Abstract
Most δ-opioid receptors are located on the presynaptic terminals of primary afferent neurons in the spinal cord. However, their presence in different phenotypes of primary afferent neurons and their contribution to the analgesic effect of δ-opioid agonists are not fully known. Resiniferatoxin (RTX) is an ultra-potent transient receptor potential vanilloid type 1 channel (TRPV1) agonist and can selectively remove TRPV1-expressing primary afferent neurons. In this study, we determined the role of δ-opioid receptors expressed on TRPV1 sensory neurons in the antinociceptive effect of the δ-opioid receptor agonists [d-Pen2,d-Pen5]-enkephalin and [d-Ala2,Glu4]-deltorphin. Nociception was measured by testing the mechanical withdrawal threshold in the hindpaw of rats. Changes in the δ-opioid receptors were assessed using immunocytochemistry and the [3H]-naltrindole radioligand binding. In RTX-treated rats, the δ-opioid receptor on TRPV1-immunoreactive dorsal root ganglion neurons and afferent terminals in the spinal cord was diminished. RTX treatment also significantly reduced the maximal specific binding sites (31%) of the δ-opioid receptors in the dorsal spinal cord. Interestingly, intrathecal injection of [d-Pen2,d-Pen5]-enkephalin or [d-Ala2,Glu4]-deltorphin produced a large and prolonged increase in the nociceptive threshold in RTX-treated rats. These findings indicate that loss of TRPV1-expressing afferent neurons leads to a substantial reduction in presynaptic δ-opioid receptors in the spinal dorsal horn. However, the effect of δ-opioid agonists on mechano-nociception is paradoxically potentiated in the absence of TRPV1-expressing sensory neurons. This information is important to our understanding of the cellular sites and mechanisms underlying the spinal analgesic effect of δ-opioid agonists.
INTRODUCTION
The spinal cord dorsal horn is a critical site for the nociceptive transmission and analgesic actions of µ- and δ-opioid receptor agonists. Similar to µ-opioid receptors, the δ-opioid receptors are distributed in the dorsal root ganglion (DRG) neurons and their central terminals in the superficial dorsal horn of the spinal cord (Abbadie et al., 2002; Wang and Wessendorf, 2001; Zhang et al., 1998). Activation of δ-opioid receptors inhibits nociceptive transmission at the spinal level. For example, intrathecal administration of the δ-opioid receptor agonist produces a potent analgesic effect in acute and chronic pain models (Holdridge and Cahill, 2006; Hurley et al., 1999; Malmberg and Yaksh, 1992). Furthermore, spinally administered δ-opioid agonists inhibit the evoked response of spinal dorsal horn neurons (Khan et al., 2002). The δ-opioid receptors are located both presynaptically on the central terminals of primary afferents and postsynaptically on the spinal dorsal horn neurons (Abbadie et al., 2002; Besse et al., 1990). However, the presence of δ-opioid receptors in different phenotypes of nociceptive afferent neurons and their relative contribution to the analgesic effect produced by spinally administered δ-opioid agonists are not fully known.
The capsaicin receptor, transient receptor potential vanilloid type 1 channel (TRPV1), is essential for the detection of thermal nociception. Mice deficient in TRPV1 show an impaired response to noxious heat (Caterina et al., 2000). Resiniferatoxin (RTX), originally extracted from the cactus-like plant Euphorbia resinifera, is an ultra-potent TRPV1 agonist (Szallasi and Blumberg, 1999). RTX treatment induces a long-lasting impairment of thermal nociception (Chen and Pan, 2006b; Pan et al., 2003). However, mechano-nociception remains largely intact in the absence of TRPV1-expressing sensory neurons (Chen and Pan, 2006b; Chen et al., 2007b). Unlike capsaicin, which only removes certain primary sensory neurons in neonatal rats, RTX can remove TRPV1-expressing sensory neurons in adult rats (Chen and Pan, 2006b; Pan et al., 2003). The use of RTX is a preferred approach since it circumvents the developmental plasticity induced by capsaicin treatment in neonatal animals. Thus, RTX has become a valuable tool for studying mechanical pain transmission through non-TRPV1 sensory neurons and for studying the functional importance of presynaptic opioid receptors in the spinal cord.
We have shown that RTX treatment leads to a substantial reduction in the number of presynaptic µ-opioid receptors in the spinal dorsal horn (Chen and Pan, 2006b). Paradoxically, the analgesic effect of µ-opioid receptor agonists is significantly potentiated in RTX-treated rats (Chen and Pan, 2006b). In the spinal cord, the ratio of pre- to postsynaptic δ-opioid receptors is greater than that of µ-opioid receptors (2:1 vs. 1:1, respectively) (Abbadie et al., 2002). Because many δ-opioid receptors may be located on TRPV1-expressing afferent neurons, RTX treatment could remove many presynaptic δ-opioid receptors in the spinal dorsal horn. In the present study, we determined the relative contribution of presynaptic δ-opioid receptors to the analgesic effect of spinally administered δ-opioid agonists. We found that RTX treatment caused a substantial reduction in presynaptic δ-opioid receptors in the spinal cord but it failed to attenuate the effect of δ-opioid agonists on mechano-nociception. This study provides new evidence that the δ-opioid receptors on non-TRPV1-expressing primary afferents and spinal dorsal horn neurons play an important role in the modulation of mechano-nociception.
METHODS
Animals
Male rats (Harlan Sprague-Dawley, Indianapolis, IN) weighing 200 – 220 g were used in this study. Rats received a single intraperitoneal injection of RTX (200 µg/kg, LC Laboratories, Woburn, MA) under isoflurane (2% in O2) anesthesia. RTX was dissolved in a mixture of 10% Tween-80 and 10% ethanol in normal saline (Chen and Pan, 2006b; Pan et al., 2003). We have shown that systemic RTX treatment deletes primary afferent neurons without any evident effect on spinal dorsal horn neurons that express TRPV1 in adult rats (Chen and Pan, 2006b). Rats in the control group received intraperitoneal injection of the vehicle. The experiments were conducted 4–5 weeks after RTX and vehicle injections unless stated otherwise. The surgical preparation and experimental protocols were approved by the University of Texas M. D. Anderson Cancer Center and conformed to the National Institutes of Health guidelines on the ethical use of animals.
Intrathecal catheters (PE-10 polyethylene tubing) were inserted in RTX- and vehicle-treated rats during isoflurane-induced anesthesia. In each case, the catheter was advanced 8 cm caudally through an incision in the cisternal membrane and secured to the musculature at the incision site. The rats were allowed to recover for 5–7 days before being used to test the antinociceptive effect of the specific δ-opioid agonists [d-Pen2,d-Pen5]-enkephalin (DPDPE) and [d-Ala2,Glu4]-deltorphin (DELT). Only animals with no evidence of neurological deficits after catheter insertion were studied. Drugs for intrathecal injection were dissolved in normal saline and administered in a volume of 5 µl followed by a 10 µl flush with normal saline. Repeated intrathecal injections in the same animals were separated by 4–5 days. DPDPE and DELT were obtained from Tocris (Ellisville, MO).
