Summary
Adaptive changes in serotonin2A (5-HT2A) receptor signaling are associated with the clinical response to a number of psychiatric drugs including atypical antipsychotics and selective serotonin reuptake inhibitors. The present study examined possible mechanisms of agonist-induced desensitization of 5-HT2A receptors in rat hypothalamic paraventricular nucleus (PVN) after 4 and 7 days of treatment with 1 mg/kg (-)-1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane HCl (DOI). The magnitude of 5-HT2A receptor-mediated oxytocin release decreased 78% after 4 days and 61% after 7 days of DOI treatment. Similarly, the magnitude of ACTH release following 1 mg/kg DOI decreased by 31% after 4 days and 38% after 7 days of DOI treatment. Treatment with DOI for either 4 or 7 days caused a significant decrease (by approximately 50%) in the high affinity 5-HT2A receptor binding as measured by 125I-DOI binding compared to saline-treated control rats. In contrast, western blot analysis demonstrated a significant increase in 5-HT2A receptor protein levels with 4 or 7 days of DOI treatment to 167% and 191% of control levels respectively. Real time quantitative RT-PCR analysis revealed a small but nonsignificant increase in the levels of 5-HT2A mRNA following treatment with DOI for 4 or 7 days. Taken together, the 5-HT2A receptor-stimulated hormone responses, agonist binding data and western blot data suggest that although agonist treatment increases the levels of 5-HT2A receptor protein in the cell membrane, there is a reduction in the population of 5-HT2A receptors capable of high-affinity binding and mediating a functional response.
Keywords: serotonin, serotonin2A receptors, desensitization, ligand binding, protein expression
Introduction
Adaptive changes in 5HT2A receptor signaling hypothesized to underlie the mechanism of action of several drug treatments for neuropsychiatric disorders (Dean and Hayes, 1996). For example, several antipsychotic drugs, such as olanzapine, desensitize 5-HT2A receptors (Kuoppamaki, et al., 1995;Roth and Ciaranello, 1991) while serotonin reuptake blockers (e.g., fluoxetine), alter the maximal efficacy of 5-HT2A receptor signaling (Damjanoska, et al., 2003;Li, et al., 1993;Tilakaratne, et al., 1995). Although the molecular mechanisms that underlie the adaptive changes in 5-HT2A receptor signaling are not well understood, they may contribute to the clinical effectiveness of these drugs. 5-HT2A receptors are unique in their regulation as sustained treatment with either agonists or antagonists induce their desensitization. The studies in this report examine the regulation of 5-HT2A receptors in the hypothalamic paraventricular nucleus (PVN) following sustained treatment with a 5-HT2A receptor agonist in rats, to provide insight into the molecular mechanisms that regulate the sensitivity of 5-HT2A receptor signaling in vivo.
As an integral part of the limbic system, the PVN plays an important role in mood modulation (Herman and Cullinan, 1997;Saphier and Feldman, 1986). The neuroendocrine response to serotonergic activation has been used as a diagnostic tool to examine the functioning of serotonergic neurons in the brains of patients suffering from mood disorders (Lerer, et al., 1999). The presence of 5-HT2A receptors in the hypothalamic PVN is supported by autoradiographic (Appel, et al., 1990), in situ hybridization (Gundlah, et al., 1999;Wright, et al., 1995) and immunohistochemical labeling studies (Zhang, et al., 2002). 5-HT2A receptors in the hypothalamic PVN mediate the neuroendocrine responses to an acute peripheral injection of the selective 5-HT2A/C receptor agonist, DOI. This was demonstrated using both intra-PVN and peripheral injections of the selective 5-HT2A receptor antagonist MDL 100,907 which dose-dependently inhibited the effect of DOI (1 mg/kg, s.c.) on hormone secretion (Hemrick-Luecke and Evans, 2002;Van de Kar, et al., 2001;Zhang et al., 2002). Thus, plasma hormone levels can be used as an index of the function of 5-HT2A receptor signaling in the hypothalamic PVN.
Previous data from our laboratory demonstrated that sustained treatment with DOI produces a desensitization of 5-HT2A receptor signaling in the PVN, as indicated by reduced levels of plasma oxytocin and adrenocorticotrophic hormone (ACTH) following a challenge injection of 1 mg/kg DOI (Damjanoska, et al., 2004). Interestingly, sustained agonist treatment in previous studies had shown reductions in agonist and antagonist binding to 5-HT2A receptors (with greater reductions in agonist binding than antagonist binding) in the cortex (McKenna, et al., 1989). However, the effect of chronic agonist treatment on the binding properties of 5-HT2A receptors in hypothalamic PVN is not known.
In the present study, we further explore the mechanisms involved in the regulation of 5-HT2A receptor function in the hypothalamic PVN. The dose-response effects for 5-HT2A receptor-mediated increases in plasma hormones were examined to further verify the desensitization response to sustained agonist treatment. In order to determine the impact of sustained agonist treatment on 5-HT2A receptors, we also examined 5-HT2A receptor agonist binding measured using autoradiography, the levels of 5-HT2A receptors in the membrane measured using Western blots and the levels of 5-HT2A receptor mRNA using real time quantitative RT-PCR.
Methods
Animals
Male Sprague-Dawley rats (225-275 g; Harlan Laboratories, Indianapolis, IN) were housed two per cage in an environment controlled for temperature, humidity, and lighting (7 AM-7 PM). Food and water were provided ad libitum. Eight to nine rats were used per experimental group. All procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals as approved by the Loyola University Institutional Animal Care and Use Committee.
