Abstract
A dilemma in behavioral brain mapping is that conventional techniques immobilize the subject, extinguishing all but the simplest behaviors. This is avoided if brain activation is imaged after completion of the behavior and tissue capture of the tracer. A single-pass flow tracer proposed for positron emission tomography (PET) is a radiolabeled copper(II) complex of pyruvaldehyde bis(N4-methylthiosemicarbazone), [Cu64]-PTSM. [Cu64]-PTSM reaches steady-state cerebral distribution more rapidly than the metabolic tracer [18F]-fluorodeoxyglucose, allowing imaging with substantially greater temporal resolution. Using dual-label autoradiography, this study compares the relative regional cerebral blood flow tracer distribution (CBF-TR) of [64Cu]-PTSM to that of the classic perfusion tracer [14C]-iodoantipyrine in a rat model during treadmill walking. Rats were exposed to continuous walking on a treadmill and compared to quiescent controls. [64Cu]-PTSM was bolus injected (iv) after 1 minute, followed by a 5 minute uptake and subsequent bolus injection of [14C]-iodoantipyrine. CBF-TR was quantified by autoradiography and analyzed in the three-dimensionally reconstructed brain by statistical parametric mapping, as well as by region-of-interest analysis. A high homology was found between the [64Cu]-PTSM and [14C]-iodoantipyrine patterns of cerebral activation in cortical and subcortical regions. For white matter, however, [64Cu]-PTSM showed lower perfusion than [14Cu]-iodoantipyrine. [64Cu]-PTSM is a useful tracer for functional brain mapping in freely-moving subjects. Its application in conjunction with PET promises to increase our understanding of the neural circuitry of behaviors dependent on locomotion.
1. INTRODUCTION
A central dilemma in functional neuroimaging of animal behaviors has been the fact that conventional neuroimaging protocols using positron emission tomography (PET), single photon emission computer tomography (SPECT) and functional magnetic resonance imaging (fMRI) generally require immobilization of the subject, which eliminates all but the simplest activity and introduces the additional variables of restraint stress. The result is that brain function of normal mammalian behaviors such as aggression, mating, foraging and social interaction – all requiring locomotion – remains poorly understood. Brain mapping in freely behaving subjects is of interest because such activated brain states may serve to accentuate pathological differences that only manifest partially while a subject is in the resting state (Holschneider and Maarek, 2004).
The problem of behavioral restraint can be solved if radiotracers are administered during, and regional brain activation is imaged after, completion of the behavioral task. Since imaging of brain metabolism using radiotracers typically is done after tracer uptake is complete and relatively imperturbable, this method is suitable for neuroimaging in nontethered, ambulatory subjects. This approach has been applied to functional brain mapping in humans during treadmill walking using [18F]-fluoro-2-deoxyglucose PET (Ishii et al., 1993; Mishina et al., 1999), as well as in animals engaged in a variety of behaviors using [18F]- or [14C]-2-dexoglucose (2-DG) (Gonzalez-Lima et al., 1993; Jacobs et al., 1995; Prins and Hovda, 2001; Schwartzman et al., 1986). The primary drawback of radiolabeled 2-DG is that the duration of the uptake and trapping of the tracer is around 25–45 minutes (Mori et al., 1990), which is suboptimal given the fact that many behaviors are more short-lived. In principle, delivery of a radiotracer that reaches a cerebral equilibrium in a shorter time frame than 2-DG, would allow imaging of behaviors with a greater temporal resolution.
One compound which has been proposed as a single-pass flow tracer for non-invasive PET applications is the copper(II) complex of pyruvaldehyde bis(N4-methylthiosemicarbazone), Cu-PTSM (Mathias et al., 1990; Young et al., 1994). Copper-PTSM, has been used to assess cerebral and myocardial perfusion in humans and animals (Di Rocco et al., 1993; Green et al., 1988; Green et al., 1990; Mathias et al., 1990; McCarthy et al., 1999; Okazawa et al., 1994). When labeled with [61Cu] (t1/2 = 3.3 hrs.), [64Cu] (t1/2 = 12.7 hrs.), or [67Cu] (t1/2 = 58.5 hrs.), Cu-PTSM might be appropriate for neuroimaging in freely moving subjects. [64Cu] is the most versatile of all the copper radionuclides owing to its unique decay scheme, which combines electron capture (41%), β− (40%), and β+ (19%). These copper labeled complexes show high lipophilicity and are extracted into the brain with a high efficiency (Extraction fraction 0.90–0.76 for cerebral blood flows of 36 –89 ml/min/100 g) (Mathias et al., 1990; Okazawa et al., 1994; Wallhaus et al., 1998). Data suggests a microsphere-like retention of Cu-PTSM, likely due to the irreversible binding of copper to intracellular thiols, with a concomitant reduction of copper (II) to copper (I) (Blower et al., 1996). After ~2–3 min., greater than 95% of the radioactivity in blood is due to metabolites, and these do not penetrate the blood brain barrier (Mathias et al., 1990) (Okazawa et al., 1994). No redistribution of [64Cu] is observed in baboon brain during 2 hours of imaging (Mathias et al., 1990). Although Cu-PTSM shows some initial backdiffusion and a slight underestimation in the high-flow range (Okazawa et al., 1994), it is not as severe as that with technetium-99m-hexamethylprophylene amine oxime ([99mTc]-HMPAO) (Yonekura et al., 1988) or the 99m-technetium-ethyl-cysteinate dimer ([99mTc]-ECD) (Di Rocco et al., 1993). Presence in some species of Cu-PTSM binding to albumin has been reported (Mathias et al., 1995), resulting in a correction factor being necessary for assessment of absolute regional CBF (Okazawa et al., 1996). However, for qualitative studies, such as would typically be adequate for constraint-free imaging, the relative distribution of Cu-PTSM correlates well with regional CBF obtained by 15O-water PET (Okazawa et al., 1994).
To date, Cu-PTSM has not been applied to functional neuroimaging in freely-moving subjects, though local increases in perfusion have been demonstrated in the sensorimotor cortex of the immobilized monkey in response to vibratory stimulation of the forepaw (Green et al., 1990). The goal of the current study was to use dual label autoradiography to compare the relative CBF tracer distribution (CBF-TR) of [64Cu]-PTSM, which requires intracellular capture to be retained in brain, to that of [14C]-iodoantipyrine, a freely diffusible, classic perfusion tracer. Regional cerebral perfusion was mapped across the entire brain following injection into rats while engaged in an active locomotor task or at rest.
2. RESULTS
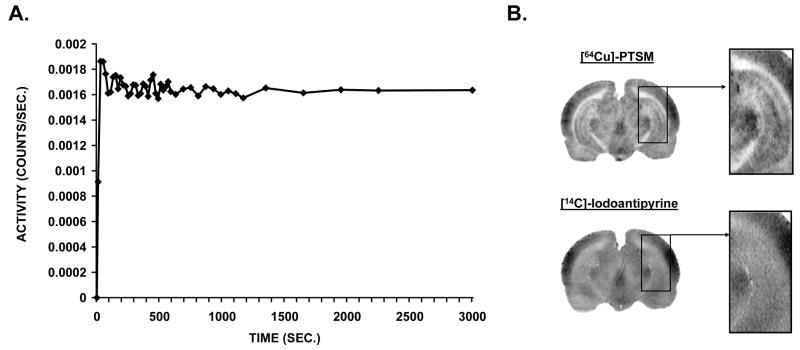
To confirm the rapid capture and retention of [64Cu]-PTSM, a dynamic PET study of whole brain uptake of the tracer was performed in an anesthetized rat (Fig. 1A). This showed rapid initial increase in [64Cu] activity in whole brain, followed by a plateau of such activity after ~200 sec., which was maintained over 50 minutes. Sample autoradiographs are shown in figure 1B, and suggest excellent spatial resolution for the [64Cu] tracer compared to the [14C] tracer.