Behavioral assessment of nociception
To quantitatively assess the thermal sensitivity, rats were placed on the glass surface of a thermal testing apparatus (IITC, Woodland Hills, CA). The rats were allowed to acclimate for 30 min before testing. The temperature of the glass surface was maintained constant at 30°C. A mobile radiant heat source located under the glass was focused onto the hindpaw of each rat. The paw-withdrawal latency was recorded by a timer, and the mean value from both hindpaws was determined. The cutoff time point of 30 s was used to prevent tissue damage (Chen and Pan, 2003; Pan et al., 2003).
The nociceptive mechanical threshold was measured using an Ugo Basil Analgesimeter (Varese, Italy). The test was performed by applying a noxious pressure to the hindpaw. By pressing a pedal that activated a motor, the force increased at a constant rate on the linear scale. When the animal responded by withdrawal of the paw or vocalization, the pedal was immediately released and the nociceptive threshold read on a scale. The cutoff weight of 400 g was used to avoid tissue injury (Chen and Pan, 2006b; Chen et al., 2007b). Both hindpaws were tested in each rat, and the mean value was used as the nociceptive withdrawal threshold.
Double fluorescence labeling of δ-opioid receptors and IB4 in the DRGs and spinal cord
To determine the effect of RTX treatment on the δ-opioid receptors and Griffonia simplicifolia isolectin B4 (IB4)-positive DRG neurons and primary afferent terminals in the spinal dorsal horn, double fluorescence labeling of δ-opioid receptors and IB4 [a marker for unmyelinated primary neurons and afferent fibers (Kitchener et al., 1994; Kitchener et al., 1993)] was performed in the lumbar DRGs and spinal cords from three RTX-treated and three vehicle-treated rats 4 weeks after treatment. As described previously (Chen and Pan, 2006b; Chen et al., 2007b), each rat was intracardially perfused with 4% paraformaldehyde and 10% sucrose in 0.1 M PBS (pH 7.4) while under deep anesthesia with pentobarbital sodium (60 mg/kg, i.p.). The lumbar spinal cord and the DRGs were quickly removed and postfixed in the same fixative solution and cryoprotected in 30% sucrose in PBS for 48 hours at 4 °C. The spinal cord and DRG tissues were cut into 25- and30-µm-thick sections, respectively. The sections were collected free-floating in 0.1 M PBS and blocked in 4% normal goat serum in PBS for 1 hour. Then sections were incubated with the primary antibody (rabbit anti-δ opioid receptors, dilution 1:1000, Neuromics; Minneapolis, MN) diluted in PBS solution containing 4% normal goat serum and 0.1% TX-100 for 2 hours at room temperature and overnight at 4°C. Subsequently, sections were rinsed in PBS and incubated with the secondary antibody (Alexa Fluor-488 conjugated to goat anti-rabbit IgG, dilution: 5 µg/ml; Molecular Probes, Eugene, OR) for 1.5 hour. The sections were rinsed again and incubated with Alexa Fluor-594 conjugated to IB4 (dilution: 2 µg/ml, Molecular Probes) for 2 hours at room temperature. Finally, the sections were rinsed and mounted on slides, dried, and coverslipped. Omission of the primary antibody resulted in negative labeling in all the sections examined.
Double immunofluorescence labeling of TRPV1 and δ-opioid receptors in the DRG and spinal cord
Double immunofluorescence labeling of δ-opioid receptors and TRPV1 was performed in L4 and L5 DRGs and the lumbar spinal cord from three RTX-treated and three vehicle-treated rats. The tissue preparation techniques were the same as described above. The DRG and spinal cord sections were incubated with a mixture of primary antibodies (guinea pig anti-TRPV1, dilution 1:1,000; and rabbit anti-δ opioid receptors, dilution 1:1000, Neuromics) diluted in PBS solution containing 4% normal goat serum and 0.1% TX-100 for 2 hours at room temperature and overnight at 4°C. Subsequently, spinal sections were rinsed in PBS and incubated with the mixture of secondary antibodies (Alexa Fluor-488 conjugated to goat anti-guinea pig IgG; and Alexa Fluor-594 conjugated to goat anti-rabbit IgG, dilution: 5 µg/ml; Molecular Probes) for 1.5 hours. For the DRG sections, a different mixture of secondary antibodies (Alexa Fluor-488 conjugated to goat anti-rabbit IgG; and Alexa Fluor-594 conjugated to goat anti-guinea pig IgG, dilution: 5 µg/ml; Molecular Probes) was used. The sections were then rinsed in PBS for 30 min, mounted on slides, dried, and cover-slipped. Omission of the primary antibodies resulted in negative labeling in the DRG and spinal cord.
The sections were examined on a laser scanning confocal microscope (Zeiss LSM 510, Germany), and areas of interest were photodocumented. Alexa Fluor-488 and Alexa Fluor-594 fluorochromes were excited at 488- and 543-nm wavelengths, respectively. Immunoreactivity was examined under the conditions of optimal resolution (small pinhole, thin optical slice, and high-numerical-aperture water- or oil-immersion objective). The pinhole diameter was set to 1 airy unit (which corresponds to 1.2 µm depth of field). To completely rule out crosstalk between the fluorescent detection channels, the multitracking configuration was used.
[3H]-Naltrindole membrane bindings in the spinal cord
To determine if RTX treatment would alter the binding number and affinity of the δ-opioid receptors in the spinal cord, saturation binding of [3H]-naltrindole, a specific radioligand for δ- opioid receptors (Contreras et al., 1993), was carried out using the dorsal spinal cord tissue membranes. Four RTX- and four vehicle-treated rats were decapitated after being anesthetized with 2–3% isoflurane. The whole spinal cord was quickly harvested, and the dorsal half was dissected and used for the binding experiment. The tissue was homogenized in ice-cold 50 mM Tris-HCl buffer containing 3 mM MgCl2 and 1 mM EGTA (pH 7.4) and disrupted by sonication. The homogenate was then centrifuged at 500 g for 10 min at 4°C. The pellet was discarded and the supernatant was centrifuged at 48,000 g for 20 min at 4°C. The pellet was resuspended in Tris-HCl buffer and was centrifuged again as described in the preceding text. The final pellet was resuspended in 50 mM Tris-HCl buffer containing (in mM) 3 MgCl2, 100 NaCl, and 0.2 EGTA (pH 7.7) and disrupted by sonication for 5 s. The protein content was measured by means of the method of Bradford using the bovine serum albumin as the standard (Protein Assay Kit II, Bio-Rad Laboratories, Hercules, CA). Saturation radioligand binding experiments were performed using 200-µl aliquots of tissues and increasing concentrations of [3H]-naltrindole (35 Ci/mmol, PerkinElmer Life Sciences, Inc., Boston, MA) from 10 to 1,200 pM. Nonspecific binding was determined with 1 µM naloxone (Sigma, St. Louis, MO). Incubation was performed in duplicate in Tris-HCl buffer at 25°C for 60 min. The reaction was terminated by filtration through Whatman GF/B filters on a cell harvester with ice cold Tris-HCl buffer (pH 7.4). Radioactivity was measured by immersion of filters in the scintillation fluid and quantified using a liquid scintillation counter (LS 6500, Beckman Coulter, Fullerton, CA).
