Abstract
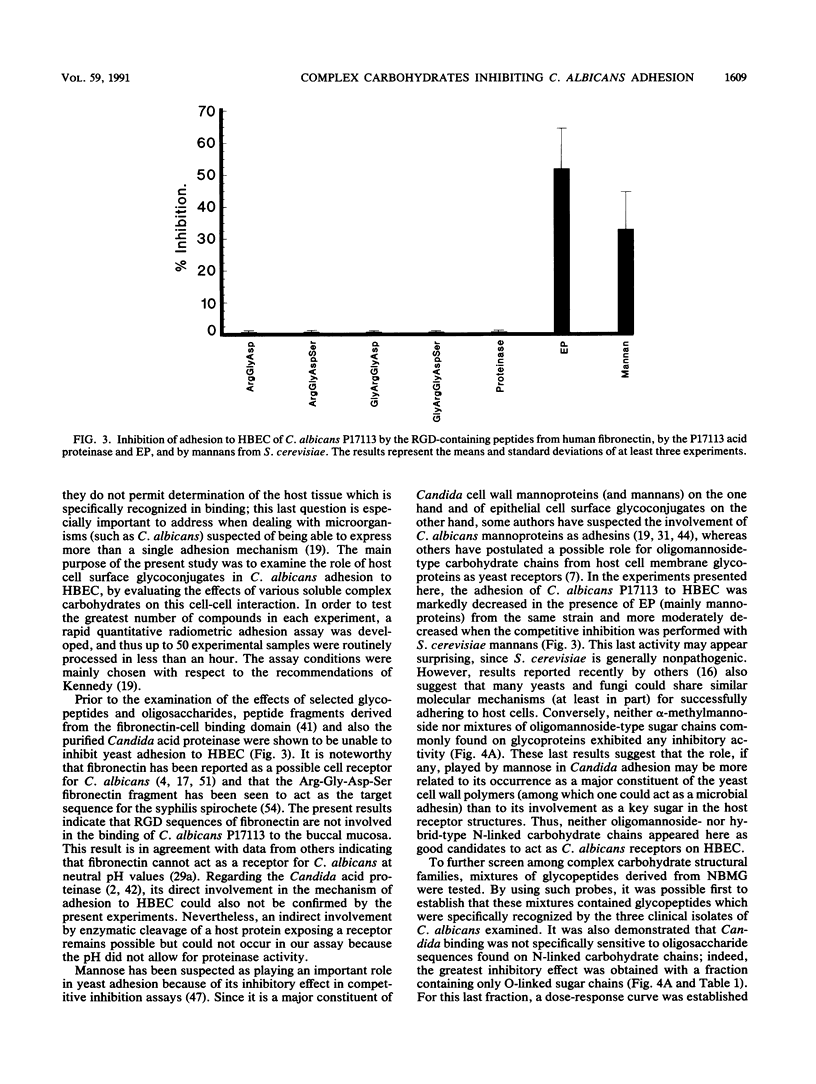
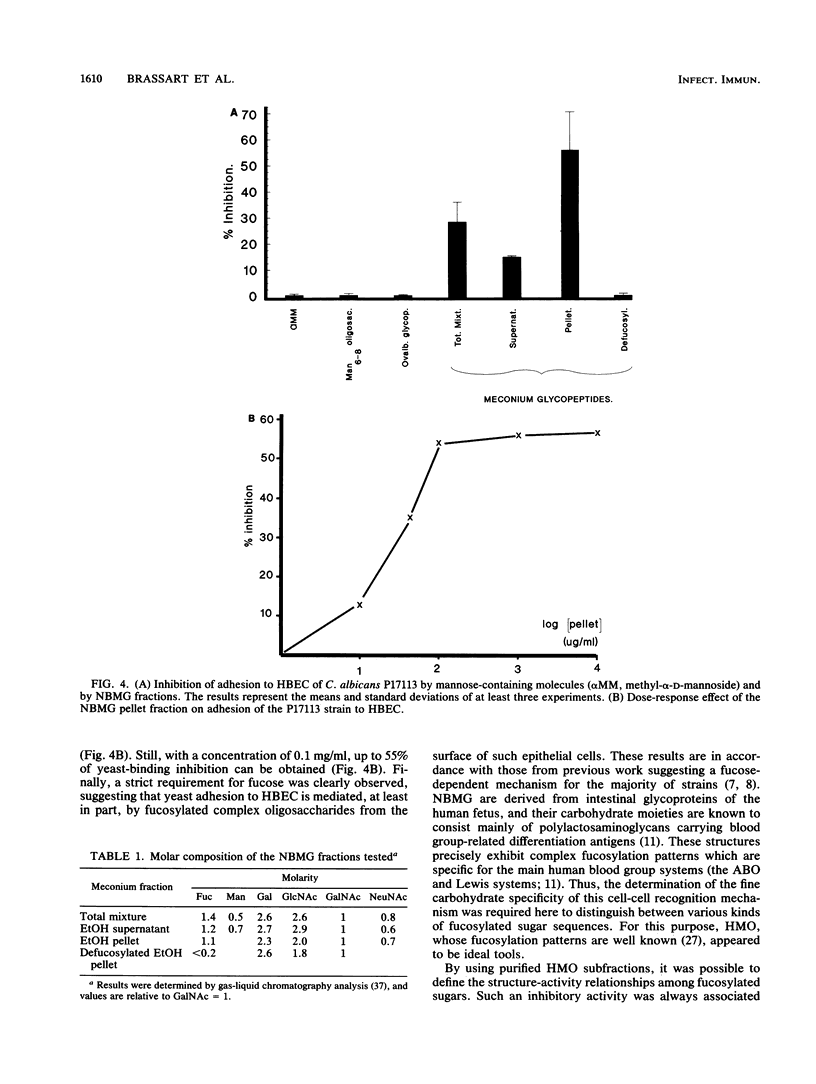
The role of cell surface glycoconjugates as possible adhesion receptors for Candida albicans yeasts on human buccal epithelial cells was investigated by using a quantitative radiometric assay involving 14C-metabolically labeled microorganisms. Various structurally defined soluble glycopeptides and oligosaccharides were tested at a low concentration (1 mg/ml) for their ability to competitively inhibit yeast adhesion to such exfoliated cells. Comparisons were also made with various molecular species previously proposed to act as adhesion molecules. A preparation of glycopeptides derived from pooled human newborn meconiums inhibited the attachment (up to 55%) of all three clinical isolates examined. The mild hydrolysis of fucosyl residues from the above mixture totally abolished its inhibitory potency. By using human milk oligosaccharide probes, the minimal structural requirement for activity was found to be the Fuc alpha 1----2Gal beta determinant (the H sugar sequence found on all blood group substances of the ABO [H] system). By contrast, the fucosylated determinants of the Lewis blood group system were found to be totally inactive. Total adhesion inhibitions were never obtained in the present experiments, suggesting that H disaccharide-bearing cell surface glycoconjugates could act as host receptors for C. albicans on human buccal epithelial cells as a part of a mechanism involving multireceptor specificities.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borg M., Rüchel R. Expression of extracellular acid proteinase by proteolytic Candida spp. during experimental infection of oral mucosa. Infect Immun. 1988 Mar;56(3):626–631. doi: 10.1128/iai.56.3.626-631.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouali A., Robert R., Tronchin G., Senet J. M. Binding of human fibrinogen to Candida albicans in vitro: a preliminary study. J Med Vet Mycol. 1986 Aug;24(4):345–348. doi: 10.1080/02681218680000511. [DOI] [PubMed] [Google Scholar]