Fig. 1.
(A) Time-activity curve for whole brain in a dynamic positron emission tomography (PET) study of a rat. Whole brain relative activity versus time shows the microsphere-like character of the [64Cu]-PTSM tracer, which was intravenously injected (1.25 mCi/kg) at time zero and subsequently was trapped in the brain, reaching plateau levels of activity within ~200 seconds. (B) Representative autoradiographs of a single slice obtained after dual tracer injection of [64Cu]-PTSM and [14C]-iodoantipyrine in a single rat, with an inset showing detail of the hippocampus.
2.1. SPM analysis of treadmill walking versus rest
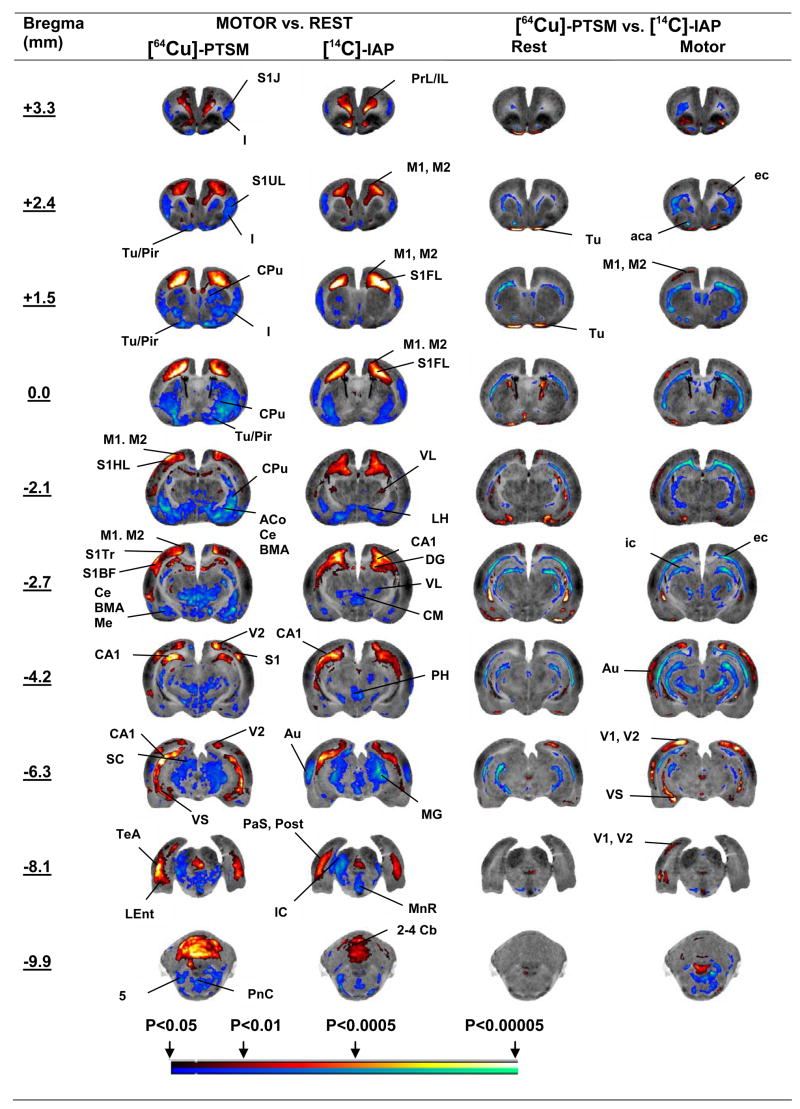
Changes in functional brain activation in response to treadmill walking are shown in figure 2 in the form of significant T-score differences of the regional CBF mapped onto coronal brain sections (Fig. 2, columns 2–3). A comprehensive list of significant group differences is shown in Table 1 (columns 2–3, P < 0.01). Both tracers demonstrated significant increases in CBF-TR in response to treadmill walking in the following cortical areas: motor (primary, secondary, M1, M2), parietal (medial association, MPtA), prelimbic/infralimbic (PrL/IL), somatosensory (primary, forelimb, hindlimb, trunk, S1FL, S1HL, S1Tr), and visual (secondary, V2). Subcortical increases in CBF-TR were noted for both tracers in the dorsal CA1 region of the hippocampus, the subicular complex (pre-, post-, para-, and ventral subiculum, PrS, Post, PaS, VS), the thalamus (anterior ventrolateral, VL) and the midline cerebellar lobules 1–5. Significant decreases (P < 0.01) were noted broadly in the auditory (Au), insular (I), olfactory (Tu), piriform (Pir), somatosensory (primary jaw, lip, S1J, S1UL) cortices, as well as the amygdala (cortical, basomedial, basolateral, central, medial, AA, ACo, CxA, PLCo, BMA, BLA, Ce, Me), caudate-putamen (CPu), globus pallidus (GP), lateral hypothalamus (LH), inferior and superior colliculi (IC, SC), the lateral lemniscus (ll), medial geniculate (MG), pontine reticular nucleus (PnC), midline raphe (MnR, CLi), thalamus (ventromedial VM, centromedial CM, rhomboid Rh, reuinens Re, posterior ventrolateral, VL) and trigeminal nucleus (Mo5, Pr5, Sp5).
Fig. 2.
Regions of statistically significant differences of functional brain activation in rats (SPM analysis) during treadmill walking (n = 9) or rest (n = 9) autoradiographically imaged following dual tracer injection of [64Cu]-PTSM and [14C]-iodoantipyrine ([14C]-IAP). Depicted are representative coronal slices (anterior–posterior coordinates relative to bregma). Colored overlays show statistically significant positive (red) and negative (blue) differences (voxel level, P < 0.05, Cluster >150 contiguous voxels). Right sides of the images represent the left sides of the brain. Abbreviations are those from the Paxinos and Watson (2005) rat atlas: 5 (trigeminal nucleus, motor, sensory), 2–4 Cb (2nd, 3rd, 4th cerebellar lobule), ACo (anterior cortical amygdaloid n.), BMA (basomedial amygdaloid n.), CA1 (hippocampus), CPu (striatum), CxA (cortical amygdala), ec (external capsule), GPE (external globus pallidus), GPI (internal globus pallidus), I (insular cortex), ic (internal capsule), IC (inferior colliculus), LEnt (lateral entorhinal cortex), LH (lateral hypothalamus), M1, M2 (primary, secondary motor cortex), MG (medial geniculate), MnR (median raphe), S1UL, S1FL, S1Tr (primary somatosensory cortex, lip, forelimb, trunk), Pir (piriform cortex), PnC (pontine reticular n., caudal), PrL (prelimbic cortex), SC (superior colliculus), S1 (primary somatosensory cortex), S (subiculum), TeA (temporal association cortex), Tu (olfactory cortex), V1, V2 (primary, secondary visual cortex), VM (ventromedial thalamic nucleus), VS (ventral subiculum)(Paxinos and Watson, 2005).
Table 1.
