Abstract
This study assessed the influence of mineral trioxide aggregate (MTA) on adaptive immune responses. BALB/c mice were immunized with heat-killed Fusobacterium nucleatum (Fn) in MTA or other control adjuvants, and serum IgG responses to Fn were measured. Either Fn- or Peptostreptococcus anaerobius (Pa)-reactive memory T cells (Tm) were pre-incubated in vitro with/without MTA and restimulated with Fn or Pa. Tm proliferation and cytokine production were assessed. Compared to control groups, IgG-antibody responses were upregulated in mice immunized with Fn in MTA in a similar manner to animals immunized with Fn in Freund's adjuvant or aluminum hydroxide adjuvant. While MTA did not affect the upregulated expression of IL-10, TNF-α or RANKL by Tm, it suppressed the proliferation of Pa- or Fn-Tm and inhibited their production of Th1- or Th2-signature cytokines. MTA upregulated the adaptive humoral immune responses, but had little or no effect on pro- or anti-inflammatory cytokine production by Tm.
Keywords: MTA, IgG antibody, T cells, cytokine, bacteria
Introduction
Bacterial infection at either gingival and radicular tissues results in the development of inflammatory lesions of periodontal or periradicular tissue, which are accompanied by the natural infiltration of a variety of immune cells (1;2). In general, innate immune cells play an antibacterial role at the acute stage of infection, while adaptive immune cells are committed to eliminating bacteria at the chronic stage of infection. Adaptive immune responses, which include antibody production and cell-mediated immune responses, are considered to be the host protective means against bacterial infection at human periradicular lesions of endodontic origin (3). As the early acute stage of the periradicular infectious lesion phases into the chronic stage, bone resorption develops around the root end of a tooth, together with a corresponding increase in the infiltration of adaptive immune cells (4).
The application of mineral trioxide aggregate (MTA) to the root-end associated with a periradicular lesion caused by bacterial infection can only be considered optimally effective if it does not compromise either innate or adaptive immune response. Our group previously demonstrated that MTA does not affect the activity of macrophages. More specifically, MTA did not show any effects on the antibacterial activities of either M1- or M2-type macrophages, including bacterial phagocytosis, reactive oxygen and nitrogen species production or arginase activity (5). However, the effects, if any, that MTA exerts on adaptive immune cells are still to be elucidated.
To determine the presence or absence of such effects, our experimentation was principally based on the application of aluminum salts, which are known to be potent adjuvants for immunization with protein antigens. While aluminum hydroxide (Al(OH)3) is most commonly used as an immune adjuvant, some studies showed that aluminum oxide (Al2O3) also possesses adjuvant effects (6). Aluminum hydroxide is included as an adjuvant in some vaccines (e.g., Alhydrogel), since it contributes to induction of a good antibody response. However, it has little capacity to stimulate cellular immune responses, important for protection against many pathogens (7). Very interestingly, MTA is also composed of aluminum oxide, as well as other mineral components, such as lime (CaO) and silica (SiO2) (8). It has been reported that aluminum in cation form (Al3+) is detected in distilled water incubated with MTA (9). It is therefore conceivable that aluminum cation is released from MTA under physiological conditions. Based on these lines of evidence, we were initially led to hypothesize that MTA could augment host protective antibody responses, while suppressing tissue destructive cellular immune responses due to its adjuvant effects by the presence of aluminum oxide (Al2O3). To test this hypothesis, the present study focused on assessing the influence of MTA on (1) the induction of in vivo IgG antibody response to F. nucleatum, and (2) the cytokine production pattern of memory T cells, which were primed by two bacteria commonly found in periradicular lesions, F. nucleatum or P. anaerobius.
Materials and Methods
MTA preparation
Ângelus MTA (Odonto-lógika, Londrina, Paraná, Brazil) paste was prepared from the mixture of MTA powder and distilled water in a sterile condition, according to manufacturer's instructions. By the method previously published (10), the MTA paste was then inserted into both ends of sterilized glass capillary tubes (Ø = 1.2 mm; length = 10 mm) so that the contact surface with the cell culture medium could be standardized (area = 2.26 mm2). Empty capillary tubes without MTA were used as negative controls.
Animals
BALB/c mice (6- to 8-week-old males, n=6/group) were utilized. Animals were kept in conventional cages and maintained at controlled ambient temperature. Food and water were offered ad libitum. The protocol for this animal experiment was approved by The Forsyth Institute's animal ethics committee.
Antigen preparation
Two types of bacteria, F. nucleatum ATCC 10953 and P. anaerobius ATCC 27337, were chosen to represent Gram-negative and Gram-positive endodontic pathogens, respectively (11). F. nucleatum and P. anaerobius were grown in blood agar plate (Becton, Dickinson, Franklin Lake, NJ), harvested during the log growth phase and counted using a spectrophotometer (Thermo Spectronic Genesys, Waltham, MA) (1 OD = 8×108CFU/mL). After re-suspension of F. nucleatum and P. anaerobius in phosphate buffered saline (PBS), the bacteria were killed at 100°C and used as heat-killed (HK) bacterial antigen, following a previously published method (12).
