Abstract
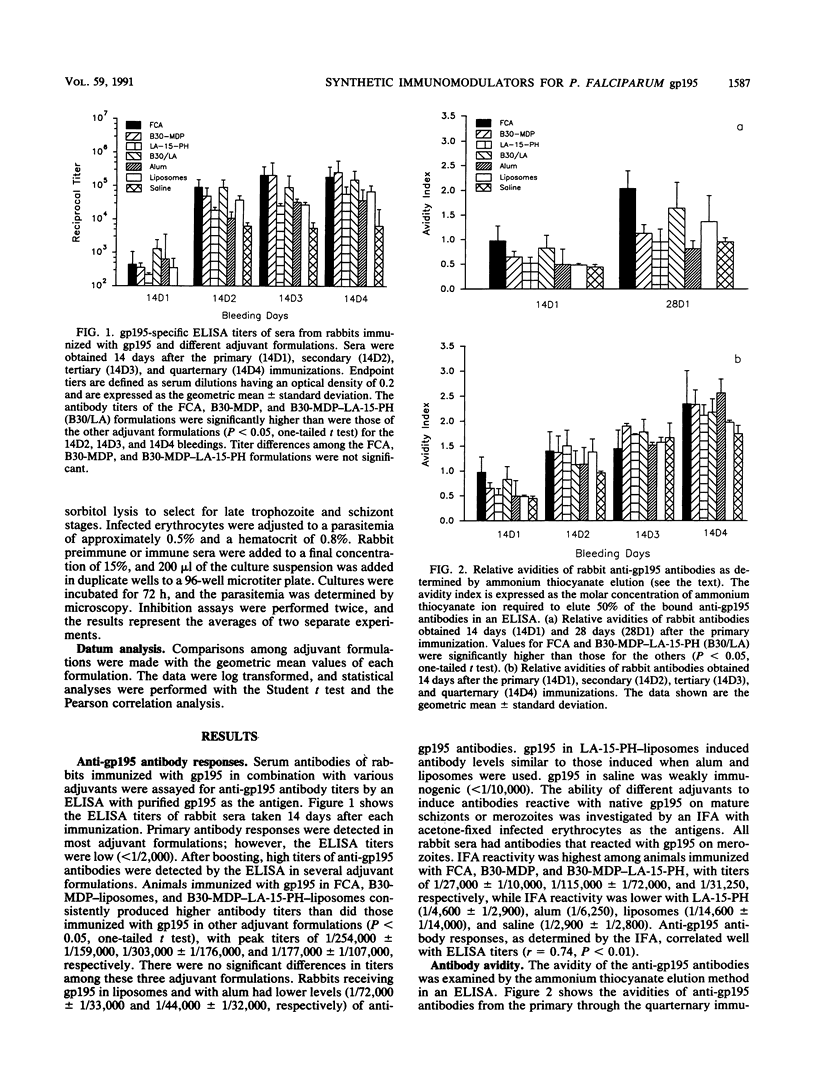
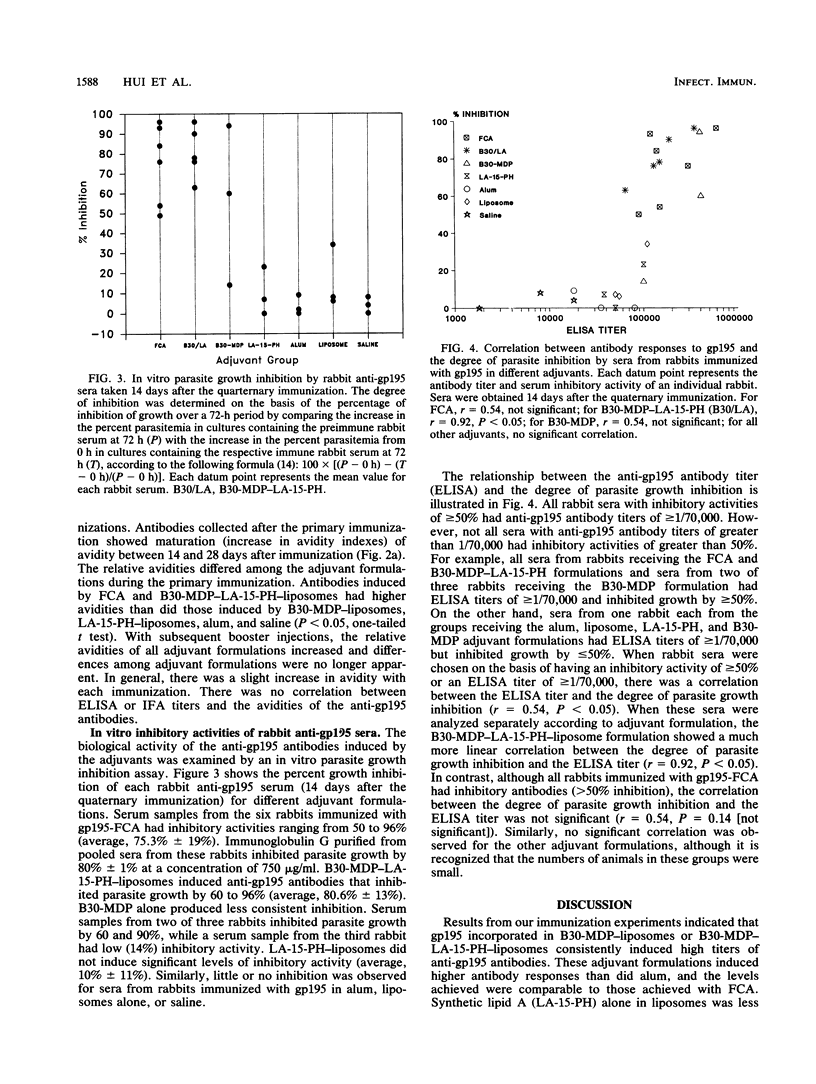
The Plasmodium falciparum major merozoite surface protein (gp195) is a protective antigen against lethal malaria. However, increasing evidence indicates that the efficacy of a malaria vaccine will require a strong adjuvant that is safe for human use. We compared the efficacies of two low-toxicity synthetic immunomodulators, B30-MDP (a lipophilic muramyl dipeptide derivative) and LA-15-PH (a synthetic equivalent of monophosphoryl lipid A), with that of Freund complete adjuvant (FCA) in eliciting an antibody response to gp195. Rabbits were immunized with native gp195 and B30-MDP, LA-15-PH, or the two in combination, with liposomes as the vehicle. Aluminum hydroxide and FCA were used as reference adjuvants. Results showed that adjuvant formulations based on B30-MDP alone or in combination with LA-15-PH induced high antibody titers to gp195, as compared with FCA. LA-15-PH alone was less effective. Aluminum hydroxide induced significantly lower antibody titers. The functional activity of the rabbit anti-gp195 antibodies induced by different adjuvants was evaluated in an in vitro parasite growth inhibition assay previously shown to correlate with anti-gp195 immunity in the Aotus monkey model. All rabbits immunized with B30-MDP-LA-15-PH and two of three rabbits immunized with B30-MDP alone produced sera that strongly inhibited parasite growth. The degree of growth inhibition was similar to that with FCA. The antibody titers of the rabbits receiving B30-MDP-LA-15-PH strongly correlated with the degree of in vitro growth inhibition. Our findings provided strong evidence that adjuvant formulations based on synthetic B30-MDP and LA-15-PH can replace FCA as adjuvants in stimulating protective immunity specific for gp195.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alving C. R., Richards R. L., Moss J., Alving L. I., Clements J. D., Shiba T., Kotani S., Wirtz R. A., Hockmeyer W. T. Effectiveness of liposomes as potential carriers of vaccines: applications to cholera toxin and human malaria sporozoite antigen. Vaccine. 1986 Sep;4(3):166–172. doi: 10.1016/0264-410x(86)90005-8. [DOI] [PubMed] [Google Scholar]
- Aprile M. A., Wardlaw A. C. Aluminium compounds as adjuvants for vaccines and toxoids in man: a review. Can J Public Health. 1966 Aug;57(8):343–360. [PubMed] [Google Scholar]