Statistical analysis
Data are presented as means ± SEM. Paw withdrawal thresholds in response to thermal and mechanical stimulation before and after RTX treatment were compared using an unpaired Student’s t-test. The effect of DPDPE and DELT on the mechanical withdrawal threshold was determined by two-way ANOVA followed by post hoc analysis. Because the time course and duration of the effect produced by DPDPE and DELT were different between the vehicle- and RTX-treated groups, the ED50 value was calculated by integrating the area under the dose-effect curve (Chen and Pan, 2006b). The [3H]-naltrindole saturation binding data were processed using nonlinear regression analysis (Prism; GraphPad Software, San Diego, CA) to calculate the maximal specific binding (Bmax) and dissociation constant (Kd), and paired Student’s t-test was used to determine the difference in Bmax and Kd between the control and RTX-treated groups. Differences were considered to be statistically significant when P < 0.05.
RESULTS
Effect of RTX on the paw withdrawal latency before and after RTX treatment
Systemic injection of RTX caused a large increase in the paw withdrawal latency in response to the noxious heat stimulus. After RTX treatment, the thermal paw withdrawal latency was significantly increased from an average of 9.16 ± 1.68 s before RTX injection to 27.43 ± 2.67 s (P < 0.05) 3 days after RTX injection (n = 22). The diminished thermal sensitivity lasted for more than 5 weeks. By contrast, intraperitoneal injection of the vehicle did not significantly alter the paw withdrawal threshold in response to the noxious heat. The latency was 9.24 ± 1.33 and 9.31 ± 1.29 s (n = 18) before and 3 days after vehicle injection. Because the thermal sensitivity was diminished in RTX-treated rats, the analgesic effect of the δ-opioid agonists was tested using only the pressure stimulus.
Effect of RTX treatment on the immunoreactivity of δ-opioid receptors and TRPV1 in DRG neurons and spinal dorsal horn
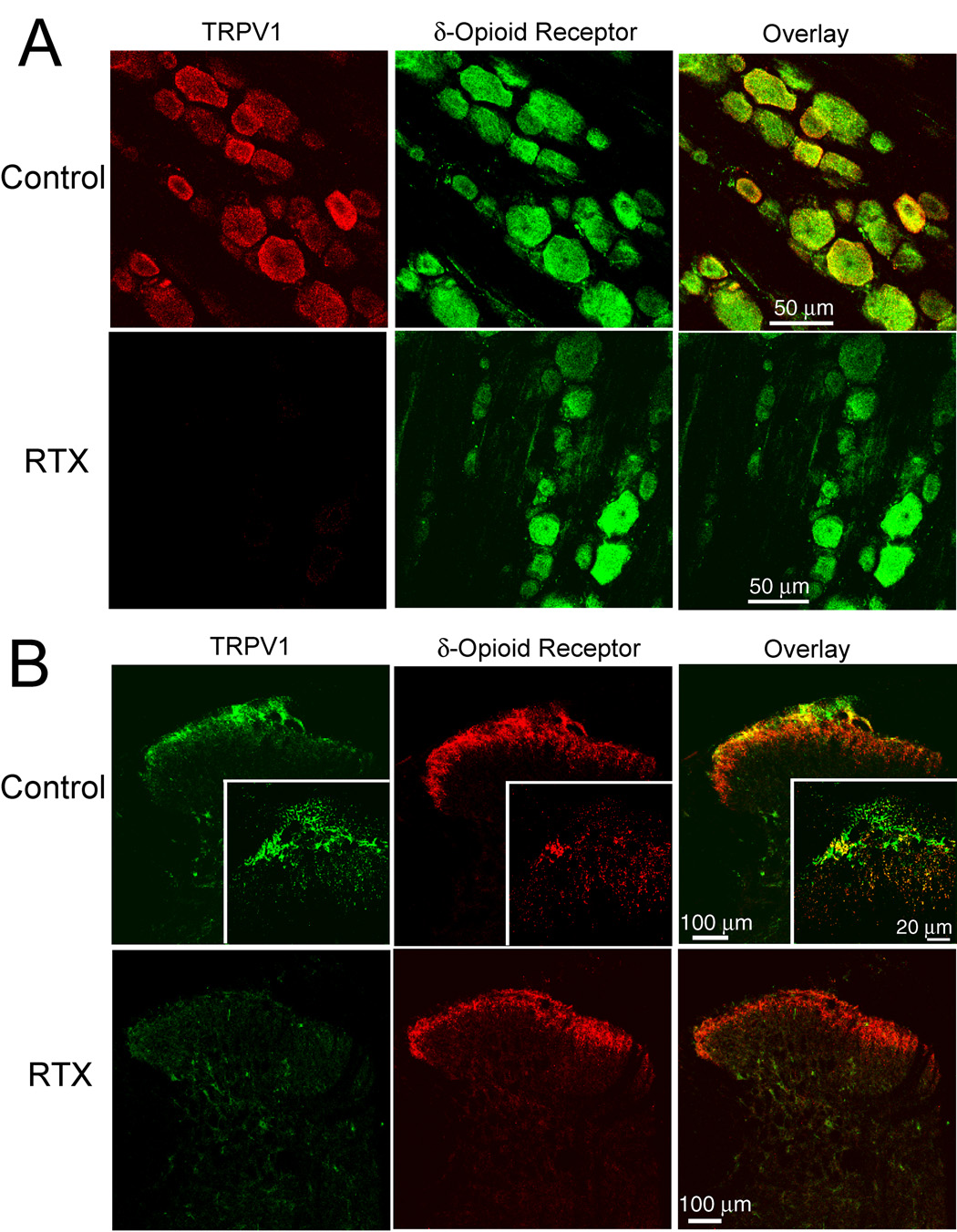
In vehicle-treated rats, the δ-opioid receptor immunoreactivity was present in DRG neurons of different sizes ranging from 20 to 50 µm. Also, dense TRPV1 immunoreactivity was mainly found in small- and medium-sized DRG neurons and colocalized with the δ-opioid receptor immunoreactivity (Fig. 1A). To quantify the reduction in the δ-opioid receptor-immunoreactive DRG neurons by RTX, the total number of δ-opioid receptor-immunoreactive cell bodies was counted from six confocal images randomly selected from each DRG in three RTX- and three vehicle-treated rats. This analysis showed an approximately 32.1% decrease (from 474.5 ± 32.4 cells in the vehicle-treated group to 321.8 ± 27.1 cells in the RTX-treated group, n = 3 rats in each group, P < 0.05, paired Student’s t-test) in the number of DRG neurons immunoreactive to δ-opioid receptors in RTX-treated rats. In the RTX-treated group, DRG neurons immunoreactive to TRPV1 were abolished. Furthermore, DRG neurons that were immunoreactive to both TRPV1 and δ-opioid receptors were not detected in RTX-treated rats (Fig. 1A).
Fig. 1.
Confocal images showing the effect of RTX on TRPV1- and δ-opioid receptor-immunoreactive dorsal root ganglion (DRG) neurons and afferent terminals in the spinal cord. A: representative confocal images showing δ-opioid receptor (green)- and TRPV1 (red)-immunoreactivity DRG neurons from one vehicle control and one RTX-treated rat. B: confocal images showing TRPV1 (green)- and δ-opioid receptor (red)-immunoreactivity in afferent terminals in the spinal dorsal horn of one vehicle control and one RTX-treated rat. Co-localization of the TRPV1- and δ-opioid receptor-immunoreactivity is indicated in yellow when two images are digitally merged. All images are single confocal optical sections.