Immunization with bacterial antigens
(A) Immunization with F. nucleatum for examination of IgG antibody response
A total of 4 groups of BALB/c mice (6- to 8-week-old males, n=6/group) were immunized with heat-killed F. nucleatum (3×108 CFU/mouse, s.c. injection) in a mixture of 1) PBS, 2) Freund's adjuvant (Difco Laboratories, Detroit, MI), 3) aluminum hydroxide adjuvant (Alum) (Sigma, St. Louis, MO), or 4) MTA (100mg/mL) every two weeks, for a total of two immunizations (see Fig. 1 B). A third booster immunization was carried out by an injection (s.c.) of heat-killed F. nucleatum suspended in PBS alone, two weeks after the second immunization. In particular, for the group receiving Freund's adjuvant, Freund's complete adjuvant and Freund's incomplete adjuvant were used for primary and secondary immunizations, respectively. Otherwise, the same composition of Alum or MTA was used for primary and secondary immunization. Blood was collected on days 0, 14, 28 and 32 and serum obtained. IgG antibody reactions to F. nucleatum present in the blood serum specimens were determined by ELISA, as described below (timetable is shown in Fig. 1 B).
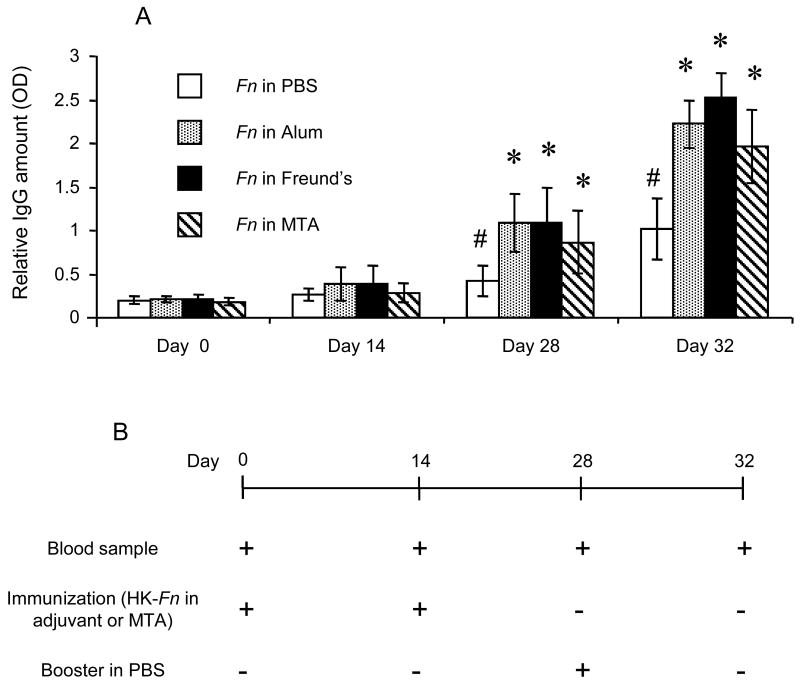
Figure 1. Influence of MTA on IgG antibody response to endodontic pathogen immunization (A).
BALB/c mice received immunizations with heat killed-F. nucleatum in PBS (Fn+PBS), heat killed-F. nucleatum in Freund's adjuvant (Fn+Freund's), heat killed-F. nucleatum in aluminum hydroxide adjuvant (Fn+Alum) or heat killed-F. nucleatum in MTA (Fn+MTA). MTA injection alone without bacteria was used as control. The schedule for immunization is indicated in the diagram inserted in the figure (B). Column and bar indicate the mean and SD of relative IgG titer value from six different mice, respectively. The methods to measure IgG antibody are described in the materials and methods section.
* indicates that the value of the column is statistical different than the value of heat killed-F. nucleatum in PBS group, in the same day, by t-test (p<0.01).
# indicates that the value of the column is statistically higher than the same group in day 0, by t-test (p<0.05).
(B) Immunization with F. nucleatum and P. anaerobius for examination of bacterial antigen-specific memory T cell response
In order to develop antigen-specific memory type T cells, two groups of animals were immunized with 1) heat-killed F. nucleatum or 2) heat-killed P. anaerobius, following the same protocol as described above for the analysis of serum antibody reaction. Two days after booster injection with F. nucleatum or P. anaerobius suspended in PBS alone, these animals were sacrificed, and mononuclear lymphocytes were isolated from the cervical and auxiliary lymph nodes so that memory T cells specific to F. nucleatum or P. anaerobius could be primed in vitro, as described below.
Measurement of serum IgG antibody responses to bacterial antigens using ELISA
The wells of ELISA plates were coated with heat-killed F. nucleatum and heat-killed P. anaerobius in 0.2M sodium bicarbonate buffer (pH 9.6) and incubated at 4°C overnight. To optimize the assay system, previous baseline experiments set the concentration of heat-killed F. nucleatum and heat-killed P. anaerobius at 107CFU/mL. The wells of ELISA plates were subjected to blocking with 1% bovine serum albumin (Sigma) and 1% sucrose (Sigma) in PBS supplemented with 0.05% Tween 20 (PBST). Blood serum diluted in PBST was incubated in the wells of ELISA plates for 1 hour at room temperature. Then, each well was reacted with horseradish peroxidase (HRPO)-conjugated anti-mouse IgG (Sigma) for 1 hour at room temperature. O-Phenylenediamine dihydrochloride (OPD; Sigma) in 0.1M citrate buffer solution (pH 5.5) supplemented with 2μL/mL of 30% H2O2 was applied as a substrate. Colorimetric reaction developed in the wells of ELISA plates was halted by addition of 2N H2SO4, and color densities were measured using a plate reader at OD 490 nm (Biokinetics reader EL312e; Bio-Tek Instruments, Winooski, VT). The results were expressed as optical density of the immunoglobulin isotype tested.