- Audibert F. M., Przewlocki G., Leclerc C. D., Jolivet M. E., Gras-Masse H. S., Tartar A. L., Chedid L. A. Enhancement by murabutide of the immune response to natural and synthetic hepatitis B surface antigens. Infect Immun. 1984 Jul;45(1):261–266. doi: 10.1128/iai.45.1.261-266.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballou W. R., Hoffman S. L., Sherwood J. A., Hollingdale M. R., Neva F. A., Hockmeyer W. T., Gordon D. M., Schneider I., Wirtz R. A., Young J. F. Safety and efficacy of a recombinant DNA Plasmodium falciparum sporozoite vaccine. Lancet. 1987 Jun 6;1(8545):1277–1281. doi: 10.1016/s0140-6736(87)90540-x. [DOI] [PubMed] [Google Scholar]
- Bomford R. The comparative selectivity of adjuvants for humoral and cell-mediated immunity. II. Effect on delayed-type hypersensitivity in the mouse and guinea pig, and cell-mediated immunity to tumour antigens in the mouse of Freund's incomplete and complete adjuvants, alhydrogel, Corynebacterium parvum, Bordetella pertussis, muramyl dipeptide and saponin. Clin Exp Immunol. 1980 Feb;39(2):435–441. [PMC free article] [PubMed] [Google Scholar]
- Chang S. P., Hui G. S., Kato A., Siddiqui W. A. Generalized immunological recognition of the major merozoite surface antigen (gp195) of Plasmodium falciparum. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6343–6347. doi: 10.1073/pnas.86.16.6343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough E. R., Audibert F. M., Barnwell J. W., Schlesinger D. H., Arnon R., Chedid L. A. Biologically active antibodies elicited by a synthetic circumsporozoite peptide of Plasmodium knowlesi administered in saline with a muramyl dipeptide derivative. Infect Immun. 1985 Jun;48(3):839–842. doi: 10.1128/iai.48.3.839-842.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkstra J., Mellors J. W., Ryan J. L., Szoka F. C. Modulation of the biological activity of bacterial endotoxin by incorporation into liposomes. J Immunol. 1987 Apr 15;138(8):2663–2670. [PubMed] [Google Scholar]
- Hall R., Hyde J. E., Goman M., Simmons D. L., Hope I. A., Mackay M., Scaife J., Merkli B., Richle R., Stocker J. Major surface antigen gene of a human malaria parasite cloned and expressed in bacteria. 1984 Sep 27-Oct 3Nature. 311(5984):379–382. doi: 10.1038/311379a0. [DOI] [PubMed] [Google Scholar]
- Herrington D. A., Clyde D. F., Losonsky G., Cortesia M., Murphy J. R., Davis J., Baqar S., Felix A. M., Heimer E. P., Gillessen D. Safety and immunogenicity in man of a synthetic peptide malaria vaccine against Plasmodium falciparum sporozoites. Nature. 1987 Jul 16;328(6127):257–259. doi: 10.1038/328257a0. [DOI] [PubMed] [Google Scholar]