In vehicle-treated rats, the δ-opioid receptor immunoreactivity was located in laminae I and II of the spinal dorsal horn. Also, the TRPV1 immunoreactivity was localized predominantly in the lamina I and the outer zone of lamina II and colocalized with some δ-opioid receptor immunoreactivity (Fig. 1B). In RTX-treated rats, TRPV1-immunoreactive terminals were completely absent from the superficial dorsal horn of the spinal cord, and the density of the δ-opioid receptor immunoreactivity in the superficial spinal dorsal horn was substantially reduced. Notably, in the vehicle-treated rats, there was a subpopulation of TRPV1-immunoreactive dorsal horn neurons. These neurons were not immunoreactive to the δ-opioid receptors and were not affected in RTX-treated rats (Fig. 1B).
Effect of RTX treatment on the immunoreactivity of δ-opioid receptors and IB4 labeling in DRG neurons and spinal dorsal horn
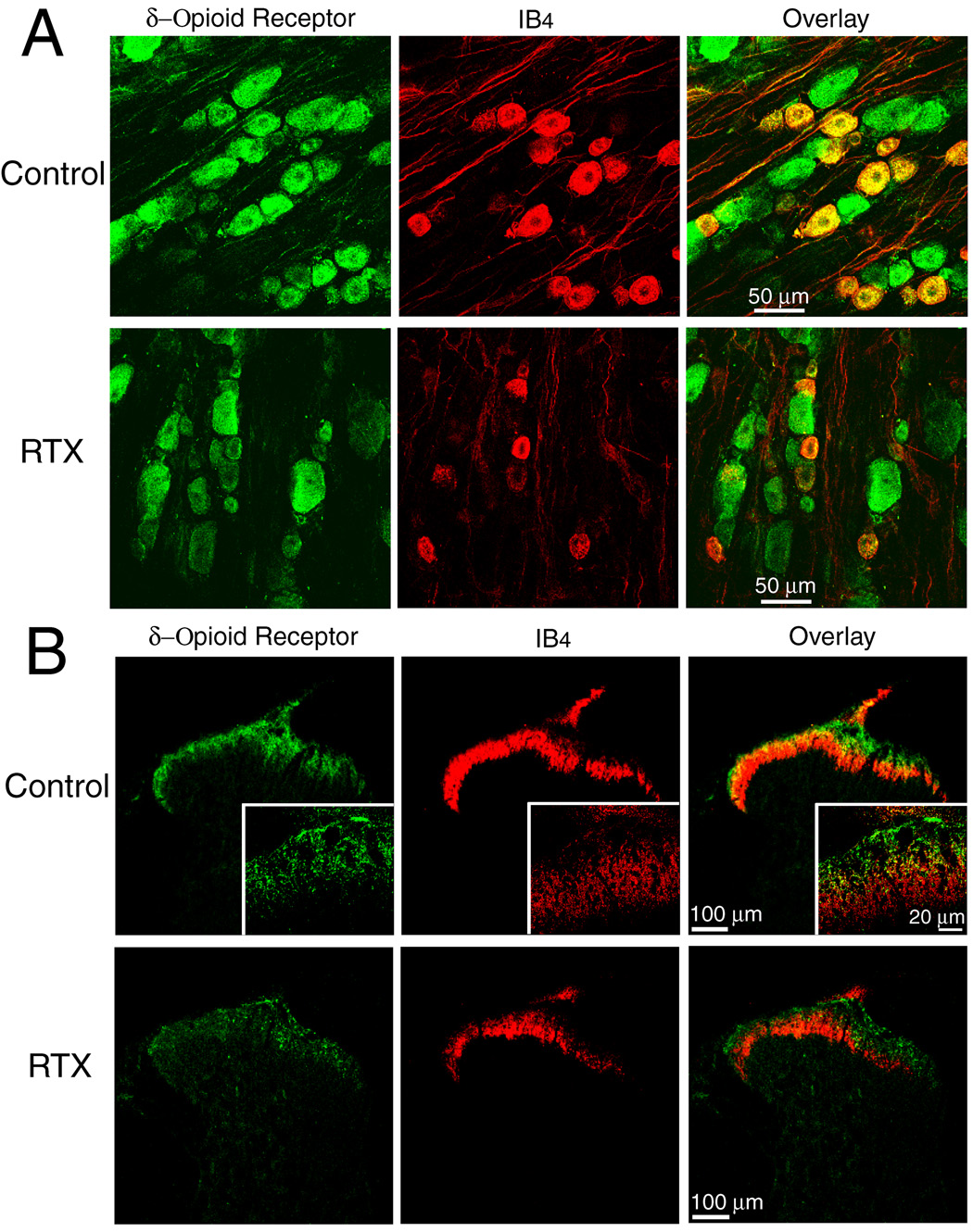
In vehicle-treated rats, IB4 labeling was mainly present in small- and medium-sized DRG neurons. The majority of the IB4-positive DRG neurons exhibited δ-opioid receptor immunoreactivity (Fig. 2A). In RTX-treated rats, the number of DRG neurons exhibiting both δ-opioid receptor immunoreactivity and IB4-labeling was reduced by approximately 68.3% (1,248 in the vehicle-treated group vs. 395 in the RTX-treated group).
Fig. 2.
Confocal images showing the effect of RTX on δ-opioid receptor- and isolectin B4 (IB4)-positive dorsal root ganglion (DRG) neurons and afferent terminals in the spinal cord. A: representative confocal images showing δ-opioid receptor-immunoreactive (green) and IB4-positive (red) DRG neurons from one control and one RTX-treated rat. B: confocal images showing δ-opioid receptor-immunoreactive (green) and IB4-positive (red) afferent terminals in the spinal dorsal horn of one vehicle control and one RTX-treated rat. Colocalization of δ-opioid receptor-immunoreactivity and IB4-labeling is indicated in yellow when two images are digitally merged. All images are single confocal optical sections.
The IB4 labeling and δ-opioid receptor immunoreactivity were concentrated in the superficial laminae of the spinal cord in vehicle-treated rats. Some of the δ-opioid receptor immunoreactivity was localized on the terminals labeled with IB4 in the lamina I and the outer zone of the lamina II (Fig. 2B). In RTX-treated rats, the δ-opioid receptor-immunoreactive terminals were largely reduced in the lamina I and the lamina II outer zone of the spinal cord. There was also a marked reduction in the density of IB4-positive terminals in the laminae I and II of the spinal cord in RTX-treated rats (Fig. 2B).