Naïve T cell culture
Lymph node cells from BALB/c mice were recovered and passed through a nylon- and glass-wool column to enrich T cells (∼90% pure) (13). These T cells (106 cells in 1mL) were cultured for 3 days in the presence or absence of MTA-filled glass capillaries in RPMI 1640 medium, supplemented with 10% fetal bovine serum (Invitrogen, Grand Island, NY); 50μM of β-mercaptoethanol; antibiotics, including penicillin, streptomycin and gentamicin (Invitrogen); and l-glutamine in a 24-well plate (Corning, New York, NY). The T cells recovered from pre-incubation in the 24-well plate were re-stimulated for additional 3 days in wells of a 96-well plate (2×105cells/well). The wells had previously been coated with monoclonal antibody (mAb) specific to TCR β-chain (6μg/mL; clone: H57-597) (Pharmingen, San Diego, CA), either with or without anti-CD28 mAb (2μg/mL, clone: 37.51) (Pharmingen). Cytokines produced in the culture supernatant on day 3 and proliferation of T cells in the last 16 hours of a total 4-day culture were verified using ELISA and [3H]-thymidine incorporation assay, respectively.
Memory T cell culture
T line cells specific for F. nucleatum and P. anaerobius were developed from lymph nodes of animals immunized with heat-killed F. nucleatum and heat-killed P. anaerobius in Freund's adjuvant following the protocol used for serum IgG antibody induction. T cells were enriched from the mononuclear cell suspension isolated from lymph nodes by passing them through a nylon wool and glass wool column (13). T cells (106 cells/mL) were first primed in vitro with Mitomycin C (Sigma) (MMC)-treated spleen antigen presenting cells (APC) (2×106 cells/mL) and F. nucleatum or P. anaerobius (107 CFU/ml) in RPMI 1640 medium supplemented with 10% FBS. After incubation for 1 week, T cells which proliferated in response to each bacterial antigen presentation were separated by gradient centrifugation using Histopaque 1083 (Sigma), and the memory phenotypes were examined using flow cytometry. These in vitro-primed memory T cells were restimulated with or without MTA capillaries in the presence of MMC-treated APC (2×106 cells/mL) and F. nucleatum or P. anaerobius (107 CFU/mL) in a 24-well plate for an additional 3 days. These MTA-exposed T cells were examined for their reactivity to respective bacterial antigen presentation. Briefly, the T cells were again isolated from APC by gradient centrifugation and stimulated (2×104 cells/well) with fresh MMC-treated APC (4×105 cells/well) in the presence or absence of F. nucleatum or P. anaerobius (2×106 CFU/well) in a 96-well plate (Corning) for 3 days. Culture supernatant isolated on day 3 was subjected to cytokine measurement using ELISA. The proliferation of T cells was evaluated by their incorporation of [3H]-thymidine (0.5 μCi/well), which was applied during the last 16 hours of a total 4-day culture.
Flow cytometry analysis
In order to evaluate the memory T cell phenotypes, the in vitro-primed T cells were incubated with anti-CD4 mAb (clone: YTS191.1, Serotec, Raleigh, NC), anti-CD44 mAb (clone: IM7, Pharmingen), followed by FITC-labeled donkey F(ab')2 anti-rat IgG (Jackson ImmunoResearch, West Grove, PA). Fluorescence data were collected using logarithmic amplification on an EPICS™ Altra flow cytometer (Beckman Coulter, Fullerton, CA).
[3H]-thymidine incorporation assay
The protocol for the measurement of T cell proliferation followed our previously published method (13). Briefly, after collection of the culture supernatant for ELISA on day 3, [3H]-thymidine (0.5 μCi/well) was applied to the T cell culture and incubated overnight (16 hours). Radioactivity incorporated into the lymphocytes was determined using a Tri-Carb liquid scintillation analyzer (model 2100TR; Packard, Meriden, CT).
Cytotoxicity assay
The freshly isolated lymph node T cells or spleen mononuclear cells (2×106 cells/mL, respectively) were incubated in a 24-well plate in the presence of a capillary filled with or without MTA for 24 hours. Cell death induced during the 24-hour incubation was measured by CytoTox-96™ Non-Radioactive cytotoxicity assay (Promega, Madison, WI). The CytoTox-96 assay quantitatively measures lactate dehydrogenase (LDH), a stable cytosolic enzyme that is released upon cell lysis in the same manner as 51Cr is released in a conventional radioactive-based cytotoxicity assay. The control cells incubated in medium alone were all killed after 24-hour incubation by addition of Lysis Solution (Promega) and provided a LDH-positive control (100% cell death). The rate of cell death induced by a capillary filled with or without MTA was calculated and expressed as (% cell death) based on the LDH-positive control.