Animal Treatments
Rats received daily injections of DOI (1 mg/kg, i.p.) for 4 or 7 days or 0.9% saline (1 ml/kg, i.p.) for 7 days. Rats receiving DOI injections for 4 days were given injections of 0.9% saline (1 ml/kg, i.p.) on the days prior to the commencement of DOI treatment. Thus, every group received injections for a total of 7 days, which allowed us to control for injection effects. DOI was purchased from Sigma (St. Louis, MO) and was dissolved in 0.9% saline. DOI is a prototypical 5-HT2A/2C receptor agonist (Leonhardt, et al., 1992;Van Wijngaarden, et al., 1990). DOI was used for sustained treatment to induce 5-HT2A receptors desensitization and for acute stimulation of hormone secretion. Twenty-four hours after the last DOI treatment, a challenge injection of DOI (0.25, 1, or 5 mg/kg, s.c.) or 0.9% saline (1 ml/kg, s.c.), was administered 15 minutes prior to sacrifice. The trunk blood was collected in centrifuge tubes containing 0.5 ml of a 0.3 M EDTA (pH 7.4) solution. The plasma samples for radioimmunoassays were stored at −80°C. Brains were quickly removed, frozen on dry ice, and stored at −80°C for biochemical and radioligand binding analyses.
Radioimmunoassay of Hormones
Plasma ACTH (Li, et al., 1993) and oxytocin (Li, et al., 1997) concentrations were determined by radioimmunoassays as previously described.
Autoradiography of 125I-DOI binding
Rats treated with the saline-challenge injection were used for the autoradiography assays. The density of 5-HT2A receptors in the PVN of the hypothalamus was determined by in vitro autoradiographic labeling using 125I-DOI ((±)-1-(2,5-dimethoxy-4-iodophenyl)-2-amino-propane HCl) as described (Appel et al, 1990) with some modifications.14 μm-thick coronal sections were mounted on Fisherbrand™ Superfrost Plus slides (Fisher Scientific) and stored at −20°C. Slides were thawed and dried in a desiccator at room temperature immediately before the assay. The brain sections were preincubated for 15 min at room temperature in a 50 mM Tris-HCl buffer pH 7.4 containing 0.5 mM EDTA, 2 mM MgCl2, 0.02% ascorbic acid and 10μM pargyline. Slides were then exposed to 0.22 nM 125I-DOI (specific activity of 2,200 Ci/mmol) for 90 min at room temperature. Non-specific binding was determined in the presence of 100 nM spiperone to define 125I-DOI binding to 5-HT2A receptors. Slides were washed twice with cold assay buffer for 10 min and rinsed with cold double-distilled H2O. After drying, the slides were apposed to Kodak Bio-Max MR film for 6 days at 4°C. A set of 125I microscales (Amersham Biosciences, Piscataway NJ) were included with each film to calibrate the gray-scale optical density readings to fmol/mg of tissue equivalent. Images were scanned and analyzed using MCID Elite 7.0 software (Imaging Research, St-Catharines, Ontario, Canada). Specific 125I-DOI binding was determined by subtracting the non-specific binding sites from the total binding sites. Although no pharmacokinetic data have been reported at the concentrations used in this study, the study by Zea-Ponce et al. suggests that the DOI may be cleared by this time point (Zea-Ponce, et al., 2002). Furthermore, the tissue washing procedures makes it unlikely that injected DOI would interfere with the 125I-DOI binding measurements. Data for each rat are the mean of four adjacent sections.
Immunoblot Analysis of 5-HT2A Receptors Proteins
Punches of the hypothalamic PVN from the treatment groups that received a challenge injection of 0.25 mg/kg DOI were used for the measurements of 5-HT2A receptor protein levels. The PVN was dissected from a 700 μm coronal section obtained using a cryostat (−10°C) as previously described (Serres, et al., 2000). Tissues were homogenized in 10 mM Tris buffer containing 0.1 M NaCl, 0.1 M EDTA, and a protease inhibitor cocktail (1:1000) (Sigma Chemical Co., St. Louis, MO). Membrane fractions were prepared as previously described (Zhang, et al., 2001). Protein concentrations in these homogenates were measured using BCA protein assay kits (Pierce, Rockford, IL).
Membrane protein samples (3μg/lane) were resolved by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis containing 0.1% SDS, 12.5% acrylamide/bisacrylamide (30:0.2), 4.6 M urea, and 375 mM Tris, pH 8.7. Gels were prepared with 4 samples from the control group (saline treatment) and 4 samples from each of the DOI treatment groups. The proteins were electrophoretically transferred for 2 hours to nitrocellulose membranes. Membranes were incubated with a blocking buffer for 1 hour at room temperature (5% non-fat dry milk and 0.1% TWEEN-20 detergent in TBS buffer). The membranes were incubated overnight at 4°C with 5-HT2A receptor antibody (serum was diluted 1:50,000) (Singh, et al., 2007). Next, the membranes were washed in PBS and incubated for 1 hour at room temperature with a horseradish peroxidase-labeled anti-mouse secondary antibody (diluted 1:10,000). The membranes were incubated with the ECL chemiluminescence substrate solution (Amersham, Arlington Heights, IL) before exposure to Kodak blue-sensitive X-ray film (Midwest Scientific, Valley Park, MO). Blots were washed and then used for detection of actin protein to verify equal loading of protein. The 5-HT2A receptor protein levels were normalized to the actin levels for each respective sample.
Films were analyzed densitometrically using the Scion Image program (Frederick, MD). Gray scale density readings were calibrated using a transmission step wedge standard. The integrated optical densities (IOD) of each band were calculated as the sum of the densities of all of the pixels within the area of the band outlined. An area adjacent to the band was used to calculate the background density of the band. The background IOD was subtracted from the IOD of each band. Each experimental sample was measured in triplicate.
RNA preparation
Twenty-five rats (8 treated with saline, 8 treated with DOI for 4 days, and 9 treated with DOI for 7 days) were used for measuring changes in 5-HT2A receptor mRNA expression in the PVN. Coronal slices of rat brain were cut in a cryostat at −12°C. A 700 μm coronal section containing the PVN was collected and the PVN was microdissected using a stereomicroscope. RNA from individual PVN was isolated with Sigma TriReagent (Sigma, St. Louis, MO) as per manufacturer's instructions. Briefly, PVN tissue was homogenized in 250 μL of Sigma TriReagent with a Kontes pellet pestle (Fisher Scientific, Pittsburgh, PA) and centrifuged at 12,000 × g for 10 minutes at 4°C. Chloroform was added to the supernatant, mixed, and allowed to sit at room temperature for 10 minutes. After centrifugation at 12,000 × g for 15 minutes at 4°C, the aqueous phase containing RNA was precipitated with isopropanol, was washed with 70% ethanol, and the air-dried pellet was resuspended in RNase-free water. Concentration of RNA was measured at 260 nm absorbance in RNase-free water on a SmartSpec Plus spectrophotometer (BioRad Laboratories, Hercules, CA). Isolated total RNA was stored in −80°C until used.