- Hui G. S., Palmer K. L., Siddiqui W. A. Use of human plasma for continuous in vitro cultivation of Plasmodium falciparum. Trans R Soc Trop Med Hyg. 1984;78(5):625–626. doi: 10.1016/0035-9203(84)90222-0. [DOI] [PubMed] [Google Scholar]
- Hui G. S., Siddiqui W. A. Serum from Pf195 protected Aotus monkeys inhibit Plasmodium falciparum growth in vitro. Exp Parasitol. 1987 Dec;64(3):519–522. doi: 10.1016/0014-4894(87)90068-3. [DOI] [PubMed] [Google Scholar]
- Kotani S., Takada H., Tsujimoto M., Ogawa T., Takahashi I., Ikeda T., Otsuka K., Shimauchi H., Kasai N., Mashimo J. Synthetic lipid A with endotoxic and related biological activities comparable to those of a natural lipid A from an Escherichia coli re-mutant. Infect Immun. 1985 Jul;49(1):225–237. doi: 10.1128/iai.49.1.225-237.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambros C., Vanderberg J. P. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J Parasitol. 1979 Jun;65(3):418–420. [PubMed] [Google Scholar]
- Lew A. M., Anders R. F., Edwards S. J., Langford C. J. Comparison of antibody avidity and titre elicited by peptide as a protein conjugate or as expressed in vaccinia. Immunology. 1988 Oct;65(2):311–314. [PMC free article] [PubMed] [Google Scholar]
- Lise D., Dubeaux C., Tello D., Mazier D., Jolivet M., Schlesinger D. H., Audibert F., Chedid L. Construction of immunogens for synthetic malaria vaccines. Biochem Biophys Res Commun. 1988 May 31;153(1):31–38. doi: 10.1016/s0006-291x(88)81185-9. [DOI] [PubMed] [Google Scholar]
- Marx P. A., Pedersen N. C., Lerche N. W., Osborn K. G., Lowenstine L. J., Lackner A. A., Maul D. H., Kwang H. S., Kluge J. D., Zaiss C. P. Prevention of simian acquired immune deficiency syndrome with a formalin-inactivated type D retrovirus vaccine. J Virol. 1986 Nov;60(2):431–435. doi: 10.1128/jvi.60.2.431-435.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masihi K. N., Lange W., Brehmer W., Ribi E. Immunobiological activities of nontoxic lipid A: enhancement of nonspecific resistance in combination with trehalose dimycolate against viral infection and adjuvant effects. Int J Immunopharmacol. 1986;8(3):339–345. doi: 10.1016/0192-0561(86)90116-5. [DOI] [PubMed] [Google Scholar]
- Morgan A. J., Epstein M. A., North J. R. Comparative immunogenicity studies on Epstein-Barr virus membrane antigen (MA) gp340 with novel adjuvants in mice, rabbits, and cotton-top tamarins. J Med Virol. 1984;13(3):281–292. doi: 10.1002/jmv.1890130310. [DOI] [PubMed] [Google Scholar]