Cytokine ELISA
Cytokine detection was measured in the culture supernatant using commercially available ELISA kits: IFN-γ (Duoset™, R&D Systems, Minneapolis, MN); TNF-α, IL-10, and RANKL (Peprotech, Rocky Hill, NJ) and IL-4 (Pharmingen).
Statistical analysis
All in vitro assays were carried out in triplicate. Data were analyzed using parametric Student's t-test. The results were considered significant when p<0.05.
Results
Effect of MTA on IgG antibody response
Immunization with heat-killed F. nucleatum in MTA upregulated IgG antibody to F. nucleatum compared to the group immunized with heat-killed F. nucleatum in control PBS (Fig. 1). As we expected, immunization with heat-killed F. nucleatum in Freund's adjuvant or in aluminum hydroxide (Alum) adjuvant upregulated the IgG antibody to F. nucleatum (Fig. 1). These results suggested that MTA possesses adjuvant effects in the induction of IgG antibody responses against F. nucleatum.
Lack of cytotoxicity to lymphocytes by MTA
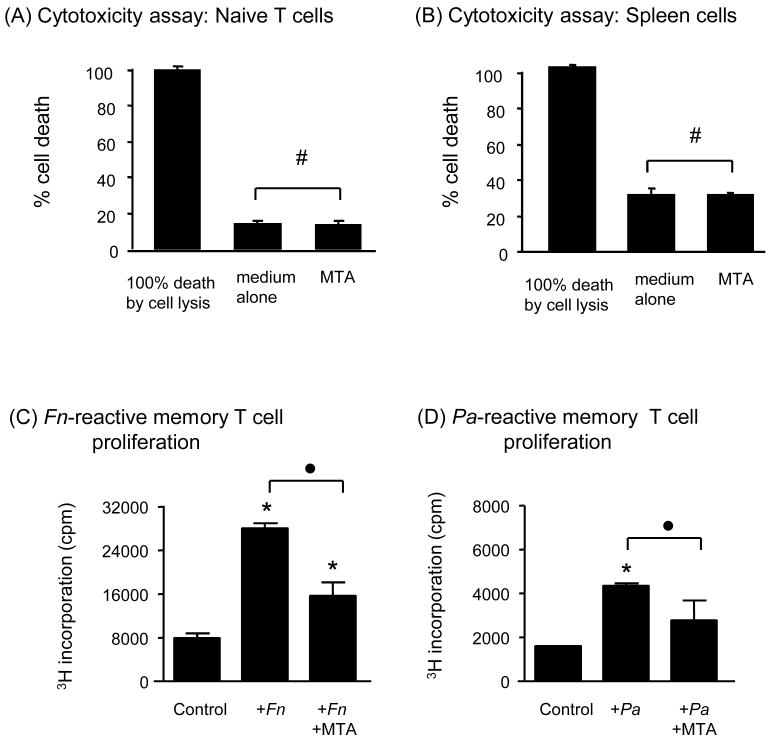
In order to evaluate the possible cytotoxic effects of MTA on the adaptive immune cells, 1) mononuclear cells isolated from spleen, which contain about 35% T cells and 60 % B cells, and 2) naïve T cells isolated from lymph nodes of BALB/c mice were incubated with or without MTA. The level of cell toxicity measured at 24 hours after MTA exposure is shown as % cell death in comparison to the positive control of 100% cell death that was induced by lysis of all cells in a well after 24 hour incubation in medium alone (Fig. 2 A, naïve T cells; B, spleen mononuclear cells). B cells are, by their own nature, more prone to die by apoptosis in the regular in vitro culture condition. Therefore, as expected, spleen cells which contain not only naïve T cells, but also B cells (Fig. 2 B), showed a higher percentage of cell death than the group of naïve T cells (Fig. 2 A). Most importantly, the presence of MTA did not affect cell death of lymphocytes induced in the in vitro culture, indicating that MTA is not cytotoxic to lymphocytes.
Figure 2. Effects of MTA on isolated lymphocytes incubated in vitro. Lack of cytotoxicity to lymphocytes by MTA (A and B).
Freshly isolated lymph node T cells (A) or spleen mononuclear cells (B) (2×106 cells/mL, respectively) were incubated in a 24-well plate in the presence capillary filled with or without MTA for 24 hours. Cell death induced during the 24-hour incubation was measure by CytoTox-96™ Non-Radioactive cytotoxicity assay. # indicates no statistical differences between the groups with and without MTA. Effects of MTA on proliferation of bacteria-specific memory T cells (C and D): F. nucleatum-reactive memory T cells (C) and P. anaerobius-reactive memory T cells (D) were established from BALB/c mice. These bacterial-reactive memory T cells were pre-incubated with or without MTA in the presence of respective bacterial antigen and APC for 3 days. After pre-incubation, the T cells were re-stimulated with respective bacterial antigen and APC for 4 days. Proliferation of bacterium-specific memory T cells was measured. Column and bar indicate the mean and SD of triplicates, respectively, of one representative experiment. Abbreviations: Fn, F. nucleatum; Pa, P. anaerobius. Column and bar indicate the mean and SD of triplicates, respectively. This figure shows one representative result out of a total of 3 experiments. * indicates that the mean number of the histogram column is statistically higher than control empty capillaries by t-test (p<0.05). • indicates the statistical differences between the two columns connected with brackets by t-test (p<0.05).