Quantitative real-time RT-PCR
Reverse transcription of RNA isolated from rat PVN was performed using SuperScript II (Invitrogen, Carlsbad, CA) as per manufacturer's instructions to generate cDNA. Equal amounts of RNA (0.5 μg) from each sample were added to respective reverse transcription reactions (20 μL total) with random hexamers as primers. Quantitative real-time PCR reactions were prepared using SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA), a 4% (v/v) concentration of cDNA product, and forward and reverse primers at a final concentration of 0.35 μM. Sequence design of primers for the housekeeping gene, GAPDH, (tgg agt cta ctg gcg tct tca c; ggc atg gac tgt ggt cat ga) were kindly provided by Dr. Phong T. Le (Stritch School of Medicine, Loyola University Chicago, Chicago, IL). Primers for our target, 5-HT2A receptor, (aac ggt cca tcc aca gag; aac agg aag aac acg atg c) were previously validated by Kindlundh-Hogberg et al., (Kindlundh-Hogberg, et al., 2006). For each sample, separate reactions were prepared for GAPDH and 5- HT2A amplification, and all reactions were performed in triplicate using the ABI 7500 Fast Real-Time PCR System (Applied Biosystems, Foster City, CA). A negative control lacking cDNA or any known DNA template was included for each primer pair. Cycling parameters were as follows: 1 cycle at 50°C for 2 minutes, 1 cycle at 95°C for 10 minutes, followed by 40 cycles of denaturing (95°C for 15 seconds) and annealing/elongation (60°C for 1 minute).
Quantification and Analysis of Real Time RT-PCR
Data analysis was performed using ABI 7500 SDS software (Applied Biosystems, Foster City, CA). Prior to sample analysis, standard curves (1-1:100,000) for both the housekeeping and target genes were examined to validate equal efficiencies of amplification for each primer set, thus allowing the use of the comparative CT method (ΔΔCT method) in the relative quantification of the target gene. Triplicate CT values for all samples were averaged across three separate experiments (n=8-9). Average CT values of the target gene were normalized to the respective average CT values of the housekeeping gene (ΔCT (sample) = CT (target) - CT (housekeeping)). Saline control ΔCT values (n=8) were averaged and used to normalize all samples (ΔΔCT (sample) = ΔCT (sample) - Average ΔCT (control)).
Statistical Analyses
Body weights were analyzed by repeated measures one-way ANOVA and a Newman-Keuls multiple range test. Hormone data (ACTH and oxytocin) were analyzed by a two-way ANOVA and a Newman-Keul's multiple range test. Western blot and receptor autoradiography data were analyzed by one-way ANOVA and a Newman-Keul's multiple range test. Relative quantification (2−ΔΔCT) values were averaged for each group and a single factor ANOVA was performed. GB-STAT software (Dynamic Microsystems, Inc., Silver Spring, MD) was used for all statistical analyses.
Results
The Effects of Sustained DOI Treatment on Body Weight
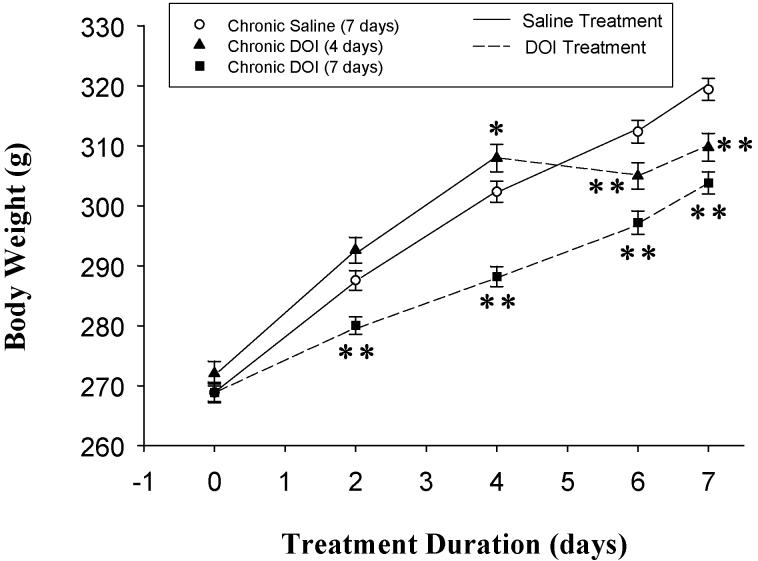
Rats were treated with 1 mg/kg DOI or saline for 7 days. A third group of rats was injected with saline for 3 days and then DOI for 4 days to examine the effects of a shorter exposure time to DOI and to control for injection effects. Rats were weighed before the initial injection and then 2, 4, 6, and 7 days after the initial injection (figure 1). Treatment with DOI significantly (p < 0.0001) attenuated the gain in body weight compared to controls as demonstrated by repeated measures ANOVA, where F(14, 476) = 120.2.
Figure 1.
Body weights were significantly decreased by treatment with 1mg/kg DOI for 4 and 7 days as analyzed by one-way ANOVA with repeated measures. Body weight was significantly higher in the 4 day DOI group before the DOI injections. This was due to 3 rats which weighed more than the other rats; 2 of these 3 rats weighed more than 1 standard deviation from the mean and one weighed more than 2 standard deviations from the mean. However, even including these heavier rats, DOI for 3 and 4 days significantly reduced body weight compared to saline-treated controls. Newman-Keuls post-hoc analysis indicates significant differences compared to saline-treated controls on the same day at p < 0.01** and p < 0.05* as shown.