Statistically significant differences by SPM analysis of functional brain activation in cortical and subcortical areas in rats imaged using dual tracer injection.
| Motor vs. Rest | [64Cu]-PTSM vs. [14C]-IAP | |||
|---|---|---|---|---|
| [64Cu]-PTSM | [14C]-IAP | Rest | Motor | |
|
CORTEX
| ||||
| Entorhinal, lateral (LEnt) | ↑/↑ | ↑/↑ | - | - |
| Motor, primary, secondary (M1, M2) | ↑/↑ | ↑/↑ | - | ↑/↑ |
| Parietal, association, medial (MPtA) | ↑/↑ | ↑/↑ | - | - |
| Prelimbic/Infralimbic (PrL/IL) | ↑/↑ | ↑/↑ | - | - |
| Somatosensory, primary, forelimb, hindlimb, trunk (S1FL, S1HL, S1Tr) | ↑/↑ | ↑/↑ | - | - |
| Temporal, association (TeA) | ↑/↑ | ↑/↑ | - | - |
| Visual, primary (V1) | - | - | - | ↑/↑ |
| Visual, secondary lateral (V2) | ↑/↑ | ↑/↑ | ↑/↑ | ↑/↑ |
| Auditory (Au) | ↓/– | ↓/↓ | - | ↑/↑ |
| Insula (I) | ↓/↓ | ↓/– | - | - |
| Olfactory (Tu) | ↓/↓ | ↓/↓ | ↑↑ | ↑↑ |
| Piriform (Pir) | ↓/↓ | ↓/↓ | ↑↑ | ↑↑ |
| Somatosensory, primary, jaw, lip (S1J, S1UL) | ↓/↓ | ↓/↓ | - | - |
| Somatosensory, primary, barrel field (S1BF) | ↑/↑ | - | - | - |
|
| ||||
|
SUBCORTEX
| ||||
| Hippocampus (CA1, dentate gyrus DG) | ↑/↑ | ↑/↑ | - | - |
| Subicular complex (PaS, Post, PrS, VS) | ↑/↑ | ↑/↑ | - | ↑/↑ |
| Thalamus, ventrolateral anterior portion (VL) | ↑/↑ | ↑/↑ | - | - |
| Amygdala, anterior area, cortical anterior, cortical posterior(AA, ACo, CxA, PLCo) | ↓/↓ | ↓/↓ | ↑/↑ | ↑/↑ |
| Amygdala, basomedial, basolateral, central, medial (BMA, BLA, Ce, Me) | ↓/↓ | ↓/↓ | - | - |
| Caudate-putamen (CPu) | ↓/↓ | ↓/↓ | - | - |
| Globus pallidus (GP) | ↓/↓ | – | - | - |
| Hypothalamus, lateral, posterior (LH, PH) | ↓/↓ | ↓/↓ | - | - |
| Inferior colliculus (IC) | ↓/↓ | ↓/↓ | - | - |
| Lateral lemniscus (ll) | ↓/– | ↓/– | - | - |
| Medial geniculate (MG) | ↓/↓ | ↓/↓ | - | - |
| Pontine reticular n. (PnC) | ↓/↓ | ↓/↓ | - | - |
| Raphe, median, caudal linear (MnR, CLi) | ↓ | ↓ | - | - |
| Superior colliculus (SC) | ↓/↓ | ↓/↓ | - | - |
| Thalamus, ventrolateral, posterior portion (VL) | ↓/↓ | ↓/↓ | - | - |
| Thalamus, ventromedial, centromedial (VM, CM) and midline (rhomboid, Rh, reuinens, Re) | ↓/↓ | ↓/↓ | - | - |
| White matter (aca, ec, fmj, fmj, ic) | - | - | ↓/↓ | ↓/↓ |
| 5 n. (Mo5, Pr5, Sp5) | ↓/↓ | ↓/↓ | - | - |
| Cerebellar lobules 1–5 (1–5Cb) | ↑/↑ | ↑/↑ | - | - |
Column 2: Treadmill versus rest imaged using [64Cu]-PTSM, Column 3: Treadmill versus rest imaged using [14C]-iodoantipyrine ([14C]-IAP), Column 4: [64Cu]-PTSM versus [14C]-IAP perfusion map differences during the resting condition, Column 5: [64Cu]-PTSM versus [14C]-IAP perfusion map differences during treadmill walking. Significant increases or decreases are noted, respectively, for the right and left hemispheres (Right/Left). Significance is shown at the voxel level (P < 0.01) for clusters of > 150 contiguous voxels. Abbreviations are taken from the Paxinos and Watson rat atlas (Paxinos and Watson, 2005).
2.2. Cortical ROI analysis of treadmill walking versus rest
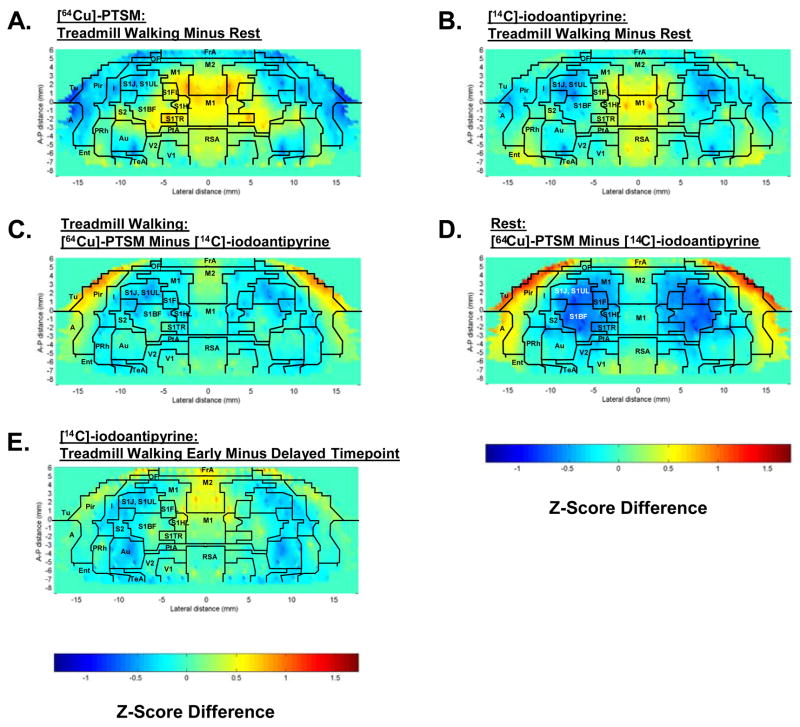
Maps of the Z-score differences of regional cerebral blood flow-related tissue radioactivity (CBF-TR) of the flattened cortex for rats during locomotion and rest are shown for each of the two tracers in figure 3. Results confirm those of the SPM analyses. Significant relative increases (P < 0.05) in response to treadmill walking were observed for both [64Cu]-PTSM and [14C]-iodoantipyrine in primary and secondary motor cortex (M1, M2), primary somatosensory cortex of the forelimbs, hindlimbs and trunk (S1FL, S1HL, S1Tr), lateral entorhinal cortex (LEnt), temporal association cortex (TeA), and retrosplenial/secondary visual transition cortex (RS/V2). CBF-TR was increased also locally in S1BF for the [64Cu]-PTSM. Significant decreases (P < 0.05) were noted in auditory (Au), insular (I), posterior olfactory (Tu), piriform (Pir), and primary somatosensory cortices of the jaw and lip region (S1J, S1UL), as well as the cortical amygdala. There were no statistical differences between the locomotor and control group in nontransformed cerebral blood flow tracer distribution (CBF-TR) or Z-scores of CBF-TR calculated by slice or globally across all cortical regions.
Fig. 3.