Effect of MTA on memory T cells
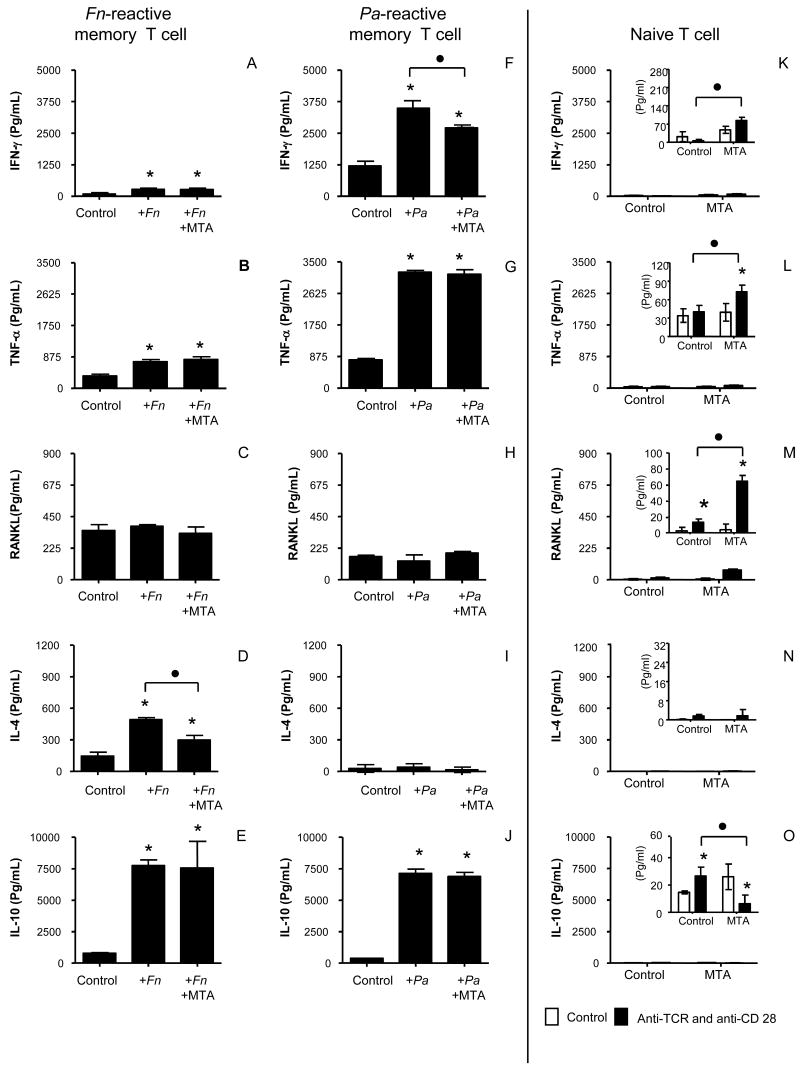
The memory T cells reactive to F. nucleatum or P. anaerobius from immunized BALB/c mice were tested for specificity by proliferation and cytokine expression in response to specific bacterial antigen. F. nucleatum-reactive memory T cells did not show proliferation to P. anaerobius-antigen presentation, and P. anaerobius-reactive memory T cells proliferation did not respond to F. nucleatum-antigen presentation (not shown). Also, flow cytometry results indicated that both F. nucleatum- and P. anaerobius-reactive memory T cells express CD44 (c.a. 90% of total of each T cells) and CD4 (c.a. 60% of total of each T cells) (data not shown). Both F. nucleatum- and P. anaerobius-reactive BALB/c memory T cells showed increased proliferation (Fig. 2C and D). Expression of cytokines in response to antigen (Fig. 3A and F, IFN-γ; B and G, TNF-α; E and J, IL-10) was also examined in memory cells. F. nucleatum-reactive memory T cells presented high IL-4 and low IFN-γ expression, whereas P. anaerobius-reactive memory T cells produced high IFN-γ and low IL-4 levels. Both memory T cell subsets showed elevated RANKL expression when they were incubated with APC, irrespective of the presence of bacteria (Fig. 3C and H). Having established these basal proliferation patterns and cytokine expression profiles by F. nucleatum- and P. anaerobius-reactive memory T cells, the influence of MTA could then be evaluated for the basal responses of these memory type T cells to their specific-antigen presentation.
Figure 3. Effects of MTA on cytokine production by bacteria-specific memory T cells and naïve T cells.
