helpful discussions, Matt Ashbach for technical assistance, and Drs. Phil Green, Barry Hall, Mickey Fry, Lance Encell, and Al Mildvan for critical analysis of this manuscript. This work was supported by the Medical Scientist Training Program (National Institutes of Health, National Institute of General Medical Sciences, 5T3207266) to P.H.P and grants form the National Cancer Institute to L.A.L. (R35 CA39903 and CA78885).
Abbreviations
- Taq pol I
Thermus aquaticus DNA polymerase I
- WT
wild-type
References
- 1.Pelletier H, Sawaya M R, Kumar A, Wilson S H, Kraut J. Science. 1994;264:1891–1903. [PubMed] [Google Scholar]
- 2.Beese L S, Derbyshire V, Steitz T A. Science. 1993;260:352–355. doi: 10.1126/science.8469987. [DOI] [PubMed] [Google Scholar]
- 3.Patel P H, Jacobo-Molina A, Ding J, Tantillo C, Clark A D, Jr, Raag R, Nanni R G, Hughes S H, Arnold E. Biochemistry. 1995;34:5351–5363. doi: 10.1021/bi00016a006. [DOI] [PubMed] [Google Scholar]
- 4.Li Y, Korolev S, Waksman G. EMBO J. 1998;17:7514–7525. doi: 10.1093/emboj/17.24.7514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reha-Krantz L J, Nonay R L. J Biol Chem. 1994;269:5635–5643. [PubMed] [Google Scholar]
- 6.Astatke M, Grindley N D, Joyce C M. J Mol Biol. 1998;278:147–165. doi: 10.1006/jmbi.1998.1672. [DOI] [PubMed] [Google Scholar]
- 7.Wang J, Sattar A K, Wang C C, Karam J D, Konigsberg W H, Steitz T A. Cell. 1997;89:1087–1099. doi: 10.1016/s0092-8674(00)80296-2. [DOI] [PubMed] [Google Scholar]
- 8.Takehsita S, Sato M, Toba M, Masahashi W, Hashimoto-Gotoh T. Gene. 1987;61:63–74. doi: 10.1016/0378-1119(87)90365-9. [DOI] [PubMed] [Google Scholar]
- 9.Suzuki M, Baskin D, Hood L E, Loeb L A. Proc Natl Acad Sci USA. 1996;93:9670–9675. doi: 10.1073/pnas.93.18.9670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grimm E, Arbuthnot P. Nucleic Acids Res. 1995;23:4518–4519. doi: 10.1093/nar/23.21.4518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Desai U J, Pfaffle P K. BioTechniques. 1995;19:780–782. ,784. [PubMed] [Google Scholar]
- 12.Patel P H, Preston B D. Proc Natl Acad Sci USA. 1994;91:549–553. doi: 10.1073/pnas.91.2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dube D K, Loeb L A. Biochemistry. 1989;28:5703–5707. doi: 10.1021/bi00440a001. [DOI] [PubMed] [Google Scholar]
- 14.Encell L P, Landis D M, Loeb L A. Nat Biotech. 1999;17:143–147. doi: 10.1038/6142. [DOI] [PubMed] [Google Scholar]
- 15.Drake J W. Proc Natl Acad Sci USA. 1991;88:7161–7164. [Google Scholar]
- 16.Ochman H, Wilson A C. J Mol Evol. 1988;26:74–86. doi: 10.1007/BF02111283. [DOI] [PubMed] [Google Scholar]
- 17.Sweasy J B, Loeb L A. Proc Natl Acad Sci USA. 1993;90:4626–4630. doi: 10.1073/pnas.90.10.4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim B, Hathaway T R, Loeb L A. J Biol Chem. 1996;271:4872–4878. doi: 10.1074/jbc.271.9.4872. [DOI] [PubMed] [Google Scholar]
- 19.Black M E, Newcomb T G, Wilson H-M P, Loeb L A. Proc Natl Acad Sci USA. 1996;93:3525–3529. doi: 10.1073/pnas.93.8.3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Landis D M, Loeb L A. J Biol Chem. 1998;273:25809–25817. doi: 10.1074/jbc.273.40.25809. [DOI] [PubMed] [Google Scholar]
- 21.Christians F C, Loeb L A. Proc Natl Acad Sci USA. 1996;93:6124–6128. doi: 10.1073/pnas.93.12.6124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mao E F, Lane L, Lee J, Miller J H. J Bacteriol. 1997;179:417–422. doi: 10.1128/jb.179.2.417-422.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.LeClerc J E, Li B, Payne W L, Cebula T A. Science. 1996;274:1208–1211. doi: 10.1126/science.274.5290.1208. [DOI] [PubMed] [Google Scholar]
- 24.Gibbons R J, Kapsimalis B. J Bacteriol. 1967;93:510–512. doi: 10.1128/jb.93.1.510-512.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taddei F, Radman M, Maynard-Smith J, Toupance B, Gouyon P H, Godelle B. Nature (London) 1997;387:700–702. doi: 10.1038/42696. [DOI] [PubMed] [Google Scholar]
- 26.Ochman H, Elwyn S, Moran N A. Proc Natl Acad Sci USA. 1999;96:12638–12643. doi: 10.1073/pnas.96.22.12638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guttman D S, Dykhuizen D E. Science. 1994;266:1380–1383. doi: 10.1126/science.7973728. [DOI] [PubMed] [Google Scholar]