Cortical flat maps of the color-coded average Z-score differences for rats during treadmill walking (n = 9) or rest (n = 9). Functional brain activation is shown during treadmill walking, i.e. Z-scoreMotor minus Z-scoreRest, for (A) [64Cu]-PTSM and (B) [14C]-iodoantipyrine. Also depicted are differences in cortical activation between the tracers, i.e. Z-score[64Cu]PTSM minus Z-score[14C]iodoantipyrine. (C) during treadmill walking and (D) at rest. (E) depicts differences in cortical activation during treadmill walking between [14C]-iodoantipyrine when this tracer was injected early (after 1 minute of walking, i.e., the same time point used for [64Cu]-PTSM) or after 6 minutes of walking. Superimposed on the maps are the borders of the main cortical areas: A, cortical amygdaloid nucleus; Au, auditory; FrA, frontal association; I, insular; Ent, entorhinal; M1, primary motor; M2, secondary motor; OF, orbital frontal; PtA, parietal association; Pir, piriform; PRh, perirhinal; RSA, retrosplenial. Primary somatosensory mapping: S1FL, the forelimbs; S1HL, the hindlimbs; S1TR, the trunk; S1BF, the barrel fields; S1J/S1UL, the jaw, lip, and oral region; S2, secondary somatosensory; TeA, temporal association; Tu, olfactory; V1, primary visual; V2, secondary visual (Paxinos and Watson, 2005).
2.3. SPM analysis of [64Cu]-PTSM versus [14C]-iodoantipyrine
Isolated significant increases (P < 0.01) in CBF-TR of the [64Cu] tracer compared to the [14C] tracer were noted at rest and during treadmill walking in anterior olfactory/piriform, visual (V1, V2), motor (M1, M2), and auditory cortices and the ventral subiculum (Fig. 2, columns 4–5; Table 1, columns 4–5). Significant decreases (P < 0.01) were noted in white matter throughout the brain (e.g. external and internal capsules, anterior commissures, forcepts major and minor, ec, ic, ac, fmj, fmi).
2.4. Cortical ROI analysis of [64Cu]-PTSM versus [14C]-iodoantipyrine
Copper-64-PTSM compared to [14C]-iodoantipyrine showed significant increases in CBF-TR (P < 0.05) both during treadmill walking and at rest in broad areas of olfactory (Tu), piriform (Pir), amygdaloid (A) and secondary motor (M2) cortices. Isolated significant increases were also noted in visual cortex, though most regions remained below the significance threshold. Isolated significant changes (P < 0.05) were noted in ROIs within primary somatosensory cortex of the jaw and lip region (decreased CBF-TR, S1J, S1UL) and barrel field (increased CBF-TR, S1BF).
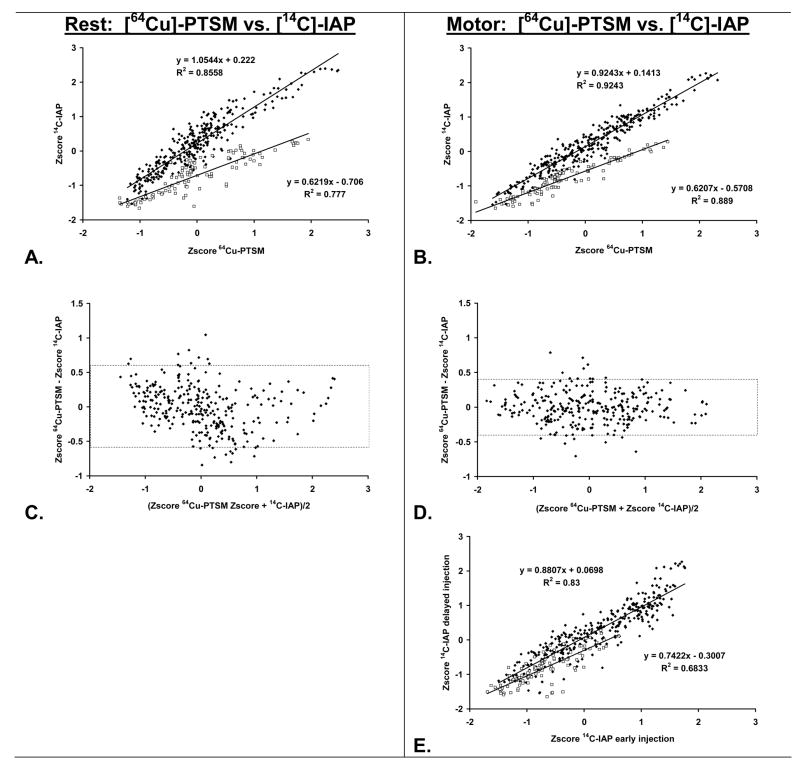
Scatterplots of the relationship of cortical Z-scores based on the [64Cu] tracer compared to the [14C] tracer revealed a bifurcated pattern, with clustering for olfactory, piriform, amygdaloid and secondary motor areas that was separate from that of all other cortex (Fig. 4).
Fig. 4.
Row 1: Linear correlation of cortical perfusion as measured using [64Cu]-PTSM or [14C]-iodoantipyrine in (A) the resting and (B) the locomotor state. Depicted are the group average Z-scores of cerebral blood flow–related tissue radioactivity (CBF-TR) for olfactory (Tu), piriform (Pir), secondary motor (M2) and amygdaloid cortices (ACo)( □, 102 ROIs), as well as all other cortical regions (◆, 318 ROIs). Row 2: Illustration of agreement between the cortical perfusion patterns of [64Cu]-PTSM and [14C]-iodoantipyrine in (C) the resting and (D) the locomotor state. Shown are Bland-Altman plots and their 95% limits of agreement (stippled line) for all cortical areas, excluding Tu, Pir, M2, and ACo. Row 3: (E) shows correlation of cortical perfusion as measured using [14C]-iodoantipyrine when the tracer was injected after 1 minute or 6 minutes of treadmill walking.
2.4.1. All Cortex (outside from olfactory, piriform, secondary motor and amygdaloid areas)
At rest (Fig. 4A), the linear regression line had a slope of 1.05 (1.01 to 1.10: 95%, R2 = 0.86, P < 0.000001) and an intercept of 0.22 (0.19 to 0.26: 95%, P < 0.000001), whereas during treadmill walking (Fig. 4B) the regression line had a slope of 0.92 (0.89 to 0.95: 95%, R2 = 0.92, P < 0.000001) and an intercept of 0.14 (0.12 to 0.17: 95%, P < 0.000001). The group mean Z-score for the [64Cu] tracer did not differ significantly from that of the [14C] tracer (Rest: −0.00 ± 0.75, 0.00 ± 0.83, P = 0.94, Locomotor: −0.01 ± 0.84, −0.01 ± 0.84, P = 0.98) (Figs. 4C, D). Using [14C] as the reference method, bias according to Bland and Altman was 0.00 ± 0.32 at rest and 0.00 ± 0.22 during the treadmill challenge. Limits of agreement covering 95% of the expected differences were −0.62 to 0.63 at rest, and −0.44 to 0.44 during the treadmill challenge.
2.4.2. Olfactory, piriform, secondary motor and amygdaloid cortices
These demonstrated a perfusion pattern in which the Z-scores derived from the [64Cu] tracer were greater than those derived from the [14C] tracer (Figs. 4A, B). At rest, the regression line had a slope of 0.62 (1.01 to 1.10: 95%, R2 = 0.78, P < 0.000001) and an intercept of −0.71 (−0.19 to 0.26: 95%, P < 0.000001), whereas during treadmill walking the regression line had a slope of 0.62 (0.89 to 0.95: 95%, R2 = 0.89, P < 0.000001) and an intercept of −0.57 (0.12 to 0.17: 95%, P < 0.000001).