- Naylor P. T., Larsen H. S., Huang L., Rouse B. T. In vivo induction of anti-herpes simplex virus immune response by type 1 antigens and lipid A incorporated into liposomes. Infect Immun. 1982 Jun;36(3):1209–1216. doi: 10.1128/iai.36.3.1209-1216.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patarroyo M. E., Amador R., Clavijo P., Moreno A., Guzman F., Romero P., Tascon R., Franco A., Murillo L. A., Ponton G. A synthetic vaccine protects humans against challenge with asexual blood stages of Plasmodium falciparum malaria. Nature. 1988 Mar 10;332(6160):158–161. doi: 10.1038/332158a0. [DOI] [PubMed] [Google Scholar]
- Perrin L. H., Merkli B., Loche M., Chizzolini C., Smart J., Richle R. Antimalarial immunity in Saimiri monkeys. Immunization with surface components of asexual blood stages. J Exp Med. 1984 Aug 1;160(2):441–451. doi: 10.1084/jem.160.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter C. W., Jennings R., Phair J. P., Clarke A., Stuart-Harris C. H. Dose-response relationship after immunization of volunteers with a new, surface-antigen-adsorbed influenza virus vaccine. J Infect Dis. 1977 Mar;135(3):423–431. doi: 10.1093/infdis/135.3.423. [DOI] [PubMed] [Google Scholar]
- Pullen G. R., Fitzgerald M. G., Hosking C. S. Antibody avidity determination by ELISA using thiocyanate elution. J Immunol Methods. 1986 Jan 22;86(1):83–87. doi: 10.1016/0022-1759(86)90268-1. [DOI] [PubMed] [Google Scholar]
- Richards R. L., Hayre M. D., Hockmeyer W. T., Alving C. R. Liposomes, lipid A, and aluminum hydroxide enhance the immune response to a synthetic malaria sporozoite antigen. Infect Immun. 1988 Mar;56(3):682–686. doi: 10.1128/iai.56.3.682-686.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson E. C., Banerji B., Seid R. C., Jr, Levin J., Alving C. R. Interactions of lipid a and liposome-associated lipid A with Limulus polyphemus amoebocytes. Infect Immun. 1983 Mar;39(3):1385–1391. doi: 10.1128/iai.39.3.1385-1391.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robey W. G., Arthur L. O., Matthews T. J., Langlois A., Copeland T. D., Lerche N. W., Oroszlan S., Bolognesi D. P., Gilden R. V., Fischinger P. J. Prospect for prevention of human immunodeficiency virus infection: purified 120-kDa envelope glycoprotein induces neutralizing antibody. Proc Natl Acad Sci U S A. 1986 Sep;83(18):7023–7027. doi: 10.1073/pnas.83.18.7023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Pescador L., Burke R. L., Ott G., Van Nest G. The effect of adjuvants on the efficacy of a recombinant herpes simplex virus glycoprotein vaccine. J Immunol. 1988 Sep 1;141(5):1720–1727. [PubMed] [Google Scholar]