F. nucleatum-reactive memory T cells and P. anaerobius-reactive memory T cells were established from BALB/c mice. These bacterial-reactive memory T cells were pre-incubated with or without MTA in the presence of respective bacterial antigen and APC for 3 days. After pre-incubation, the T cells were re-stimulated with respective bacterial antigen and APC for 4 days (A-E, F. nucleatum-reactive memory T cells; F-J, P. anaerobius-reactive memory T cells). Otherwise, naïve T cells isolated from lymph nodes of BALB/c mice (K-O) were pre-incubated with or without MTA for 3 days. After pre-incubation, T cells were activated with immobilized anti-TCR MAb and anti-CD28 MAb for 4 days. Production of cytokines, including IFN-γ, TNF-α, RANKL, IL-4 and IL-10 by TCR/CD28-activated T cells, were measured in the culture supernatant. Column and bar indicate the mean and SD of triplicates, respectively. As to the figures for cytokine expression profile of naïve T cells (K-O), the inserted figures show the magnified y-axis scale for the detected amount of cytokines, while the y-axis of external figures keep the same scale as the one used for memory T cells. In other words, the insert and external figures for naïve T cells show the same data in different scales. Abbreviations: Fn, F. nucleatum; Pa, P. anaerobius. Column and bar indicate the mean and SD of triplicates, respectively. This figure shows one representative result out of a total of 3 experiments. * indicates that the mean number of the histogram column is statistically higher than control empty capillaries by t-test (p<0.05). • indicates the statistical differences between the two columns connected with brackets by t-test (p<0.05).
The exposure of BALB/c memory T cells to MTA suppressed the antigen-specific proliferation of both F. nucleatum- and P. anaerobius-reactive memory T cells (Fig. 2C and D). MTA exposure also inhibited IL-4 production by F. nucleatum-reactive memory T cells (Fig. 3D) and inhibited IFN-γ production by P. anaerobius-reactive BALB/c memory T cells (Fig. 3F). However, exposure to MTA did not affect the expression of RANKL, TNF-α and IL-10 by antigen-stimulated BALB/c memory T cells (Fig. 3B, C and E, F. nucleatum-reactive memory T cells; G, H and J, P. anaerobius-reactive memory T cells). These results therefore indicate that MTA exerts most of its influence on the T cell growth factors (IFN-γ and L-4), while the expression of the inflammatory cytokine, TNF-α, the bone destructive cytokine, RANKL, and the anti-inflammatory cytokine, IL-10, all remained unaffected by MTA. This lack of influence of MTA on the expression of RANKL, TNF-α and IL-10 by these two different types of memory T cells indicates that MTA may have no effects on the inflammation mediated by memory T cells in the context of periradicular lesions.
Effect of MTA on the Naïve T cell reactions to TCR/CD28 activation
In addition to memory type T cells, which appear to be the predominant T cells in periradicular lesions (4), naïve T cells may also be involved in the process of cell-mediated immune responses to pathogens present in the root canal system. Therefore, the present study also examined the influence of MTA on the activity of naïve T cells, including the effects of MTA exposure on the proliferation of naïve T cells in response to TCR/CD28 activation, as well as their cytokine expression pattern. First, in terms of proliferation, naïve T cells stimulated with TCR/CD28 showed an incorporation of [3H]-thymidine level which was comparable to that demonstrated by the proliferation of Th1-and Th2-type memory T cells (not shown). However, in contrast to the memory type T cells, where MTA suppressed the antigen-specific proliferation, the pre-exposure of MTA slightly increased TCR/CD28-mediated proliferation of naïve T cells compared to the control naïve T cells cultured without MTA (not shown). Second, while naïve T cells and Th1- or Th2-type memory T cells were applied in equal numbers to the respective proliferation assay, naïve T cells stimulated with TCR/CD28 showed significantly lower expression of all examined cytokines, including IFN-γ, IL-4, IL-10, TNF-α and RANKL, than the Th1- or Th2-type memory T cells activated with their specific antigens (Fig. 3K, L, M, N and O). Although pre-exposure of MTA slightly increased IFN-γ, TNF-α and RANKL expression by TCR/CD28-activated naïve T cells (Fig. 3, K, L and M; see the inset figures with magnified scale), the expression level of these three cytokines was still negligibly lower than that expressed by the two types of activated memory T cells.
Discussion
Periradicular lesions are characterized by inflammation in the connective tissues accompanied by bone destruction around the infected root-end of teeth (4;14). The cellular infiltration of the periradicular lesion has been characterized by a variety of cell types, mainly represented by macrophages and T and B lymphocytes (15;16). MTA is applied to the end of an infected tooth root canal where it is directly in contact with host immune cells infiltrating the inflammatory lesions of periradicular connective tissues. Therefore, it is plausible that immune responses at the periradicular lesions could be influenced by the presence of this endodontic material. The effects of MTA on cytokine expression by host immune cells in response to endodontic pathogens had not previously been addressed until we reported the influence of MTA on cytokine production by bacteria-stimulated mouse macrophages (17). Based on this study, it was observed that MTA did not affect TNF-α, IL-12 and IL-10 cytokine production by M1 or M2 macrophages under the conditions used in the present study, i.e., F. nucleatum and P. anaerobius stimulation (17). Since the influence of MTA on innate immune scavenger cells, or macrophages, had been previously addressed, it was intriguing to evaluate its potential effects on adaptive immune responses, including T cell and B cell responses. Accordingly, the present study demonstrated that MTA appears to only minimally affect memory T cell activity to specific bacterial antigen presentation. However, we originally hypothesized that MTA might increase adaptive immune response because one of its components, aluminum oxide, is reported to possess adjuvant effects. In terms of B cell-mediated humoral responses, this hypothesis was confirmed by IgG antibody responses to F. nucleatum which were upregulated by the presence of MTA.