2.5. ROI analysis of [14C]-iodoantipyrine early versus delayed injection
The fact that the [64Cu] and [14C] tracers were injected serially, rather than simultaneously, may have contributed to the observed differences in olfactory, piriform, secondary motor and amygdaloid cortices. We investigated this by injecting a separate group of treadmill running rats (n = 8) with [14C]-iodoantipyrine at the same time point previously used for the [64Cu]-PTSM injections (after 1 minute of running). Given the dynamic nature of behavior, we hypothesized that brain activation would differ when the [14C] tracer was injected at 1 minute versus 6 minutes after treadmill exposure. Differences would be the same as those observed for the [64Cu] versus [14C] comparison.
Results confirmed the hypothesis
[14C]-iodoantipyrine showed significant differences (P < 0.05) in cortical regions in animals injected early compared to those injected late during treadmill walking (Fig. 3 E). Significant relative increases in CBF-TR were seen in broad areas of olfactory, piriform and secondary motor cortex. Isolated significant activations were also noted in amygdaloid cortex. Significant decreases were observed in primary somatosensory cortex of the jaw and lip region (S1J, S1UL), as well as in auditory cortex (Au). Scatterplots (Fig. 4E) of the differences in the early versus delayed [14C] injection confirmed that the slope of the Z-score values for cortical regions of the piriform, olfactory, secondary motor and amygdaloid areas (slope 0.74, 95%: 0.64 to 0.84, R2 = 0.68) differed from the slope of the Z-scores of all other cortex sampled (slope 0.88, 95%: 0.84 to 0.92, R2 = 0.83, F1,416 = 5.21, P < 0.025).
3. DISCUSSION
Treadmill walking elicited activation in motor circuits (primary motor cortex, ventrolateral thalamus, midline cerebellum), primary somatosensory circuits (forelimb, hindlimb, and trunk cortex), as well as visual circuits (visual cortex, superior colliculus). This was consistent with the complex nature of this locomotor task, which involves integration of neural circuits subserving motor, sensory, and visual functions. Activations/deactivations noted in the current study replicated those reported in our earlier work mapping changes in regional cerebral perfusion in response to Rotarod treadmill walking (Holschneider et al., 2003; Nguyen et al., 2004).
Our study demonstrated a high homology between the patterns of cerebral activation during a treadmill challenge as imaged using [64Cu]]-PTSM and [14C]-iodoantipyrine. Homology in terms of magnitude and distribution of the functional brain activation was high in both cortical and subcortical regions, except for white matter where the [64Cu] compared to the [14C] tracer showed relatively smaller levels of activation. This may be due to the higher lipid content of white matter, which though it may facilitate tracer extraction may also decrease the efficiency with which the tracer is subsequently trapped. Our results are consistent with prior reports that suggest that a flow underestimation (in absolute terms) for Cu-PTSM is apparent in white matter regions of the brain (Mathias et al., 1990). The slope of the line correlating the cortical distribution of the [64Cu] and [14C] tracers (excluding piriform, olfactory, amygdaloid and secondary motor cortices) was 1.05 at rest and 0.92 during the locomotor challenge. This is in general agreement with prior work showing a slope of 1.01 (R2 =0.96) in immobilized gerbils (Green et al., 1988). In addition, the Bland and Altman plots showed that the cortical perfusion Z-scores for the [64Cu] tracer did not differ significantly from those of the [14C] tracer, though at rest there was a nonsignificant trend for Z-scores of the [64Cu] tracer to be lower than those of the [14C] tracer.
The slope of the line correlating the distribution of the [64Cu] and [14C] tracers in the olfactory, piriform, amygdaloid, and secondary motor cortices was 0.62, which was lower that of the other cortical regions. This discrepancy may be due to differences in the timing of the tracer injections during the behavioral challenge. The injection of [14C]-iodoantipyrine lagged behind that of [64Cu]-PTSM by 5 minutes. This sequence of administration was designed to ensure full uptake of [64Cu] tracer in the rat brain (as suggested by the dynamic PET study) prior to administration of the [14C] tracer and subsequent euthanasia. Prior work in gerbils in the immobilized, resting state has suggested that administration of [67Cu]-PTSM and [125I]-iodoantipyrine show a similar correlation, whether the tracers are given simultaneously (slope = 1.0, R2 = 0.98) or sequentially with a 3 minute delay (slope = 1.01, R2 = 0.96) (Green et al., 1988). This led us to believe that differences in our study may have been attributable to differences in the animal’s behavioral state between the two tracer injections. The effects of olfactory novelty and its associated exploratory behavior may have been present to a greater extent in the initial minutes following exposure to the Rotarod or the transport cage in which animals were imaged at rest. In addition, some attenuation of motor circuit activation cannot be ruled out at the later time points due to habituation. The increased activation in olfactory, piriform, secondary motor, and amgydaloid cortices with the [64Cu] tracer compared to the [14C] tracer suggests increased attention being focused on these sensory modalities immediately following the administration of the [64Cu] tracer. We confirmed this observation in a separate set of animals in which a bolus of [14C]-iodoantipyrine was administered at the same time point, previously used for [64Cu]-PTSM injection. In this case, comparison of the brain maps from the early compared to the late time points for injection of the [14C] tracer confirmed a relative activation of olfactory, piriform, secondary motor, and amygdaloid cortices during the early compared to the late time point. Isolated differences during treadmill walking (e.g. deactivation of auditory cortex and nonsignificant activation of visual cortex in the [14C] early-versus-late comparison, but not in the [64Cu] versus [14C] comparison) may have been due to differences between the temporal resolution of the [64Cu] tracer (~2–3 min.) and the [14C] tracer (~10 sec.).
A limitation of our study was that we did not obtain measures of absolute CBF, but rather patterns of relative CBF as indicated by tissue radioactivity. This is because direct arterial blood sampling was not practical in the freely moving animal. Hence, the significant changes indicate relative changes in the distribution of brain perfusion during the treadmill task, and no conclusions can be made regarding absolute flow.
One limitation of [64Cu]-PTSM for human applications is its radiation dose. Following injection, Cu accumulates slowly in the liver following injection. Dosimetric calculations for human subjects (Williams et al., 2005) have shown that [64Cu]-PTSM administration yields an effective dose (3.8 mSv/100 MBq) and critical organ dose (20 mGy/100 MBq in liver), which is higher than that of [18F]-fluoro-2-deoxyglucose (effective dose 1.9 mSv/100 MBq, critical dose in bladder 7.3 mGy/100 MBq) and H215O (effective dose 0.1 mSv/100 MBq, critical dose in heart 0.19 mGy/100 MBq). This higher dose is likely due to the long half-life of the [64Cu] isotope and its negative beta decay branch (branching ratio = 39%). A lower effective dose is obtained for [61Cu]-PTSM (effective dose 2.5 mSv/100 MBq), which is comparable to that of [18F]-fluoro-2-deoxyglucose, and likely due to its shorter half-life ([61Cu] t 1/2 = 3.3 hrs.). Future work in human subjects will need to evaluate whether adequate images can be obtained with lower doses of the tracer, possibly by extending the imaging period. Furthermore, most facilities would likely need to purchase the isotope commercially (e.g. MDS Nordion, Ontario, Canada), though the half-life of [64Cu] (t1/2 = 12.7 h) makes shipping over considerable distance feasible.