The Effect of Sustained DOI Treatment on 5-HT2A Receptor-Mediated Hormone Responses
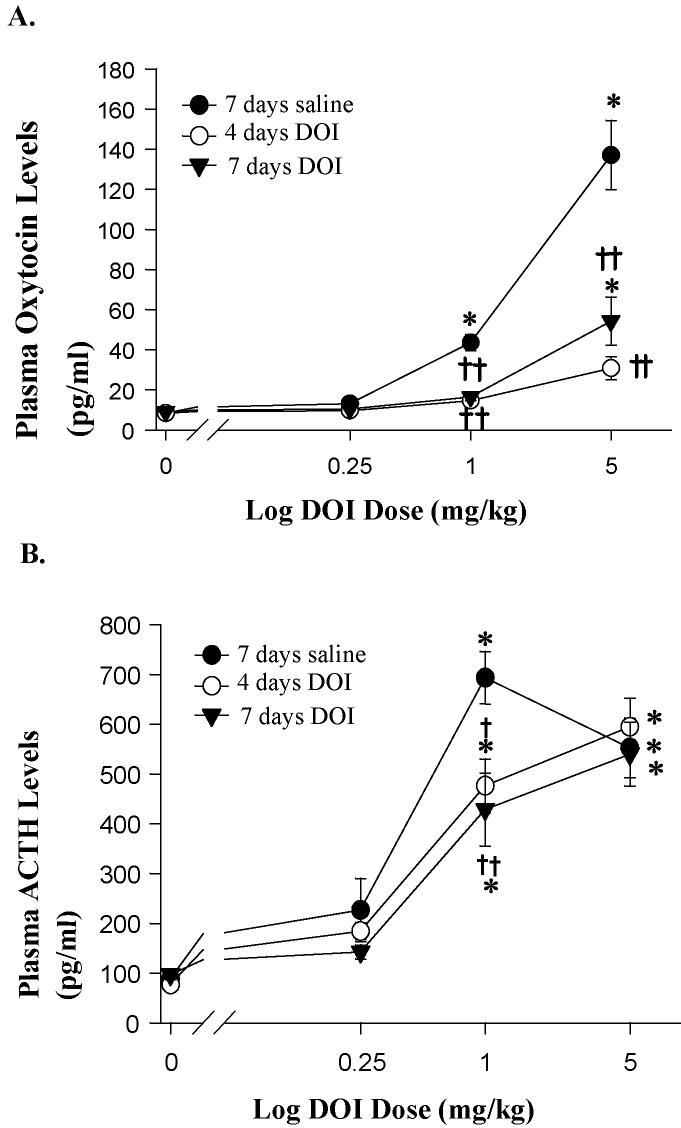
Challenge doses of DOI (0.25, 1, 5 mg/kg) were administered 15 minutes prior to the collection of plasma samples. DOI challenge injections produced a dose-dependent increase in oxytocin levels in rats treated with saline for 7 days (F(3,82) = 86.56, p < 0.0001). The DOI challenge injections in saline pretreated rats increased plasma levels of oxytocin by 56% at the 0.25 mg/kg dose, 418% at the 1 mg/kg dose (p < 0.01), and 1538% at 5 mg/kg dose (p < 0.01) (figure 2A).
Figure 2.
Hormone responses to challenge injections to a range of doses of DOI (0.25, 1.0 and 5.0 mg/kg, s.c.) after sustained DOI treatment (1 mg/kg, i.p.) for 4 or 7 days. The data represent the mean ± SEM per group for oxytocin (A) and ACTH (B) levels (n = 6-11). When comparing groups given the same chronic treatment but different challenge injections, there is a significant effect of a challenge injection of DOI when compared to a challenge injection of saline (p < 0.01 indicated by *). When comparing groups given the same challenge injection, there is a significant effect of sustained DOI treatment as indicated by † for p < 0.05 and †† for p < 0.01 (as analyzed by two-way ANOVA and Newman-Keuls' multiple range test).
Treatment with DOI for 4 or 7 days did not alter basal plasma levels of oxytocin or ACTH (figure 2). Treatment with DOI for 4 and 7 days caused a significant reduction in DOI-mediated increases in plasma oxytocin levels (F(2,82) = 42.21, p < 0.0001) (figure 2A). There was also a significant interaction between the chronic treatment and the DOI challenge injection on plasma oxytocin levels (F(6,82) = 21.16, p < 0.0001). DOI treatment decreased the plasma oxytocin levels to challenge injections of 5 mg/kg DOI by 78% (p < 0.01) after 4 days and 61% (p < 0.01) after 7 days of DOI treatment (figure 2).
The DOI challenge injections increased ACTH levels in rats treated with saline for 7 days (F(3,86) = 82.14, p < 0.0001). The DOI challenge injections increased plasma levels of ACTH by 157%, 688% (p < 0.01), and 527% (p < 0.01) at 0.25, 1, and 5 mg/kg, respectively (figure 2B). Treatment with DOI for 4 and 7 days caused a significant reduction in DOI-mediated increases in plasma ACTH levels (F(2,86) = 3.77, p < 0.05) (Figure. 2B). There was also a significant interaction between the sustained treatment and the DOI challenge injection on plasma ACTH levels (F(6,86) = 2.29, p < 0.05). Sustained DOI treatment decreased the response of plasma ACTH levels to a challenge injection of 1 mg/kg DOI by 31% (p < 0.05) after 4 days and 38% (p < 0.01) after 7 days of DOI treatment. Sustained DOI treatment did not change ACTH levels at any other DOI challenge doses when compared to saline-treated controls.
The Effects of Sustained Treatment with DOI on 125I-DOI-labeled 5-HT2A Receptors
Autoradiography with 125I-DOI was used to assess the high affinity state of 5-HT2A receptors in the PVN of the hypothalamus in rats treated with 1 mg/kg DOI for 4 or 7 days or treated with saline for 7 days (figure 3). Treatment with DOI caused a significant decrease in 125I-DOI binding in the PVN (F(2,10) = 15.60, p = 0.0008, figure 3). Both 4 and 7 days of sustained treatment with DOI reduced the binding of 125I-DOI compared to saline treated control rats by approximately 50% (p < 0.01).
Figure 3.
