Abstract
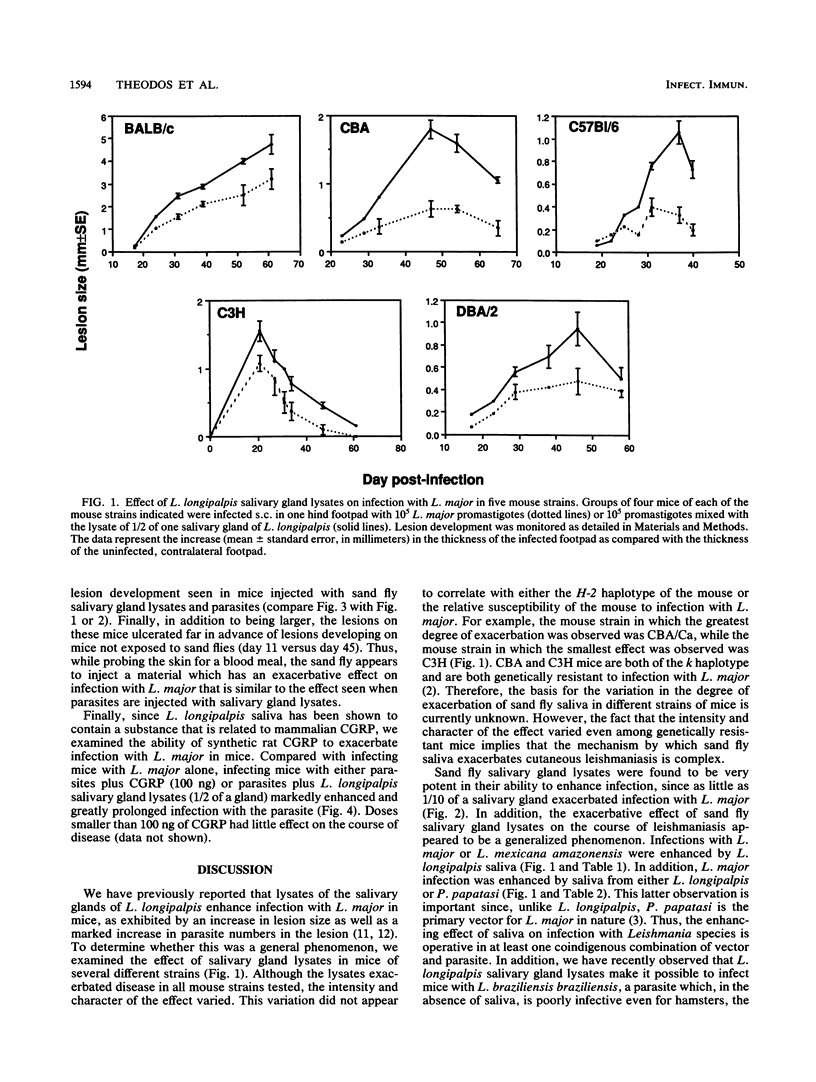
Salivary gland lysates of the sand fly Lutzomyia longipalpis markedly enhance the course of infection with Leishmania major in mice. Here we examine various parameters of this phenomenon. The exacerbative effect of L. longipalpis salivary gland lysates occurred in five different mouse strains; however, the character of the effect varied from one strain to another. Consistent exacerbation of infection was achieved with as little as 1/10 of a gland. The exacerbative effect applied to more than one Leishmania species and to more than one species of sand fly, since salivary gland lysates of L. longipalpis enhanced infection with L. mexicana amazonensis and salivary gland lysates of Phlebotomus papatasi enhanced infection with L. major. A synthetic rat calcitonin gene-related peptide was also found to exacerbate infection with L. major but was found to be approximately 100-fold less potent than saliva in mediating this effect. In addition, lesions induced at skin sites at which L. longipalpis had probed for a blood meal exhibited an exacerbated course of infection similar to that seen when parasites were injected with sand fly salivary gland lysates.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Howard J. G., Hale C., Chan-Liew W. L. Immunological regulation of experimental cutaneous leishmaniasis. 1. Immunogenetic aspects of susceptibility to Leishmania tropica in mice. Parasite Immunol. 1980 Winter;2(4):303–314. doi: 10.1111/j.1365-3024.1980.tb00061.x. [DOI] [PubMed] [Google Scholar]
- Howard J. G. Immunological regulation and control of experimental leishmaniasis. Int Rev Exp Pathol. 1986;28:79–116. [PubMed] [Google Scholar]
- Nong Y. H., Titus R. G., Ribeiro J. M., Remold H. G. Peptides encoded by the calcitonin gene inhibit macrophage function. J Immunol. 1989 Jul 1;143(1):45–49. [PubMed] [Google Scholar]
- Ribeiro J. M. Role of saliva in blood-feeding by arthropods. Annu Rev Entomol. 1987;32:463–478. doi: 10.1146/annurev.en.32.010187.002335. [DOI] [PubMed] [Google Scholar]
- Ribeiro J. M., Vachereau A., Modi G. B., Tesh R. B. A novel vasodilatory peptide from the salivary glands of the sand fly Lutzomyia longipalpis. Science. 1989 Jan 13;243(4888):212–214. doi: 10.1126/science.2783496. [DOI] [PubMed] [Google Scholar]
- Sacks D. L., Perkins P. V. Identification of an infective stage of Leishmania promastigotes. Science. 1984 Mar 30;223(4643):1417–1419. doi: 10.1126/science.6701528. [DOI] [PubMed] [Google Scholar]
- Samuelson J., Lerner E., Tesh R., Titus R. A mouse model of Leishmania braziliensis braziliensis infection produced by coinjection with sand fly saliva. J Exp Med. 1991 Jan 1;173(1):49–54. doi: 10.1084/jem.173.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titus R. G., Ceredig R., Cerottini J. C., Louis J. A. Therapeutic effect of anti-L3T4 monoclonal antibody GK1.5 on cutaneous leishmaniasis in genetically-susceptible BALB/c mice. J Immunol. 1985 Sep;135(3):2108–2114. [PubMed] [Google Scholar]
- Titus R. G., Ribeiro J. M. Salivary gland lysates from the sand fly Lutzomyia longipalpis enhance Leishmania infectivity. Science. 1988 Mar 11;239(4845):1306–1308. doi: 10.1126/science.3344436. [DOI] [PubMed] [Google Scholar]
- Titus R. G., Ribeiro J. M. The role of vector saliva in transmission of arthropod-borne disease. Parasitol Today. 1990 May;6(5):157–160. doi: 10.1016/0169-4758(90)90338-5. [DOI] [PubMed] [Google Scholar]
- Warburg A., Schlein Y. The effect of post-bloodmeal nutrition of Phlebotomus papatasi on the transmission of Leishmania major. Am J Trop Med Hyg. 1986 Sep;35(5):926–930. doi: 10.4269/ajtmh.1986.35.926. [DOI] [PubMed] [Google Scholar]