[3H]-Naltrindole saturation binding in spinal cords of RTX- and vehicle-treated rats
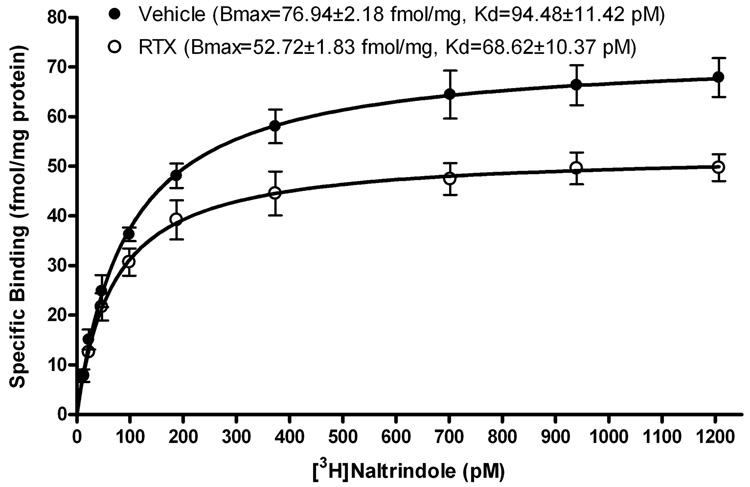
To determine how RTX treatment changes the number of the binding site and affinity of δ-opioid receptors in the spinal cord, [3H]-naltrindole saturation binding was analyzed using dorsal spinal cord membranes from RTX- and vehicle-treated rats. In brief, membrane homogenates were prepared from individual rats (n = 4 rats in each group) 4 weeks after vehicle or RTX injection. [3H]-Naltrindole exhibited a single and saturable high-affinity binding in dorsal spinal cord tissues from both the RTX- and vehicle-treated groups (Fig. 3). The maximal specific binding (Bmax) of [3H]-naltrindole in RTX-treated rats was significantly less than that in the control group (52.72 ± 1.83 vs. 76.94 ± 2.18 fmol/mg protein, P < 0.05). Furthermore, there was a small decrease in the dissociation constant (Kd) value in RTX-treated rats (68.62 ± 10.37 pM), compared with that in vehicle-treated rats (94.48 ± 11.42 pM, P > 0.05).
Fig. 3.
Effect of RTX treatment on the δ-opioid receptor binding in the dorsal spinal cord. Comparison of specific [3H]-naltrindole binding to rat dorsal spinal cord membranes from rats treated with vehicle or RTX (n = 4 replicates in each group).
Effect of DPDPE on mechanical nociception in RTX- and vehicle-treated rats
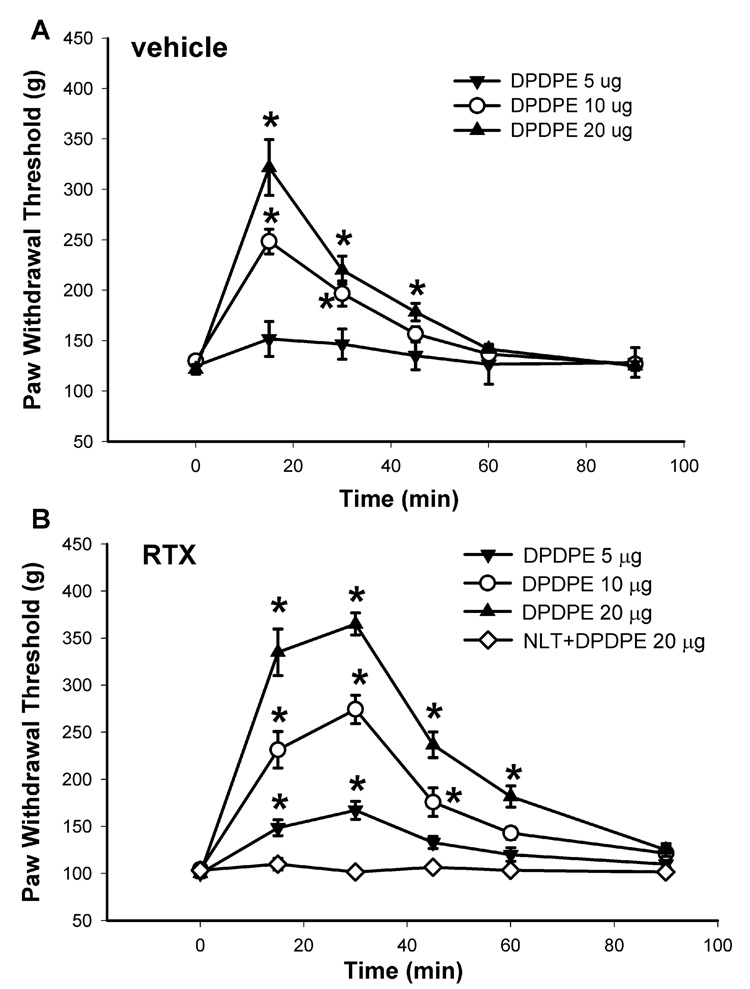
Intrathecal administration of 5, 10, and 20 µg of the specific δ-opioid receptor agonist DPDPE (Drower et al., 1991; Glaum et al., 1994), dose-dependently increased the paw withdrawal threshold in response to the noxious mechanical stimulus in both vehicle- and RTX-treated rats (Fig. 4). However, the time course and the duration of the effect of DPDPE on the mechanical threshold in the RTX-treated group were notably different from those in the vehicle-treated group. In vehicle-treated rats, the peak effect of 20 µg DPDPE appeared within 15 min after intrathecal injection, and the effect lasted for about 45 min (Fig. 4A). In contrast, the maximal effect of 20 µg DPDPE did not appear until 30 min after intrathecal injection, and the effect lasted for more than 60 min in RTX-treated rats (Fig. 4B). By integrating the area under the dose-effect curve, the estimated ED50 value (95% confidence limits) of DPDPE in vehicle- and RTX-treated groups was 9.87 (7.32–16.63) and 5.81 (2.83–11.45) µg, respectively.
Fig. 4.
The dose-response effect of intrathecal injection of the δ-opioid agonist DPDPE on the nociceptive withdrawal threshold in vehicle- and RTX-treated rats. A: time course of the effect of intrathecal injection of 5 (n = 6), and 10 (n = 7), and 20 µg (n = 7) DPDPE on the nociceptive withdrawal threshold in the vehicle-treated rats. B: time course of the effect of intrathecal injection of 5 (n = 7), and 10 (n = 7), and 20 µg (n = 8) DPDPE on the nociceptive threshold in RTX-treated rats. Note that intrathecal injection of 20 µg DPDPE failed to alter significantly the withdrawal threshold in the presence of 25 ìg naltrindole (n = 7). The nociceptive threshold was assessed by testing the withdrawal response of the hindpaw to a noxious pressure stimulus. Data are presented as means ± SEM. *, P < 0.05 compared with the respective baseline control. NLT, naltrindole.
To ensure that the potentiated analgesic effect of DPDPE in RTX-treated rats was mediated by the δ-opioid receptors, the highly specific δ-opioid receptor antagonist naltrindole (Kohno et al., 1999; Malmberg and Yaksh, 1992) was used. Intrathecal pretreatment with 20 µg naltrindole 15 min before DPDPE administration abolished the antinociceptive effect produced by intrathecal injection of 20 µg of DPDPE in RTX-treated rats (Fig. 4B).