Conclusions
For PET applications at present there are few available tracers that meet the requirements necessary for constraint-free neuroimaging, including (a) high extraction fraction in brain, (b) prolonged retention with a fixed distribution, (c) inability of radiolabeled metabolites to cross the blood brain barrier, (d) half-life sufficiently short to minimize radiation exposure and allow for rescanning of the subject as its own control, and yet sufficiently long to allow time for tracer administration during occurrence of the behavior and subsequent positioning of the subject in the scanner. Our study demonstrated that [64Cu]-PTSM meets all of these criteria in addition to being suitable for imaging with PET. We observed a high degree of homology between the patterns of cerebral activation during a treadmill challenge as imaged using [64Cu]-PTSM or [14C]-iodoantipyrine. Correlation was high in both cortical and subcortical regions; however, in white matter [64Cu]-PTSM underestimated relative perfusion compared with [14C]-iodoantipyrine. These results indicate that [64Cu]-PTSM is suitable for functional brain mapping studies in freely moving animals.
4. EXPERIMENTAL PROCEDURES
4.1. Animals
Male Sprague Dawley rats (Harlan Sprague–Dawley Labs, Indianapolis, IN, U.S.A.), weighing 350 to 375 g, were used. Experimental protocols were reviewed and approved by the Animal Research Committee of the University of Southern California. The rats were group housed before surgery and singly housed after surgery on a 12-hour light period between 6:00 A.M. and 6:00 P.M., with free access to water and rodent chow. Rats were trained 5 days/week over 2 weeks, as previously described (Holschneider et al., 2003; Nguyen et al., 2004), to run on the Rotarod (Columbus Instruments, Columbus, OH, U.S.A.), a rotating cylindrical rod, such that they were able to consistently run at 20 rpm (7.9 cm/s) for 30 minutes without falling.
4.2. Surgery
After the training period, animals were implanted under isoflurane anesthesia (1.3%) with catheters (5F silastic). A catheter was inserted into the external jugular vein, and advanced into the superior vena cava. To provide a percutaneous port, the proximal end of the catheter was tunneled through the subcutaneous space to the back, and there externalized in the infrascapular, dorsal midline and capped with a stainless steel plug. The skin surrounding the catheter was sutured. On postoperative day 3, functional brain mapping was initiated, after randomization of the animals to a treadmill challenge (n = 9) or quiescent condition (n = 9).
4.3. Radiotracers
[64Cu] was obtained through the Research Resource in Radionuclide Research at the Mallinckrodt Institute at Washington University, St. Louis. The PTSM ligand was prepared according to standard methods (Petering et al., 1964). One mole (or fraction thereof) of pyruvaldehyde (Sigma-Aldrich, St. Louis, MO) in an aqueous solution was added dropwise under gentle stirring over a period of 30–40 minutes to an aqueous solution of 2.2. moles of N4-dimethylthiosemicarbazide, and 5 % glacial acetic acid at 50° – 60° C. The di-carbonyl derivative was recrystallized by dissolving the crude product in methanol at boiling temperature and quickly adding an equal volume of water. Chemical identity of the compound was confirmed by mass spectroscopy.
Complexes of [64Cu] and the PTSM ligand were prepared at the University of Southern California PET center according to a modification of the method of Green et al (Green et al., 1988) (Anderson and Bergmann, 1994). PTSM was labeled with [64Cu] by adding PTSM (1 mg/mL in dimethyl sulfoxide) to 1–5 mCi of [64Cu] in 0.1 N sodium acetate (pH 5.5) buffer (1 mg PTSM per mCi [64Cu]) followed by 5 minute incubation at 50°C. [64Cu]-PTSM was purified on a C-18 SepPak cartridge, using 85% ethanol as the elution solvent. The ethanol was evaporated and the activity was reconstituted in phosphate-buffered saline and passed through a 0.22 mm Millipore filter into a sterile multidose vial for use in animal experiments. Purity of the compound was confirmed for each synthesis by thin layer chromatography and remained >98% throughout the study.
[14C]-iodoantipyrine (4-[N-methyl-14C] iodoantipyrine in ethanol) was obtained from Amersham Biosciences (#ARC-0126, Piscataway, NJ, U.S.A., 99% purity as confirmed by thin layer chromatography), and after evaporation of the ethanol solvent reconstituted in 0.9% saline (100 μCi/kg in 300μL of 0.9% saline).
4.4. Time Activity Curve
Tracer uptake and retention in whole brain was examined in a single rat using dynamic PET imaging. The animal was anesthetized with isoflurane (1.1%), and a catheter was placed in a femoral vein. [64Cu]-PTSM (1.25 mCi/kg, i.v.) was injected while the animal remained anesthetized, and a dynamic scan was obtained over 50 minutes using a micro-PET R4 Rodent scanner (Concorde Microsystems, Inc., Knoxville, TN). Body temperature was monitored continuously using a rectal probe and maintained at 36.5°C with a heating pad placed under the animal on the scanner bed.
4.5. Functional Neuroimaging
Brain mapping was conducted between noon and 5 pm under standard laboratory lighting conditions with a background noise level of 60 dB. Rats placed in a metal transport cage with a wire mesh top and habituated for 30 minutes to the experimental room . The Rotarod spindle and transport cage were wiped with a cloth dampened with 1% ammonia between animals to minimize olfactory cues. Novelty effects were minimized due to prior exposure of the rats to both the Rotarod and the transport cage.
At the start of the experiment, animals were refamiliarized with treadmill walking on the Rotarod for 2 minutes at 8 rpm (3.1 cm/s). Thereafter, the rats were removed and the percutaneous port was quickly connected to an external catheter linked to an infusion pump placed behind the Rotarod, following which, the animal was returned to the rotating rod. Bolus administration of the [64Cu]-PTSM tracer (4 mCi/kg, 0.4 ml over 10 sec., i.v.) was performed via the infusion pump after 1 minute, with the animal continuing to walk on the Rotarod for 5 additional minutes, the time required for brain uptake of the isotope to reach a stable plateau (Fig. 1A).
Injection of [14C]-iodoantipyrine was performed in the walking animal 5 min. after injection of the [64Cu] tracer, and was followed immediately by injection of a euthanasia solution (1.0 mL of pentobarbital 50 mg/kg, 3 mol/L potassium chloride, i.v.). Administration of [14C]-iodoantipyrine could not be concomitant with the [64Cu]-PTSM injection because, unlike the [64Cu] tracer, the [14C] tracer is diffusible and requires rapid euthanasia and brain removal to maximize spatial resolution needed for detailed brain mapping. Injection of the euthanasia solution resulted in cardiac arrest within ~10 seconds, a precipitous decrease of arterial blood pressure, termination of brain perfusion, and death (Holschneider et al., 2002). Control animals rested quietly in the transport cage placed directly next to the Rotarod, and received the same handling sequence for connection of the external catheter, as well as the same sequence of tracer injections.
4.6. Autoradiography
The brains were rapidly removed, flash frozen in methylbutane at −55°C, embedded in OCT compound (Miles Inc., Elkhart, IN, U.S.A.), and cut at −18 °C in a cryostat in 20-μm-thick coronal sections with an interslice distance of 300 μm. Sections were heat-dried on glass slides and exposed for 15 hours at room temperature to Kodak Ektascan films in spring-loaded x-ray cassettes. This short duration allowed us to obtain excellent [64Cu]-PTSM images, while minimizing the extent of “contamination” by the weaker [14C]. One week later, slices were reexposed to Kodak Ektascan film for exposure to the [14C] isotope. At this time point, decay of the [64Cu] isotope in the slices was assumed to be complete (>10 half-lives of the [64Cu], t½ = 12.7 hours), such that images obtained were the results of [14C] exposure only. Absence of a visually apparent image for the [14C] tracer after 15 hours of exposure for each animal confirmed that [14C] background in the [64Cu] images, as well as [64Cu] background in the subsequently acquired [14C] images was negligible. Reexposure to a third autoradiography film for a duration of 15 days following the decay of the [64Cu] isotope, allowed us to obtain images of the [14C]-iodoantipyrine distribution in the brain.