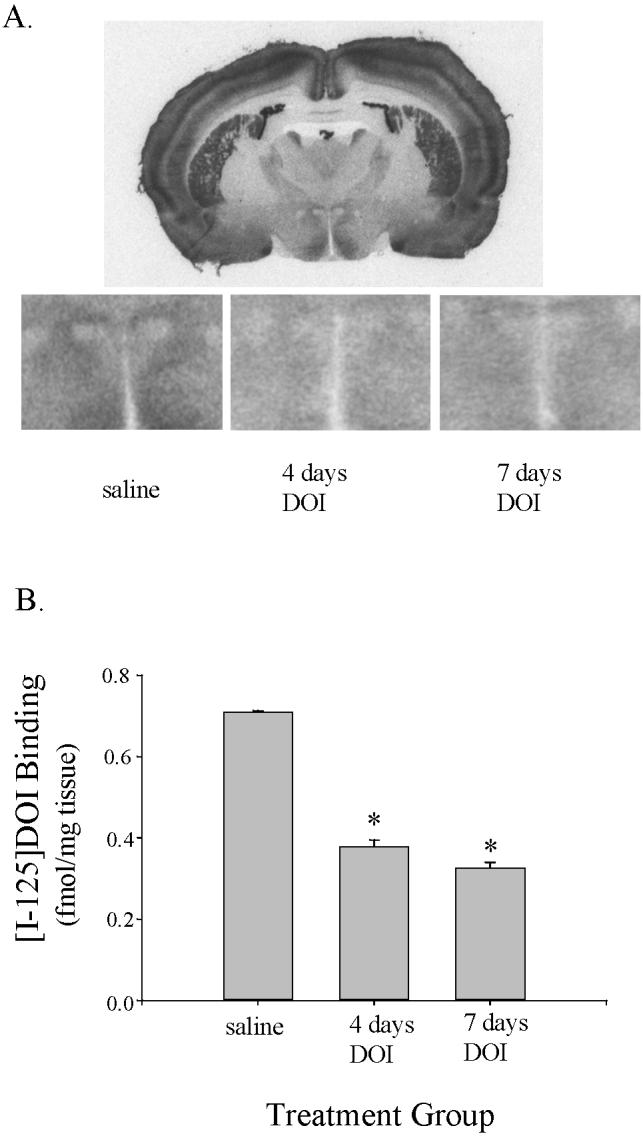
125I-DOI autoradiography was performed in tissue sections from rats treated with saline for 7 days, and DOI for 4 or 7 days (A) A full coronal section is shown for a representative saline-treated rat. The box on this coronal section indicates the region of the PVN of the hypothalamus. Higher power images of the PVN of the hypothalamus are shown for a representative saline-treated rat, a rat treated with DOI for 4 days and rat treated with DOI for 7 days. (B) DOI binding was significantly reduced in rats treated with DOI for 4 or 7 days compared to rats treated with saline for 7 days (* indicated p < 0.01).
The Effect of Sustained DOI Treatment on 5-HT2A Receptor Protein Levels
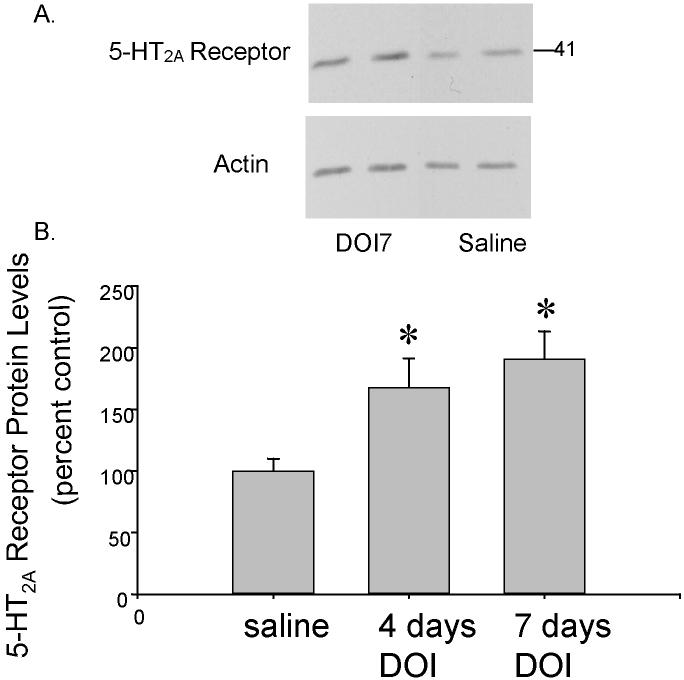
5-HT2A receptor protein levels were significantly increased by sustained treatment with DOI (F(2,21) = 5.73, p < 0.01, figure 4). Neuman-Keuls post-hoc tests revealed significant increases in 5-HT2A receptor protein levels between the saline-treated control group and the group treated with DOI for 4 days (167% of control levels ± 23.8% = the mean ± SEM, p < 0.05) and between the saline-treated control group and the group treated with DOI for 7 days (191% of control levels ± 22.4% = the mean ± SEM, p < 0.05).
Figure 4.
Western blots using 5-HT2A Receptor Antibodies. (A) A representative western blot of 5-HT2A receptor protein levels in the PVN of rats treated with DOI for 7 days (DOI7) or saline for 7 days. (B) 5-HT2A receptor protein levels were significantly increased in the rats treated with DOI for 4 and 7 days compared to saline-treated rats (p<0.05).