Aluminum salt has been used as a vaccine adjuvant to induce antibody responses to bacteria such as Corynebacterium diphtheriae, Clostridium tetani, Pertussis, Haemophilus influenzae type b, Pneumococcus conjugates, and HeP. anaerobiustitis A and B (18). F. nucleatum immunization in the presence of MTA resulted in upregulation of IgG antibody responses to the bacterium to an extent comparable to F. nucleatum immunization in PBS. Since IgG-positive cells represent 70% of the immunoglobulin-producing cells in periradicular granulomas and radicular cysts (19), it is conceivable that locally produced IgG antibody in association with the use of MTA could, in fact, contribute to antibacterial activity in periradicular lesions. In other words, to the extent that sustained IgG antibody response repels bacterial infection and thus retains the host antimicrobial responses at the periradicular lesions, the overall MTA effect on IgG antibody response, as indicated above, should benefit the host.
In terms of the influence of MTA on naïve T cells, the present study demonstrated that MTA increased the production of RANKL, IFN-γ and TNF-α by TCR/CD28-activated naïve T cells. However, such increased amount of cytokine production by TCR/CD28-activated naive T cells in the presence of MTA was negligibly lower than the same cytokines produced by memory T cells, whereas both naïve and memory T cells showed comparable levels of proliferation. It is plausible that both inflammatory and anti-inflammatory cytokines derived from activated memory T cells would more prominently affect periradicular lesions than those same cytokines derived from naïve T cells, supported by the report that majority of T cells in the periradicular lesion is memory type T cells (4).
We addressed whether MTA could alter the expression level of pro- and anti-inflammatory cytokines by either F. nucleatum- or P. anaerobius-reactive memory T cells. Significantly, MTA suppressed the proliferation of these two different types of memory T cells along with the diminished expression of T cell differentiation-promoting cytokines, IL-4 or IFN-γ, for F. nucleatum- or P. anaerobius-reactive memory T cells, respectively. Even though both types of memory T cells expressed IL-10 and RANKL (TNF-α only for P. anaerobius-reactive memory T cells), it is significant that MTA, under the conditions tested, was not observed to alter the expression of these anti-inflammatory and bone destructive cytokines. It is noteworthy that IL-10-/- mice had significantly greater infection-stimulated bone resorption in vivo compared with wild-type mice, which indicates that this cytokine may be engaged in the down-modulation of this process (20). The fact that MTA did not interfere with the production of anti-inflammatory and bone destructive cytokines from memory T cells contradicted to our original hypothesis. Since MTA did not alter the RANKL expression from memory T cells, it is intriguing to test if MTA can influence the osteoclastogenesis induced by such RANKL produced from memory T cells in the context of periradicular lesion. This question of MTA's effects on osteoclastogenesis will be addressed in our future study.
In summary, the results of the present study have demonstrated that 1) MTA upregulated the IgG antibody responses, 2) MTA suppressed proliferation of F. nucleatum-reactive and P. anaerobius-reactive memory T cells and reduced their expression of cytokines, IL-4 and IFN-γ, respectively, and 3) MTA use results in little or no alteration of the other cytokine expression (TNF-α, RANKL and IL-10) by F. nucleatum-reactive and P. anaerobius-reactive memory T cells. These results indicated that MTA's influence on adaptive immune response still favors to the host in the context of periradicular lesion caused by bacterial infection.
Acknowledgments
We thank Dr. Ricardo Teles and Dr. Flávia Teles for their kind gift of the bacteria used in this study and Han Xiaozhe and Jean Eastcott for the flow cytometry experiments. This work was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Brazil) and NIDCR grants DE 03420, DE 14551 and DE18310.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Stern MH, Dreizen S, Mackler BF, Selbst AG, Levy BM. Quantitative Analysis of Cellular Composition of Human Periapical Granuloma. J Endod. 1981;7(3):117–22. doi: 10.1016/S0099-2399(81)80125-2. [DOI] [PubMed] [Google Scholar]
- 2.Yu SM, Stashenko P. Identification of Inflammatory Cells in Developing Rat Periapical Lesions. J Endod. 1987;13(11):535–40. doi: 10.1016/S0099-2399(87)80033-X. [DOI] [PubMed] [Google Scholar]