Effect of DELT on mechanical nociception in RTX- and vehicle-treated rats
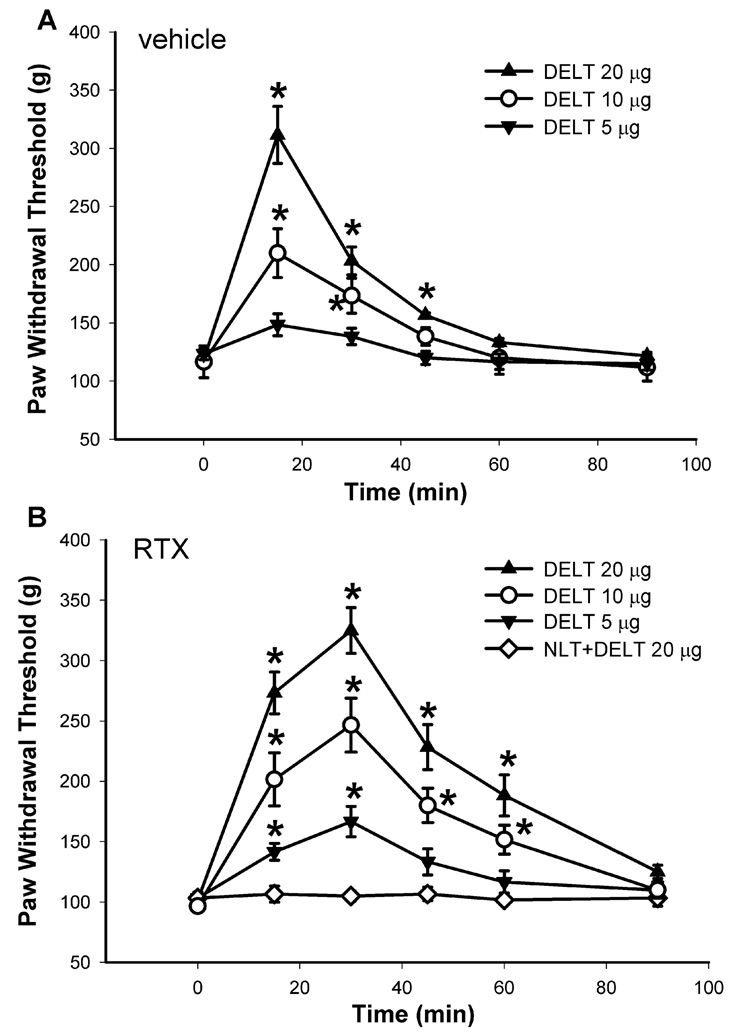
Intrathecal injection of 5, 10, and 20 µg of DELT, another selective δ-opioid receptor agonist (Stewart and Hammond, 1993), also dose-dependently increased the paw withdrawal threshold in response to the noxious mechanical stimulus in both vehicle- and RTX-treated rats. Similarly, the delayed peak effect and prolonged action of DELT upon intrathecal administration occurred in RTX-treated rats (Figs. 5A and B). The ED50 value (95% confidence limits) of DELT was decreased by approximately 2-fold, from 11.16 (7.84–18.37) in the vehicle-treated group to 5.32 (2.18–10.56) µg in the RTX-treated group.
Fig. 5.
The dose-response effect of intrathecal administration of the δ-opioid agonist DELT on the mechano-nociception in vehicle- and RTX-treated rats. A: time course of the effect of intrathecal injection of 5 (n = 7), and 10 (n = 8), and 20 µg (n = 8) DELT on the nociceptive withdrawal threshold in the vehicle-treated rats. B: time course of the effect of intrathecal administration of 5 (n = 8), and 10 (n = 8), and 20 µg (n = 7) DELT on the nociceptive threshold in RTX-treated rats. Note that intrathecal injection of 20 µg DELT failed to alter significantly the withdrawal threshold in the presence of 20 µg naltrindole (n = 6). The nociceptive threshold was determined by assessing the withdrawal response of the hindpaw to a noxious pressure stimulus. Data are presented as means ± SEM. *, P < 0.05 compared with the respective baseline control. NLT, naltrindole.
Intrathecal pretreatment with 20 µg naltrindole 15 min before DELT injection completely blocked the antinociceptive effects of 20 µg of DELT in RTX-treated rats (Fig. 5B). Intrathecal injection of 20 µg of naltrindole alone had no significant effect on the baseline withdrawal threshold in six separate rats tested (data not shown).
DISCUSSION
In the present study, we determined the contribution of presynaptic δ-opioid receptors to the analgesic effect of spinally administered δ-opioid agonists. We demonstrated that δ-opioid receptors are present in TRPV1-expressing and IB4-positive DRG neurons and their terminals. RTX treatment caused a large reduction in the amount of δ-opioid receptor-immunoreactive DRG neurons and afferent terminals in the superficial dorsal horn of the spinal cord. Membrane receptor binding experiments also showed a significant decrease (~31%) in the number of specific binding sites of the δ-opioid receptors in the spinal cords in RTX-treated rats. Surprisingly, the inhibitory effect of the δ-opioid agonists DPDPE and DELT on mechano-nociception was potentiated in RTX-treated rats. These findings suggest that, although δ-opioid receptors are present on TRPV1-expressing primary afferents, removal of these receptors does not reduce the modulation of mechano-nociception by δ-opioid agonists in the spinal cord. Thus, the δ-opioid receptors on non-TRPV1-expressing primary afferent neurons and those on postsynaptic dorsal horn neurons appears to be critically involved in the inhibition of mechano-nociceptive transmission by δ-opioid agonists.
The spinal dorsal horn is an important locus where transmission of the nociceptive information is subject to regulation by µ- and δ-opioid agonists (Chen and Pan, 2006a; Dickenson, 1995; Stewart and Hammond, 1993; Yaksh and Noueihed, 1985). Spinally administered δ-opioid agonists likely inhibit synaptic transmission through both pre- and postsynaptic mechanisms (Abbadie et al., 2002; Glaum et al., 1994; Kohno et al., 1999; Stewart and Hammond, 1993; Zachariou and Goldstein, 1996). The opioid receptors are coupled to inhibitory G proteins, and voltage-activated Ca2+ channels are an important downstream signaling target for the opioid analgesic action. One of the important analgesic mechanisms of δ-opioid receptor agonists is the inhibition of synaptic transmission by their action on voltage-activated Ca2+ channels present on the primary sensory neurons (Acosta and Lopez, 1999; Wu et al., 2008). Furthermore, the δ-opioid receptor agonists can activate G protein-coupled inwardly rectifying K+ channels (GIRK) channels in the postsynaptic dorsal horn neurons, and the analgesic effect of DPDPE is attenuated in GIRK-knockout mice (Marker et al., 2005). However, it is unclear to what extent the presynaptic δ-opioid receptors contribute to the analgesic effect of δ-opioid agonists. Furthermore, the role of δ-opioid receptors present in different phenotypes of primary afferent neurons in nociceptive modulation remains to be determined.
Based on the receptor changes caused by dorsal rhizotomy and neonatal capsaicin treatment, it has been estimated that half of µ-opioid receptors and two thirds of δ-opioid receptors in the rat spinal cord are present on primary afferent fibers (Abbadie et al., 2002). It has been shown that the release of glutamate and substance P from primary afferent neurons is inhibited by δ-opioid agonists (Glaum et al., 1994; Kohno et al., 1999; Kondo et al., 2005). Thus, the presynaptic action of δ-opioid agonists on nociceptive afferent terminals has been considered an important mechanism underlying the spinal analgesic effect of δ-opioid agonists. RTX is a well-known capsaicin analogue, and its binding is competitively inhibited by the TRPV1 antagonist capsazepine (Szallasi and Blumberg, 1999). RTX treatment can activate TRPV1 and rapidly kill primary afferent neurons through calcium influx and overloading (Olah et al., 2001; Wu et al., 2005). Our immunocytochemistry data confirmed that RTX eliminated presynaptic δ-opioid receptors on TRPV1-immunoreactive DRG neurons and their terminals in the spinal dorsal horn. Our receptor binding data suggest a 31% loss in the specific binding site of δ-opioid receptors in the spinal cord of RTX-treated rats. We also found that colocalization of δ-opioid receptors and TRPV1 co-localized in many DRG neurons and in the superficial spinal dorsal horn. As a result, RTX treatment resulted in the removal of TRPV1-expressing primary afferent nerves and caused a large reduction in δ-opioid receptors present on the central terminals of primary afferent neurons.