The Effect of Sustained DOI Treatment on 5-HT2A Receptor mRNA Levels
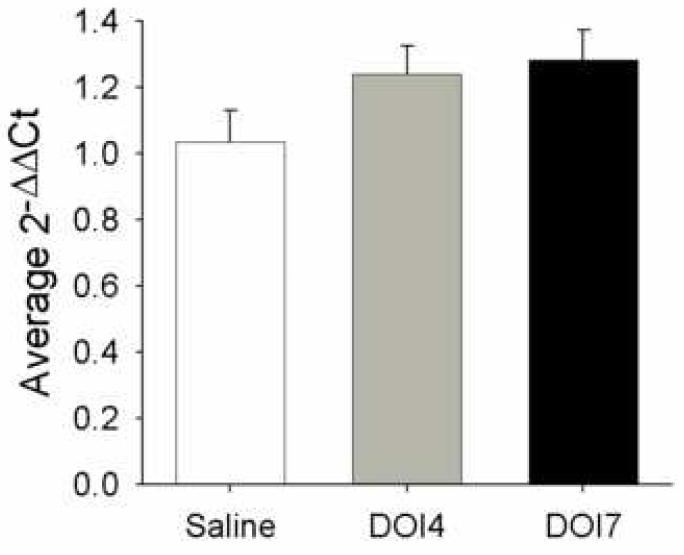
The levels of mRNA encoding for 5-HT2A receptors in the PVN were measured using real-time quantitative RT-PCR in rats treated with DOI for 4 (n=8) or 7 (n=9) days or saline (n=8) for 7 days (figure 5). The levels of 5-HT2A receptor mRNA for rats treated with DOI for 4 day were 20% higher than saline-treated controls and 22% higher for rats treated with DOI for 7 days as measured by the ΔΔCt values, however, there was no significant difference among the mRNA levels analyzed using ANOVA (F(2,22) = 2.13, p = 0.14).
Figure 5.
Real Time Quantitative RT-PCR measurements of 5-HT2A receptor mRNA. There was not a significant increase in the levels of mRNA encoding 5-HT2A receptors as measured by the ΔΔCt values in the PVN of rats treated with DOI for 4 or 7 days compared to rats treated with saline for 7 days.
Discussion
In this report, we extended our previous studies on sustained DOI-induced desensitization in the hypothalamic PVN. Sustained DOI treatment produces a desensitization of both the 5-HT2A receptor-mediated oxytocin and ACTH responses. Treatment with DOI for 4 or 7 days both caused a significant decrease in 125I-DOI binding compared to saline-treated control rats in hypothalamic PVN. In contrast, western blot analysis with a 5-HT2A receptor-selective antibody indicated that 4 or 7 days of DOI treatment increased protein levels and the levels of 5-HT2A receptor mRNA were slightly increased with 4 and 7 days of treatment with DOI, however this increase did not reach statistical significance.
Sustained treatment with DOI produced desensitization of 5-HT2A receptors in the hypothalamic PVN as assessed by the DOI-mediated ACTH and oxytocin responses. This observation is consistent with previously published studies (Damjanoska et al., 2004). Sustained agonist treatment decreased DOI-mediated responses, but with the limited number of concentrations of DOI examined and since the dose-response curve did no plateau at the maximal dose of DOI used, we are not able to calculate the Emax and the ED50 (potency).
To further examine the mechanisms underlying the impact of sustained agonist treatment on 5-HT2A receptors, we examined 5-HT2A receptor agonist binding measured using autoradiography, the levels of 5-HT2A receptors in the membrane measured using western blots and the levels of 5-HT2A receptor mRNA using real time quantitative RT-PCR. Previous studies had shown reductions in agonist and antagonist binding to 5-HT2A receptors following chronic agonist treatment in the cortex, with greater reductions in agonist binding than antagonist binding (McKenna, et al., 1989). These results in the cortex are consistent with our data in the PVN. Results from our previous studies and another group's study (Roth, et al., 1995) suggest that the desensitization of 5-HT2A receptor signaling is not likely due to altered levels of phospholipase C-coupled Gq or G11 proteins. Receptor internalization had also been demonstrated following agonist treatment providing a possible mechanism underlying the reductions in both 5-HT2A receptor agonist binding and desensitization of the hormone response (Gray and Roth, 2001).
Because down-regulation is one reported mechanism underlying receptor desensitization and in our previous study, we did not observe a decrease Gαq and Gα11 protein in the PVN (Damjanoska et al., 2004), we hypothesized that the levels of 5-HT2A receptors in the membrane are reduced in response to sustained treatment with a 5-HT2A receptor agonist. Surprisingly, we found that 4 or 7 days DOI treatment increased 5-HT2A receptor protein levels. Although there are no previous in vivo studies that report the impact of DOI or other 5-HT2A receptor agonist on 5-HT2A receptor protein levels, Akiyoshi et al (Akiyoshi, et al., 1993) found that chronic administration of DOI to cultured cerebellar granule cells induced an up-regulation of 5-HT2A receptor binding sites and 5-HT2A mRNA. Furthermore, the effects of prolonged agonist exposure on 5-HT2A receptors are dependent on the cell line used (Grotewiel and Sanders-Bush, 1994). One possible explanation for this net increase in receptor protein level is that a compensatory increase in receptor expression occurs after desensitization of the effector response. However, other regulatory mechanism(s) must be involved in preventing a population of these 5-HT2A receptors in the membrane from binding to agonist. Post-translational modifications to 5-HT2A receptors (Gray, et al., 2003), Gαq/11 proteins (Shi, et al., 2007b;Shi, et al., 2007a) or both could alter the interaction of 5-HT2A receptors with Gαq/11 proteins and thereby alter agonist binding. Our previous studies in frontal cortex of rats treated with DOI as well as in cells in culture, suggest that sustained treatment with DOI increases the phosphorylation of Gα11 protein and thereby reduces coupling to 5-HT2A receptors (Shi et al., 2007a; Shi et al., 2007b). Similar mechanisms may be at work in the PVN, but unfortunately due to the small size of the nucleus, we are unable to analyze phosphorylation of Gα11 protein using our current techniques. Alternatively post-translational modifications to 5-HT2A receptors could directly alter the binding of agonist to 5-HT2A receptors.
Using real time quantitative RT-PCR, we found a small but statistically nonsignificant increase in 5-HT2A receptor mRNA in the PVN of rats treated with DOI for 4 or 7 days. Further studies are needed unequivocally to determine if the increase in 5-HT2A receptor protein caused sustained treated with DOI is due to increased gene expression or perhaps increased stability of the protein.
In conclusion, the present data suggest that sustained agonist treatment induces desensitization of 5-HT2A receptors within the hypothalamic PVN. Sustained agonist treatment induced a reduction in agonist-labeled 5-HT2A receptors but increased the membrane associated 5-HT2A receptor protein levels. These results suggest that there is a population of 5-HT2A receptor proteins in the membrane that are functionally uncoupled as a result of sustained agonist treatment. Based on previous studies in frontal cortex and cells in culture, phosphorylation of Gα11 protein likely underlies this phenomenon (Shi et al., 2006, Shi et al., 2007). Considering the important role of 5-HT2A receptors and hypothalamic PVN in the mood and stress modulation, future studies are needed to determine the mechanisms by which membrane-associated 5-HT2A receptor protein levels are increased.