4.7. 3-D Reconstruction of the Digitized Autoradiographs
In preparation for the statistical parametric mapping (SPM) analysis, a three dimensional (3-D) reconstruction of each animal’s brain was computed using 54 serial coronal sections, selected starting at 4.8 mm anterior to bregma. Adjacent sections were aligned both manually using Adobe Photoshop CS (Adobe Systems, Inc., USA) and using TurboReg (Thevenaz et al., 1998), an automated pixel-based registration algorithm, using a non-warping geometric model that included rotations and translations (rigid-body transformation), and used nearest-neighbor interpolation. Individual 3-D images for each brain were spatially normalized into a standard space defined by a template of the rat brain. Creation of the rat brain template has been described in our previous work (Nguyen et al., 2004). Spatial normalization consisted in applying a 12 parameter affine transformation followed by a non-linear spatial normalization using 3-D discrete cosine transforms (Ashburner and Friston, 1999). Normalized images were averaged to create a mean image. This image was smoothed with a Gaussian kernel (FWHM = 3 X voxel dimension, voxel size: 64 × 64 × 300 μm3) to create the final rat-brain template. Then each initial reconstructed brain was spatially normalized to this average template.
4.8. Statistical Parametric Mapping (SPM)
SPM (Friston et al., 1990; Friston et al., 1995), a system developed for analysis of imaging data in humans, has been recently adapted by us (Nguyen et al., 2004) and others (Lee et al., 2005) for use in rat brain autoradiographs. The non-biased, voxel-by-voxel analysis of whole-brain activation using SPM was used for detection of significant changes in functional brain activation that would be difficult to predict a priori. Global differences in the amount of radiotracer delivered to the brain were adjusted by the SPM software in each animal by scaling the voxel intensities so that the mean intensity for each brain was the same (proportional scaling). Using SPM, we implemented a Student t test (unpaired) at each voxel, testing the null hypothesis that there was no effect of experimental group. Maps of positive and negative t were separately analyzed. We chose to set a significance threshold P < 0.01 (uncorrected) for individual voxels within clusters of contiguous voxels, and a minimum cluster size of 150 contiguous voxels (extent threshold). Brain regions were identified using an anatomic atlas of the rat brain (Paxinos and Watson, 2005).
4.9. Region of Interest Analysis
Region-of-interest (ROI) evaluations were performed to provide detailed mapping of the cerebral cortex using methods previously reported (Holschneider et al., 2008). In brief, autoradiographs of brain sections were digitized, the optical density was measured in each hemisphere using an optical grid for 6–10 locations spaced in 15° intervals from the midline along the cortical rim using a program customized for this purpose in MATLAB. 420 cortical regions were sampled, of the regions listed in the legend of figure 3. The animal’s tissue radioactivity values were averaged by region for the left and right hemispheres. A Z-score transformation was performed on the tissue radioactivity data to produce patterns of regional tracer concentrations for each animal (Hays, 1973). The Z-scores were calculated as: Z-scorei = (TRi − mean)/SD, where Z-scorei was the standard normal deviate of tissue radioactivity at location i, and TRi was the tissue radioactivity of location i. The mean and SD were defined in a given animal as the average and standard deviation of the data set. Group differences in regional CBF Z-scores were compared using t-tests (unpaired, 2-tailed, P < 0.05). In addition, t-tests were applied to the group means of the optical densities measured at each location, each slice (all locations in a given slice), and globally (all locations in all slices).
For display purposes, Z-scores were displayed on a color scale on two-dimensional maps of the flattened cortex (Fig. 3). In these maps, the x- and y-coordinates are obtained from measures of the anatomical distances within the autoradiographs. The x-axis (locations) represents lateral distance from the midline (in mm) along the cortical rim within a slice. The y-axis (slices) represents coronal slices, numbered from rostral to caudal, with distance relative to bregma in millimeters (positive values being rostral to this landmark). To avoid discontinuities in the graphic representation, the space between each coronal slice and the 12–20 locations within each slice, where there were no measurements, was filled with values calculated by linear interpolation (maximum interpolation distance 1000 μm).
The relation between [64Cu]-PTSM and [14C]-iodoantipyrine in the cerebral cortex was analyzed by linear regression analysis. For visual purposes, agreement between the two tracers was investigated with a Bland-Altman plot comprising two 95% bands (Bland and Altman, 1995).
Acknowledgments
We would like to acknowledge Dr. X. Chen, Michel Tohme and Ryan Park, for their technical help in conducting the dynamic PET study, Pooja Sharma for her help with the selection of the regions of interest, and Dr. Oscar U. Scremin for his comments regarding the manuscript. [64Cu] was obtained through the Research Resource in Radionuclide Research at the Mallinckrodt Institute at Washington University, St. Louis (NCI grant 5R24CA086307-03). Supported by a grants from NIMH #R21 MH073000 (DH) and NCRR S10 RR15703 (PC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson CJ, Bergmann SR. In search of the perfect PET flow tracer. J Nucl Med. 1994;35:1122–1124. [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Nonlinear spatial normalization using basis functions. Hum Brain Mapp. 1999;7:254–66. doi: 10.1002/(SICI)1097-0193(1999)7:4<254::AID-HBM4>3.0.CO;2-G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bland JM, Altman DG. Comparing methods of measurement: why plotting difference against standard method is misleading. Lancet. 1995;346:1085–7. doi: 10.1016/s0140-6736(95)91748-9. [DOI] [PubMed] [Google Scholar]
- Blower PJ, Lewis JS, Zweit J. Copper radionuclides and radiopharmaceuticals in nuclear medicine. Nucl Med Biol. 1996;23:957–80. doi: 10.1016/s0969-8051(96)00130-8. [DOI] [PubMed] [Google Scholar]
- Di Rocco RJ, Silva DA, Kuczynski BL, Narra RK, Ramalingam K, Jurisson S, Nunn AD, Eckelman WC. The single-pass cerebral extraction and capillary permeability-surface area product of several putative cerebral blood flow imaging agents. J Nucl Med. 1993;34:641–8. [PubMed] [Google Scholar]
- Friston KJ, Frith CD, Liddle PF, Dolan RJ, Lammertsma AA, Frackowiak RS. The relationship between global and local changes in PET scans. J Cereb Blood Flow Metab. 1990;10:458–66. doi: 10.1038/jcbfm.1990.88. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes A, Worsley KJ, Poline JB, Frith CD, Frackowiak RS. Statistical parametric maps in functional imaging: A general linear approach. Human Brain Mapping. 1995;2:189–210. [Google Scholar]