- 3.Tani N, Osada T, Watanabe Y, Umemoto T. Comparative Immunohistochemical Identification and Relative Distribution of Immunocompetent Cells in Sections of Frozen or Formalin-Fixed Tissue From Human Periapical Inflammatory Lesions. Endod Dent Traumatol. 1992;8(4):163–9. doi: 10.1111/j.1600-9657.1992.tb00237.x. [DOI] [PubMed] [Google Scholar]
- 4.Stashenko P, Teles R, D'Souza R. Periapical Inflammatory Responses and Their Modulation. Crit Rev Oral Biol Med. 1998;9(4):498–521. doi: 10.1177/10454411980090040701. [DOI] [PubMed] [Google Scholar]
- 5.Rezende TM, Vieira LQ, Cardoso FP, Oliveira RR, Oliveira Mendes ST, Jorge ML, Ribeiro Sobrinho AP. The Effect of Mineral Trioxide Aggregate on Phagocytic Activity and Production of Reactive Oxygen, Nitrogen Species and Arginase Activity by M1 and M2 Macrophages. Int Endod J. 2007;40(8):603–11. doi: 10.1111/j.1365-2591.2007.01255.x. [DOI] [PubMed] [Google Scholar]
- 6.Naim JO, van Oss CJ, Wu W, Giese RF, Nickerson PA. Mechanisms of Adjuvancy: I--Metal Oxides As Adjuvants. Vaccine. 1997;15(11):1183–93. doi: 10.1016/s0264-410x(97)00016-9. [DOI] [PubMed] [Google Scholar]
- 7.Petrovsky N, Aguilar JC. Vaccine Adjuvants: Current State and Future Trends. Immunol Cell Biol. 2004;82(5):488–96. doi: 10.1111/j.0818-9641.2004.01272.x. [DOI] [PubMed] [Google Scholar]
- 8.Asgary S, Parirokh M, Eghbal MJ, Brink F. Chemical Differences Between White and Gray Mineral Trioxide Aggregate. J Endod. 2005;31(2):101–3. doi: 10.1097/01.don.0000133156.85164.b2. [DOI] [PubMed] [Google Scholar]
- 9.Tomson PL, Grover LM, Lumley PJ, Sloan AJ, Smith AJ, Cooper PR. Dissolution of Bio-Active Dentine Matrix Components by Mineral Trioxide Aggregate. J Dent. 2007;35(8):636–42. doi: 10.1016/j.jdent.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 10.Oliveira Mendes ST, Ribeiro Sobrinho AP, de Carvalho AT, Souza Cortes MI, Vieira LQ. In Vitro Evaluation of the Cytotoxicity of Two Root Canal Sealers on Macrophage Activity. J Endod. 2003;29(2):95–9. doi: 10.1097/00004770-200302000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Lana MA, Ribeiro-Sobrinho AP, Stehling R, Garcia GD, Silva BK, Hamdan JS, Nicoli JR, Carvalho MA, Farias, Lde M. Microorganisms Isolated From Root Canals Presenting Necrotic Pulp and Their Drug Susceptibility in Vitro. Oral Microbiol Immunol. 2001;16(2):100–5. doi: 10.1034/j.1399-302x.2001.016002100.x. [DOI] [PubMed] [Google Scholar]
- 12.Ribeiro Sobrinho AP, Melo Maltos SM, Farias LM, de Carvalho MA, Nicoli JR, de Uzeda M, Vieira LQ. Cytokine Production in Response to Endodontic Infection in Germ-Free Mice. Oral Microbiol Immunol. 2002;17(6):344–53. doi: 10.1034/j.1399-302x.2002.170603.x. [DOI] [PubMed] [Google Scholar]
- 13.Kawai T, Eisen-Lev R, Seki M, Eastcott JW, Wilson ME, Taubman MA. Requirement of B7 Costimulation for Th1-Mediated Inflammatory Bone Resorption in Experimental Periodontal Disease. J Immunol. 2000 Feb 15;164(4):2102–9. doi: 10.4049/jimmunol.164.4.2102. [DOI] [PubMed] [Google Scholar]
- 14.Wang CY, Stashenko P. Kinetics of Bone-Resorbing Activity in Developing Periapical Lesions. J Dent Res. 1991;70(10):1362–6. doi: 10.1177/00220345910700100901. [DOI] [PubMed] [Google Scholar]
- 15.Kawashima N, Okiji T, Kosaka T, Suda H. Kinetics of Macrophages and Lymphoid Cells During the Development of Experimentally Induced Periapical Lesions in Rat Molars: a Quantitative Immunohistochemical Study. J Endod. 1996;22(6):311–6. doi: 10.1016/S0099-2399(96)80266-4. [DOI] [PubMed] [Google Scholar]
- 16.Stashenko P, Yu SM. T Helper and T Suppressor Cell Reversal During the Development of Induced Rat Periapical Lesions. J Dent Res. 1989;68(5):830–4. doi: 10.1177/00220345890680051601. [DOI] [PubMed] [Google Scholar]
- 17.Rezende TMB, Vargas DL, Cardoso FP, Sobrinho APR, Vieira LQ. Effect of Mineral Trioxide Aggregate on Cytokine Production by Peritoneal Macrophages. International Endodontics Journal. 2005;38:896–903. doi: 10.1111/j.1365-2591.2005.01036.x. [DOI] [PubMed] [Google Scholar]
- 18.Baylor NW, Egan W, Richman P. Aluminum Salts in Vaccines--US Perspective. Vaccine. 2002 May 31;20 Suppl 3:S18–S23. doi: 10.1016/s0264-410x(02)00166-4. [DOI] [PubMed] [Google Scholar]
- 19.Pulver WH, Taubman MA, Smith DJ. Immune Components in Human Dental Periapical Lesions. Arch Oral Biol. 1978;23(6):435–43. doi: 10.1016/0003-9969(78)90074-2. [DOI] [PubMed] [Google Scholar]
- 20.Sasaki H, Hou L, Belani A, Wang CY, Uchiyama T, Muller R, Stashenko P. IL-10, but Not IL-4, Suppresses Infection-Stimulated Bone Resorption in Vivo. J Immunol. 2000 Oct 1;165(7):3626–30. doi: 10.4049/jimmunol.165.7.3626. [DOI] [PubMed] [Google Scholar]