Acknowledgements
The authors would like to thank Ms. Francesca Garcia and Cynthia Gouvion for their excellent technical assistance. This work was supported by USPHS MH068612 (NAM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akiyoshi J, Hough C, Chuang DM. Paradoxical increase of 5-hydroxytryptamine2 receptors and 5- hydroxytryptamine2 receptor mRNA in cerebellar granule cells after persistent 5-hydroxytryptamine2 receptor stimulation. Mol Pharmacol. 1993;43:349–355. [PubMed] [Google Scholar]
- Appel NM, Mitchell WM, Garlick RK, Glennon RA, Teiteler M, de Souza EB. Autoradiographic characterization of (±)-1-(2,5-dimethoxy- 4-[125I]iodophenyl)-2-aminopropane ([125I]DOI) binding to 5- HT2 and 5-HT1c receptors in rat brain. J Pharmacol Exp Ther. 1990;255:843–857. [PubMed] [Google Scholar]
- Damjanoska KJ, Heidenreich BA, Kindel GH, D'Souza DN, Zhang Y, Garcia F, Battaglia G, Wolf WA, Van de Kar LD, Muma NA. Agonist-Induced Serotonin 2A Receptor Desensitization in the Rat Frontal Cortex and Hypothalamus. J Pharmacol Exp Ther. 2004;309:1043–1050. doi: 10.1124/jpet.103.062067. [DOI] [PubMed] [Google Scholar]
- Damjanoska KJ, Van de Kar LD, Kindel GH, Zhang Y, D'Souza DN, Garcia F, Battaglia G, Muma NA. Chronic fluoxetine differentially affects 5- HT2A receptor signaling in frontal cortex, oxytocin and corticotropin releasing factor (CRF)- containing neurons in rat paraventricular nucleus. J Pharmacol Exp Ther. 2003;306:563–571. doi: 10.1124/jpet.103.050534. [DOI] [PubMed] [Google Scholar]
- Dean B, Hayes W. Decreased frontal cortical serotonin2A receptors in schizophrenia. Schizophr Res. 1996;21:133–139. doi: 10.1016/0920-9964(96)00034-5. [DOI] [PubMed] [Google Scholar]
- Gray JA, Compton-Toth BA, Roth BL. Identification of two serine residues essential for agonist-induced 5-HT2A receptor desensitization. Biochemistry. 2003;42:10853–10862. doi: 10.1021/bi035061z. [DOI] [PubMed] [Google Scholar]
- Gray JA, Roth BL. Paradoxical trafficking and regulation of 5HT2A receptors by agonists and antagonists. Brain Research Bulletin. 2001;56:441–451. doi: 10.1016/s0361-9230(01)00623-2. [DOI] [PubMed] [Google Scholar]
- Grotewiel MS, Sanders-Bush E. Regulation of serotonin2A receptors in heterologous expression systems. J Neurochem. 1994;63:1255–1260. doi: 10.1046/j.1471-4159.1994.63041255.x. [DOI] [PubMed] [Google Scholar]
- Gundlah C, Pecins-Thompson M, Schutzer WE, Bethea CL. Ovarian steroid effects on serotonin 1A, 2A and 2C receptor mRNA in macaque hypothalamus. Brain Res Mol Brain Res. 1999;63:325–339. doi: 10.1016/s0169-328x(98)00295-2. [DOI] [PubMed] [Google Scholar]
- Hemrick-Luecke SK, Evans DC. Comparison of the potency of MDL 100,907 and SB 242084 in blocking the serotonin 5-HT2 receptor agonst-induced increases in rat serum corticosterone concentrations: evidence for 5-HT2A receptor mediation of the HPA axis. Neuropharmacology. 2002;42:162–169. doi: 10.1016/s0028-3908(01)00166-6. [DOI] [PubMed] [Google Scholar]
- Herman JP, Cullinan WE. Neurocircuitry of stress: Central control of the hypothalamo- pituitary-adrenocortical axis. Trends Neurosci. 1997;20:78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- Kindlundh-Hogberg AM, Svenningsson P, Schioth HB. Quantitative mapping shows that serotonin rather than dopamine receptor mRNA expressions are affected after repeated intermittent administration of MDMA in rat brain. Neuropharmacology. 2006;51:838–847. doi: 10.1016/j.neuropharm.2006.05.026. [DOI] [PubMed] [Google Scholar]
- Kuoppamaki M, Palvimaki EP, Hietala J, Syvalahti E. Differential regulation of rat 5-HT2A and 5-HT2C receptors after chronic treatment with clozapine, chlorpromazine and three putative atypical antipsychotic drugs. Neuropsychopharmacology. 1995;13:139–150. doi: 10.1016/0893-133X(95)00049-J. [DOI] [PubMed] [Google Scholar]
- Leonhardt S, Gorospe E, Hoffman BJ, Teitler M. Molecular pharmacological differences in the interaction of serotonin with 5-hydroxytryptamine1C and 5-hydroxytryptamine2 receptors. Mol Pharmacol. 1992;42:328–335. [PubMed] [Google Scholar]
- Lerer B, Gelfin Y, Gorfine M, Allolio B, Lesch KP, Newman ME. 5-HT1A receptor function in normal subjects on clinical doses of fluoxetine: Blunted temperature and hormone responses to ipsapirone challenge. Neuropsychopharmacology. 1999;20:628–639. doi: 10.1016/S0893-133X(98)00106-7. [DOI] [PubMed] [Google Scholar]
- Li Q, Brownfield MS, Battaglia G, Cabrera TM, Levy AD, Rittenhouse PA, Van de Kar LD. Long-term treatment with the antidepressants fluoxetine and desipramine potentiates endocrine responses to the serotonin agonists 6-chloro-2-[1-piperazinyl]-pyrazine (MK-212) and (±)-1- (2,5-dimethoxy-4-iodophenyl)-2-aminopropane HCI (DOI) J Pharmacol Exp Ther. 1993;266:836–844. [PubMed] [Google Scholar]