- Gonzalez-Lima F, Helmstetter FJ, Agudo J. Functional mapping of the rat brain during drinking behavior: a fluorodeoxyglucose study. Physiol Behav. 1993;54:605–12. doi: 10.1016/0031-9384(93)90256-f. [DOI] [PubMed] [Google Scholar]
- Green MA, Klippenstein DL, Tennison JR. Copper(II) bis(thiosemicarbazone) complexes as potential tracers for evaluation of cerebral and myocardial blood flow with PET. J Nucl Med. 1988;29:1549–1557. [PubMed] [Google Scholar]
- Green MA, Mathias CJ, Welch MJ, McGuire AH, Perry D, Fernandez-Rubio F, Perlmutter JS, Raichle ME, Bergmann SR. Copper-62-labeled pyruvaldehyde bis(N4-methylthiosemicarbazonato)copper(II): synthesis and evaluation as a positron emission tomography tracer for cerebral and myocardial perfusion. J NuclMed. 1990;31:1989–1996. [PubMed] [Google Scholar]
- Hays W. Statistics for the Social Sciences. Holt, Rinehart & Winston; New York: 1973. [Google Scholar]
- Holschneider DP, Maarek JM. Mapping brain function in freely moving subjects. Neurosci Biobehav Rev. 2004;28:449–61. doi: 10.1016/j.neubiorev.2004.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holschneider DP, Maarek JM, Harimoto J, Yang J, Scremin OU. An implantable bolus infusion pump for use in freely moving, nontethered rats. Am J Physiol Heart Circ Physiol. 2002;283:H1713–9. doi: 10.1152/ajpheart.00362.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holschneider DP, Maarek JM, Yang J, Harimoto J, Scremin OU. Functional brain mapping in freely moving rats during treadmill walking. J Cereb Blood Flow Metab. 2003;23:925–32. doi: 10.1097/01.WCB.0000072797.66873.6A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holschneider DPOUS, Chialvo DR, Kay BP, Maarek J-MI. Flattened Cortical Maps of Cerebral Function in the Rat: A Region-of-Interest Approach to Data Sampling, Analysis and Display. Neurosci Lett. 2008;434:179–184. doi: 10.1016/j.neulet.2008.01.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii K, Senda M, Toyama M, Oda K, Ishii S, Ishiwata K, Sasaki T, Bando M. Brain function associated with bipedal gait--a PET study. J Cereb Blood Flow Metab. 1993;13(Suppl 1):S521. [Google Scholar]
- Jacobs B, Chugani HT, Allada V, Chen S, Phelps ME, Pollack DB, Raleigh MJ. Developmental changes in brain metabolism in sedated rhesus macaques and vervet monkeys revealed by positron emission tomography. CEREB CORTEX. 1995;5:222–233. doi: 10.1093/cercor/5.3.222. [DOI] [PubMed] [Google Scholar]
- Lee JS, Ahn SH, Lee DS, Oh SH, Kim CS, Jeong JM, Park KS, Chung JK, Lee MC. Voxel-based statistical analysis of cerebral glucose metabolism in the rat cortical deafness model by 3D reconstruction of brain from autoradiographic images. Eur J Nucl Med Mol Imaging. 2005;32:696–701. doi: 10.1007/s00259-004-1739-y. [DOI] [PubMed] [Google Scholar]
- Mathias CJ, Bergmann SR, Green MA. Species-dependent binding of copper(II) bis(thiosemicarbazone) radiopharmaceuticals to serum albumin. J NuclMed. 1995;36:1451–1455. [PubMed] [Google Scholar]
- Mathias CJ, Welch MJ, Raichle ME, Mintun MA, Lich LL, McGuire AH, Zinn KR, John EK, Green MA. Evaluation of a potential generator-produced PET tracer for cerebral perfusion imaging: single-pass cerebral extraction measurements and imaging with radiolabeled Cu-PTSM. J NuclMed. 1990;31:351–359. [PubMed] [Google Scholar]
- McCarthy DW, Bass LA, Cutler PD, Shefer RE, Klinkowstein RE, Herrero P, Lewis JS, Cutler CS, Anderson CJ, Welch MJ. High purity production and potential applications of copper-60 and copper-61. NuclMed Biol. 1999;26:351–358. doi: 10.1016/s0969-8051(98)00113-9. [DOI] [PubMed] [Google Scholar]
- Mishina M, Senda M, Ishii K, Ohyama M, Kitamura S, Katayama Y. Cerebellar activation during ataxic gait in olivopontocerebellar atrophy: a PET study. Acta Neurol Scand. 1999;100:369–76. doi: 10.1111/j.1600-0404.1999.tb01055.x. [DOI] [PubMed] [Google Scholar]
- Mori K, Schmidt K, Jay T, Palombo E, Nelson T, Lucignani G, Pettigrew K, Kennedy C, Sokoloff L. Optimal duration of experimental period in measurement of local cerebral glucose utilization with the deoxyglucose method. J Neurochem. 1990;54:307–319. doi: 10.1111/j.1471-4159.1990.tb13316.x. [DOI] [PubMed] [Google Scholar]
- Nguyen PT, Holschneider DP, Maarek JM, Yang J, Mandelkern MA. Statistical parametric mapping applied to an autoradiographic study of cerebral activation during treadmill walking in rats. Neuroimage. 2004;23:252–9. doi: 10.1016/j.neuroimage.2004.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazawa H, Yonekura Y, Fujibayashi Y, Mukai T, Nishizawa S, Magata Y, Ishizu K, Tamaki N, Konishi J. Measurement of regional cerebral blood flow with copper-62-PTSM and a three-compartment model. J Nucl Med. 1996;37:1089–1093. [PubMed] [Google Scholar]
- Okazawa H, Yonekura Y, Fujibayashi Y, Nishizawa S, Magata Y, Ishizu K, Tanaka F, Tsuchida T, Tamaki N, Konishi J. Clinical application and quantitative evaluation of generator-produced copper-62-PTSM as a brain perfusion tracer for PET. J NuclMed. 1994;35:1910–1915. [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotactic Coordinates. Elsevier Academic Press; New York, NY: 2005. [Google Scholar]
- Petering HG, Buskirk HH, Underwood GE. The anti-tumor activity of 2-keto-3-ethoxybutyraldehyde bis(thiosemicarbazone) and related compounds. Cancer Res. 1964;24:367–372. [PubMed] [Google Scholar]
- Prins ML, Hovda DA. Mapping cerebral glucose metabolism during spatial learning: interactions of development and traumatic brain injury. J Neurotrauma. 2001;18:31–46. doi: 10.1089/089771501750055758. [DOI] [PubMed] [Google Scholar]
- Schwartzman RJ, Eidelberg E, Alexander GM. Asymmetrical regional changes in energy metabolism of the central nervous system during walking. Brain Res. 1986;398:113–20. doi: 10.1016/0006-8993(86)91256-4. [DOI] [PubMed] [Google Scholar]
- Thevenaz P, Ruttimann UE, Unser M. A pyramid approach to subpixel registration based on intensity. IEEE Trans Image Process. 1998;7:27–41. doi: 10.1109/83.650848. [DOI] [PubMed] [Google Scholar]
- Wallhaus TR, Lacy J, Whang J, Green MA, Nickles RJ, Stone CK. Human biodistribution and dosimetry of the PET perfusion agent copper-62-PTSM. J Nucl Med. 1998;39:1958–1964. [PubMed] [Google Scholar]
- Williams HA, Robinson S, Julyan P, Zweit J, Hastings D. A comparison of PET imaging characteristics of various copper radioisotopes. Eur J Nucl Med Mol Imaging. 2005;32:1473–80. doi: 10.1007/s00259-005-1906-9. [DOI] [PubMed] [Google Scholar]
- Yonekura Y, Nishizawa S, Mukai T, Fujita T, Fukuyama H, Ishikawa M, Kikuchi H, Konishi J, Andersen AR, Lassen NA. SPECT with [99mTc]-d,l-hexamethyl-propylene amine oxime (HM-PAO) compared with regional cerebral blood flow measured by PET: effects of linearization. J Cereb Blood Flow Metab. 1988;8:S82–S89. doi: 10.1038/jcbfm.1988.36. [DOI] [PubMed] [Google Scholar]
- Young H, Carnochan P, Zweit J, Babich J, Cherry S, Ott R. Tissue blood flow estimation with copper(II)-pyruvaldehyde bis (N-4-methylthiosemicarbazone) and PET. J Nucl Biol Med. 1994;38:89–91. [PubMed] [Google Scholar]