Despite observing a considerable reduction in the number of δ-opioid receptors in the spinal cord, we found that intrathecal injection of two specific δ-opioid agonists, DPDPE and DELT, still had a profound effect on mechano-nociception as measured with a noxious pressure stimulus in RTX-treated rats. Whereas the peak effect of DPDPE and DELT was observed within 15 min after intrathecal injection in the vehicle-treated rats, it was not reached until 30 min after drug injection in the RTX-treated rats. Furthermore, the duration of the analgesic action of these two δ-opioid agonists was prolonged in RTX-treated rats. Thus, removal of δ-opioid receptors on TRPV1-expressing afferent terminals seems to paradoxically potentiate the analgesic effect of spinally administered δ-opioid agonists. Notably, the magnitude of potentiation of the analgesic effect of δ-opioid agonists is less than that of µ-opioid agonists in RTX-treated rats. The mechanisms responsible for increased antinociceptive effect of δ-opioid agonists in RTX-treated rats are not fully known. Our immunocytochemistry and radioligand binding data experiments showed a consistent reduction in the δ-opioid receptor number in the spinal cord of RTX-treated rats. The δ-opioid receptors are distributed mainly in the cytoplasm of primary sensory neurons (Zhang et al., 1998). The surface expression and function of δ-opioid receptors in DRG neurons are influenced by opioid treatment and in painful conditions (Gendron et al., 2006). In the inflammatory pain model, more δ-opioid receptors can be recruited to the cell membrane of spinal dorsal horn neurons (Cahill et al., 2003). RTX treatment could cause extensive glutamate release from the primary afferent terminals to the dorsal horn neurons through TRPV1 stimulation (Pan and Pan, 2004). This increased nociceptive input may promote the cell surface expression of δ-opioid receptors on the postsynaptic neurons in the spinal dorsal horn in RTX-treated rats. Also, loss of TRPV1-expressing afferent fibers may result in less nociceptive input to spinal dorsal horn neurons, resulting in an enhanced effect of δ-opioid receptor agonists in RTX-treated animals. Given that two thirds of the total δ-opioid receptors in the spinal cord are found on primary afferent terminals (Abbadie et al., 2002), we were somewhat surprised that RTX reduced only 31% of δ-opioid receptors in the spinal cord. These data may suggest that most presynaptic δ-opioid receptors are located on non-TRPV1 sensory neurons and their central terminals. Alternatively, it is possible that there may be an increase in δ-opioid receptor trafficking from the cytoplasm to the membrane surface on non-TRPV1 primary afferent terminals or a compensatory upregulation of postsynaptic δ-opioid receptors in the spinal cord.
In summary, our findings suggest that the spinal effect of δ-opioid receptor agonists on mechano-nociception is mediated by δ-opioid receptors on non-TRPV1-expressing afferent terminals and/or the postsynaptic dorsal horn neurons. Although µ- and δ-opioid agonists potently modulate the pain evoked by both mechanical and thermal stimuli, other G protein-coupled receptor agonists have differential effects on sensory modalities. For example, activation of the α2 adrenergic receptors and the muscarinic acetylcholine receptors in the spinal cord primarily reduces thermal nociception but has little effects on mechano-nociception (Chen and Pan, 2003; Chen et al., 2007a). RTX treatment can be used to study the physiological function and pharmacological modulation of mechno-nociception mediated by non-TRPV1-expressing primary afferent neurons. The present study provides another example of the disproportional correlation between the number of presynaptic δ-opioid receptors and the analgesic effect of opioid agonists in the spinal cord. These findings are important for a better understanding of the analgesic mechanisms of δ-opioid agonists.
Acknowledgments
This work was supported by grants GM64830 and NS45602 from the National Institutes of Health.
List of Abbreviations
- DELT
[d-Ala2,Glu4]-deltorphin
- DPDPE
[d-Pen2,d-Pen5]-enkephalin
- DRG
dorsal root ganglion
- IB4
Griffonia simplicifolia isolectin B4
- RTX
resiniferatoxin
- TRPV1
transient receptor potential vanilloid type 1 channel
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbadie C, Lombard MC, Besson JM, Trafton JA, Basbaum AI. Mu and delta opioid receptor-like immunoreactivity in the cervical spinal cord of the rat after dorsal rhizotomy or neonatal capsaicin: an analysis of pre- and postsynaptic receptor distributions. Brain Res. 2002;930:150–162. doi: 10.1016/s0006-8993(02)02242-4. [DOI] [PubMed] [Google Scholar]
- Acosta CG, Lopez HS. delta opioid receptor modulation of several voltage-dependent Ca(2+) currents in rat sensory neurons. J Neurosci. 1999;19:8337–8348. doi: 10.1523/JNEUROSCI.19-19-08337.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besse D, Lombard MC, Zajac JM, Roques BP, Besson JM. Pre- and postsynaptic distribution of mu, delta and kappa opioid receptors in the superficial layers of the cervical dorsal horn of the rat spinal cord. Brain Res. 1990;521:15–22. doi: 10.1016/0006-8993(90)91519-m. [DOI] [PubMed] [Google Scholar]
- Cahill CM, Morinville A, Hoffert C, O'Donnell D, Beaudet A. Up-regulation and trafficking of delta opioid receptor in a model of chronic inflammation: implications for pain control. Pain. 2003;101:199–208. doi: 10.1016/s0304-3959(02)00333-0. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- Chen SR, Pan HL. Up-regulation of spinal muscarinic receptors and increased antinociceptive effect of intrathecal muscarine in diabetic rats. J Pharmacol Exp Ther. 2003;307:676–681. doi: 10.1124/jpet.103.055905. [DOI] [PubMed] [Google Scholar]
- Chen SR, Pan HL. Blocking mu opioid receptors in the spinal cord prevents the analgesic action by subsequent systemic opioids. Brain Res. 2006a;1081:119–125. doi: 10.1016/j.brainres.2006.01.053. [DOI] [PubMed] [Google Scholar]
- Chen SR, Pan HL. Loss of TRPV1-expressing sensory neurons reduces spinal mu opioid receptors but paradoxically potentiates opioid analgesia. J Neurophysiol. 2006b;95:3086–3096. doi: 10.1152/jn.01343.2005. [DOI] [PubMed] [Google Scholar]
- Chen SR, Pan HM, Richardson TE, Pan HL. Potentiation of spinal alpha(2)-adrenoceptor analgesia in rats deficient in TRPV1-expressing afferent neurons. Neuropharmacology. 2007a;52:1624–1630. doi: 10.1016/j.neuropharm.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SR, Prunean A, Pan HM, Welker KL, Pan HL, Wang XL, Zhang HM. Resistance to morphine analgesic tolerance in rats with deleted transient receptor potential vanilloid type 1-expressing sensory neurons. Neuroscience. 2007b;16:16. doi: 10.1016/j.neuroscience.2006.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras PC, Tam L, Drower E, Rafferty MF. [3H]naltrindole: a potent and selective ligand for labeling delta-opioid receptors. Brain Res. 1993;604:160–164. doi: 10.1016/0006-8993(93)90363-r. [DOI] [PubMed] [Google Scholar]