- Scolnick E. M., McLean A. A., West D. J., McAleer W. J., Miller W. J., Buynak E. B. Clinical evaluation in healthy adults of a hepatitis B vaccine made by recombinant DNA. JAMA. 1984 Jun 1;251(21):2812–2815. [PubMed] [Google Scholar]
- Siddiqui W. A., Tam L. Q., Kan S. C., Kramer K. J., Case S. E., Palmer K. L., Yamaga K. M., Hui G. S. Induction of protective immunity to monoclonal-antibody-defined Plasmodium falciparum antigens requires strong adjuvant in Aotus monkeys. Infect Immun. 1986 Apr;52(1):314–318. doi: 10.1128/iai.52.1.314-318.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui W. A., Tam L. Q., Kramer K. J., Hui G. S., Case S. E., Yamaga K. M., Chang S. P., Chan E. B., Kan S. C. Merozoite surface coat precursor protein completely protects Aotus monkeys against Plasmodium falciparum malaria. Proc Natl Acad Sci U S A. 1987 May;84(9):3014–3018. doi: 10.1073/pnas.84.9.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui W. A., Taylor D. W., Kan S. C., Kramer K., Richmond-Crum S. M., Kotani S., Shiba T., Kusumoto S. Vaccination of experimental monkeys against Plasmodium falciparum: a possible safe adjuvant. Science. 1978 Sep 29;201(4362):1237–1239. doi: 10.1126/science.99814. [DOI] [PubMed] [Google Scholar]
- Trager W., Jensen J. B. Human malaria parasites in continuous culture. Science. 1976 Aug 20;193(4254):673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- Tsujimoto M., Kotani S., Kinoshita F., Kanoh S., Shiba T., Kusumoto S. Adjuvant activity of 6-O-acyl-muramyldipeptides to enhance primary cellular and humoral immune responses in guinea pigs: adaptability to various vehicles and pyrogenicity. Infect Immun. 1986 Sep;53(3):511–516. doi: 10.1128/iai.53.3.511-516.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujimoto M., Kotani S., Okunaga T., Kubo T., Takada H., Kubo T., Shiba T., Kusumoto S., Takahashi T., Goto Y. Enhancement of humoral immune responses against viral vaccines by a non-pyrogenic 6-O-acylmuramyldipeptide and synthetic low toxicity analogues of lipid A. Vaccine. 1989 Feb;7(1):39–48. doi: 10.1016/0264-410x(89)90009-1. [DOI] [PubMed] [Google Scholar]
- Tsujimoto M., Kotani S., Shiba T., Kusumoto S. Adjuvant activity of 6-O-acyl-muramyldipeptides to enhance primary cellular and humoral immune responses in guinea pigs: dose-response and local reactions observed with selected compounds. Infect Immun. 1986 Sep;53(3):517–521. doi: 10.1128/iai.53.3.517-521.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren H. S., Vogel F. R., Chedid L. A. Current status of immunological adjuvants. Annu Rev Immunol. 1986;4:369–388. doi: 10.1146/annurev.iy.04.040186.002101. [DOI] [PubMed] [Google Scholar]