- Li Q, Muma NA, Battaglia G, Van de Kar LD. A desensitization of hypothalamic 5-HT1A receptors by repeated injections of paroxetine: reduction in the levels of Gi and Go proteins and neuroendocrine responses, but not in the density of 5-HT1A receptors. J Pharmacol Exp Ther. 1997;282:1581–1590. [PubMed] [Google Scholar]
- McKenna DJ, Nazarali A, Himeno A, Saavedra JM. Chronic treatment with (±)DOI, a psychotomimetic 5-HT2 agonist, downregulates 5-HT2 receptors in rat brain. Neuropsychopharmacology. 1989;2:81–87. doi: 10.1016/0893-133x(89)90010-9. [DOI] [PubMed] [Google Scholar]
- Roth BL, Ciaranello RD. Chronic mianserin treatment decreases 5-HT2 receptor binding without altering 5-HT2 receptor mRNA levels. Eur J Pharmacol. 1991;207:169–172. doi: 10.1016/0922-4106(91)90093-w. [DOI] [PubMed] [Google Scholar]
- Roth BL, Palvimaki EP, Berry S, Khan N, Sachs N, Uluer A, Choudhary MS. 5-Hydroxytryptamine2A (5-HT2A) receptor desensitization can occur without down-regulation. J Pharmacol Exp Ther. 1995;275:1638–1646. [PubMed] [Google Scholar]
- Saphier D, Feldman S. Electrophysiology of limbic forebrain and paraventricular nucleus connections. Brain Res Bull. 1986;17:743–750. doi: 10.1016/0361-9230(86)90085-7. [DOI] [PubMed] [Google Scholar]
- Schmidt CJ, Sorensen SM, Kehne JH, Carr AA, Palfreyman MG. The role of 5-HT2A receptors in antipsychotic activity. Life Sci. 1995;56:2209–2222. doi: 10.1016/0024-3205(95)00210-w. [DOI] [PubMed] [Google Scholar]
- Serres F, Li Q, Garcia F, Raap DK, Battaglia G, Muma NA, Van de Kar LD. Evidence that Gz proteins couple to hypothalamic 5-HT1A receptors in vivo. J Neurosci. 2000;20:3095–3103. doi: 10.1523/JNEUROSCI.20-09-03095.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Damjanoska KJ, Singh RK, Carrasco GA, Garcia F, Grippo AJ, Landry M, Sullivan NR, Battaglia G, Muma NA. Agonist induced-phosphorylation of Galpha11 protein reduces coupling to 5-HT2A receptors. J Pharmacol Exp Ther. 2007a;323:248–256. doi: 10.1124/jpet.107.122317. [DOI] [PubMed] [Google Scholar]
- Shi J, Zemaitaitis B, Muma NA. Phosphorylation of Galpha11 protein contributes to agonist-induced desensitization of 5-HT2A receptor signaling. Mol Pharmacol. 2007b;71:303–313. doi: 10.1124/mol.106.028241. [DOI] [PubMed] [Google Scholar]
- Singh RK, Shi J, Zemaitaitis BW, Muma NA. Olanzapine increases RGS7 protein expression via stimulation of the Janus tyrosine kinase-signal transducer and activator of transcription signaling cascade. J Pharmacol Exp Ther. 2007;322:133–140. doi: 10.1124/jpet.107.120386. [DOI] [PubMed] [Google Scholar]
- Tilakaratne N, Yang ZL, Friedman E. Chronic fluoxetine or desmethylimipramine treatment alters 5-HT2 receptor mediated c-fos gene expression. Eur J Pharmacol Mol Pharmacol. 1995;290:263–266. doi: 10.1016/0922-4106(95)90003-9. [DOI] [PubMed] [Google Scholar]
- Van de Kar LD, Javed A, Zhang YH, Serres F, Raap DK, Gray TS. 5-HT2A receptors stimulate ACTH, corticosterone, oxytocin, renin, and prolactin release and activate hypothalamic CRF and oxytocin-expressing cells. Journal of Neuroscience. 2001;21:3572–3579. doi: 10.1523/JNEUROSCI.21-10-03572.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Wijngaarden I, Tulp MTM, Soudijn W. The concept of selectivity in 5-HT receptor research. Eur J Pharmacol. 1990;188:301–312. doi: 10.1016/0922-4106(90)90190-9. [DOI] [PubMed] [Google Scholar]
- Wright DE, Seroogy KB, Lundgren KH, Davis BM, Jennes L. Comparative localization of serotonin1A, 1C, and 2 receptor subtype mRNAs in rat brain. J Comp Neurol. 1995;351:357–373. doi: 10.1002/cne.903510304. [DOI] [PubMed] [Google Scholar]
- Zea-Ponce Y, Kegeles LS, Guo N, Raskin L, Bakthavachalam V, Laruelle M. Pharmacokinetics and brain distribution in non human primate of R(-)[123I]DOI, A 5HT(2A/2C) serotonin agonist. Nucl Med Biol. 2002;29:575–583. doi: 10.1016/s0969-8051(02)00306-2. [DOI] [PubMed] [Google Scholar]
- Zhang Y, D'Souza D, Raap DK, Garcia F, Battaglia G, Muma NA, Van de Kar LD. Characterization of the functional heterologous desensitization of hypothalamic 5-HT1A receptors after 5-HT2A receptor activation. J Neurosci. 2001;21:7919–7927. doi: 10.1523/JNEUROSCI.21-20-07919.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Damjanoska KJ, Carrasco GA, Dudas B, D'Souza DN, Tetzlaff J, Garcia F, Hanley NR, Scripathirathan K, Petersen BR, Gray TS, Battaglia G, Muma NA, Van de Kar LD. Evidence that 5-HT2A receptors in the hypothalamic paraventricular nucleus mediate neuroendocrine responses to (-)DOI. J Neurosci. 2002;22:9635–9642. doi: 10.1523/JNEUROSCI.22-21-09635.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]