- Dickenson AH. Spinal cord pharmacology of pain. Br J Anaesth. 1995;75:193–200. doi: 10.1093/bja/75.2.193. [DOI] [PubMed] [Google Scholar]
- Drower EJ, Stapelfeld A, Rafferty MF, de Costa BR, Rice KC, Hammond DL. Selective antagonism by naltrindole of the antinociceptive effects of the delta opioid agonist cyclic[D-penicillamine2-D-penicillamine5]enkephalin in the rat. J Pharmacol Exp Ther. 1991;259:725–731. [PubMed] [Google Scholar]
- Gendron L, Lucido AL, Mennicken F, O'Donnell D, Vincent JP, Stroh T, Beaudet A. Morphine and pain-related stimuli enhance cell surface availability of somatic delta-opioid receptors in rat dorsal root ganglia. J Neurosci. 2006;26:953–962. doi: 10.1523/JNEUROSCI.3598-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaum SR, Miller RJ, Hammond DL. Inhibitory actions of delta 1-, delta 2-, and mu-opioid receptor agonists on excitatory transmission in lamina II neurons of adult rat spinal cord. J Neurosci. 1994;14:4965–4971. doi: 10.1523/JNEUROSCI.14-08-04965.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdridge SV, Cahill CM. Spinal administration of a delta opioid receptor agonist attenuates hyperalgesia and allodynia in a rat model of neuropathic pain. Eur J Pain. 2006;15:15. doi: 10.1016/j.ejpain.2006.10.008. [DOI] [PubMed] [Google Scholar]
- Hurley RW, Grabow TS, Tallarida RJ, Hammond DL. Interaction between medullary and spinal delta1 and delta2 opioid receptors in the production of antinociception in the rat. J Pharmacol Exp Ther. 1999;289:993–999. [PubMed] [Google Scholar]
- Khan GM, Li DP, Chen SR, Pan HL. Role of spinal nitric oxide in the inhibitory effect of [D-Pen2, D-Pen5]-enkephalin on ascending dorsal horn neurons in normal and diabetic rats. J Pharmacol Exp Ther. 2002;303:1021–1028. doi: 10.1124/jpet.102.040865. [DOI] [PubMed] [Google Scholar]
- Kitchener PD, Lapiz MD, Wilson P, Snow PJ. Transganglionic labelling of primary sensory afferents in the rat lumbar spinal cord: comparison between wheatgerm agglutinin and the I-B4 isolectin from Bandeiraea simplicifolia. J Neurocytol. 1994;23:745–757. doi: 10.1007/BF01268087. [DOI] [PubMed] [Google Scholar]
- Kitchener PD, Wilson P, Snow PJ. Selective labelling of primary sensory afferent terminals in lamina II of the dorsal horn by injection of Bandeiraea simplicifolia isolectin B4 into peripheral nerves. Neuroscience. 1993;54:545–551. doi: 10.1016/0306-4522(93)90274-j. [DOI] [PubMed] [Google Scholar]
- Kohno T, Kumamoto E, Higashi H, Shimoji K, Yoshimura M. Actions of opioids on excitatory and inhibitory transmission in substantia gelatinosa of adult rat spinal cord. J Physiol. 1999;518:803–813. doi: 10.1111/j.1469-7793.1999.0803p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo I, Marvizon JC, Song B, Salgado F, Codeluppi S, Hua XY, Yaksh TL. Inhibition by spinal mu- and delta-opioid agonists of afferent-evoked substance P release. J Neurosci. 2005;25:3651–3660. doi: 10.1523/JNEUROSCI.0252-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmberg AB, Yaksh TL. Isobolographic and dose-response analyses of the interaction between intrathecal mu and delta agonists: effects of naltrindole and its benzofuran analog (NTB) J Pharmacol Exp Ther. 1992;263:264–275. [PubMed] [Google Scholar]
- Marker CL, Lujan R, Loh HH, Wickman K. Spinal G-protein-gated potassium channels contribute in a dose-dependent manner to the analgesic effect of mu- and delta- but not kappa-opioids. J Neurosci. 2005;25:3551–3559. doi: 10.1523/JNEUROSCI.4899-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olah Z, Szabo T, Karai L, Hough C, Fields RD, Caudle RM, Blumberg PM, Iadarola MJ. Ligand-induced dynamic membrane changes and cell deletion conferred by vanilloid receptor 1. J Biol Chem. 2001;276:11021–11030. doi: 10.1074/jbc.M008392200. [DOI] [PubMed] [Google Scholar]
- Pan HL, Khan GM, Alloway KD, Chen SR. Resiniferatoxin induces paradoxical changes in thermal and mechanical sensitivities in rats: mechanism of action. J Neurosci. 2003;23:2911–2919. doi: 10.1523/JNEUROSCI.23-07-02911.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan YZ, Pan HL. Primary afferent stimulation differentially potentiates excitatory and inhibitory inputs to spinal lamina II outer and inner neurons. J Neurophysiol. 2004;91:2413–2421. doi: 10.1152/jn.01242.2003. [DOI] [PubMed] [Google Scholar]
- Stewart PE, Hammond DL. Evidence for delta opioid receptor subtypes in rat spinal cord: studies with intrathecal naltriben, cyclic[D-Pen2, D-Pen5] enkephalin and [D-Ala2, Glu4]deltorphin. J Pharmacol Exp Ther. 1993;266:820–828. [PubMed] [Google Scholar]
- Szallasi A, Blumberg PM. Vanilloid (Capsaicin) receptors and mechanisms. Pharmacol Rev. 1999;51:159–212. [PubMed] [Google Scholar]
- Wang H, Wessendorf MW. Equal proportions of small and large DRG neurons express opioid receptor mRNAs. J Comp Neurol. 2001;429:590–600. doi: 10.1002/1096-9861(20010122)429:4<590::aid-cne6>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Wu ZZ, Chen SR, Pan HL. Transient receptor potential vanilloid type 1 activation down-regulates voltage-gated calcium channels through calcium-dependent calcineurin in sensory neurons. J Biol Chem. 2005;280:18142–18151. doi: 10.1074/jbc.M501229200. [DOI] [PubMed] [Google Scholar]
- Wu ZZ, Chen SR, Pan HL. Distinct inhibition of voltage-activated Ca(2+) channels by delta-opioid agonists in dorsal root ganglion neurons devoid of functional T-type Ca(2+) currents. Neuroscience. 2008 doi: 10.1016/j.neuroscience.2008.03.031. in press. [DOI] [PubMed] [Google Scholar]
- Yaksh TL, Noueihed R. The physiology and pharmacology of spinal opiates. Annu Rev Pharmacol Toxicol. 1985;25:433–462. doi: 10.1146/annurev.pa.25.040185.002245. [DOI] [PubMed] [Google Scholar]
- Zachariou V, Goldstein BD. Delta-Opioid receptor modulation of the release of substance P-like immunoreactivity in the dorsal horn of the rat following mechanical or thermal noxious stimulation. Brain Res. 1996;736:305–314. doi: 10.1016/0006-8993(96)00718-4. [DOI] [PubMed] [Google Scholar]
- Zhang X, Bao L, Arvidsson U, Elde R, Hokfelt T. Localization and regulation of the delta-opioid receptor in dorsal root ganglia and spinal cord of the rat and monkey: evidence for association with the membrane of large dense-core vesicles. Neuroscience. 1998;82:1225–1242. doi: 10.1016/s0306-4522(97)00341-2. [DOI] [PubMed] [Google Scholar]