- Bouchara J. P., Tronchin G., Annaix V., Robert R., Senet J. M. Laminin receptors on Candida albicans germ tubes. Infect Immun. 1990 Jan;58(1):48–54. doi: 10.1128/iai.58.1.48-54.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderone R. A., Scheld W. M. Role of fibronectin in the pathogenesis of candidal infections. Rev Infect Dis. 1987 Jul-Aug;9 (Suppl 4):S400–S403. doi: 10.1093/clinids/9.supplement_4.s400. [DOI] [PubMed] [Google Scholar]
- Centeno A., Davis C. P., Cohen M. S., Warren M. M. Modulation of Candida albicans attachment to human epithelial cells by bacteria and carbohydrates. Infect Immun. 1983 Mar;39(3):1354–1360. doi: 10.1128/iai.39.3.1354-1360.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins-Lech C., Kalbfleisch J. H., Franson T. R., Sohnle P. G. Inhibition by sugars of Candida albicans adherence to human buccal mucosal cells and corneocytes in vitro. Infect Immun. 1984 Dec;46(3):831–834. doi: 10.1128/iai.46.3.831-834.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley I. A., Douglas L. J. Isolation and partial characterization of an adhesin from Candida albicans. J Gen Microbiol. 1987 Mar;133(3):629–636. doi: 10.1099/00221287-133-3-629. [DOI] [PubMed] [Google Scholar]
- Douglas L. J. Surface composition and adhesion of Candida albicans. Biochem Soc Trans. 1985 Dec;13(6):982–984. doi: 10.1042/bst0130982. [DOI] [PubMed] [Google Scholar]
- Ghannoum M. A., Abu el-Teen K., Radwan S. S. Blocking adherence of Candida albicans to buccal epithelial cells by yeast glycolipids, yeast wall lipids and lipids from epithelial cells. Mykosen. 1987 Aug;30(8):371–378. doi: 10.1111/j.1439-0507.1987.tb03631.x. [DOI] [PubMed] [Google Scholar]
- Ghannoum M. A., Burns G. R., Elteen K. A., Radwan S. S. Experimental evidence for the role of lipids in adherence of Candida spp. to human buccal epithelial cells. Infect Immun. 1986 Oct;54(1):189–193. doi: 10.1128/iai.54.1.189-193.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Houte J. V. Bacterial adherence in oral microbial ecology. Annu Rev Microbiol. 1975;29:19–44. doi: 10.1146/annurev.mi.29.100175.000315. [DOI] [PubMed] [Google Scholar]
- Hazen K. C. Participation of yeast cell surface hydrophobicity in adherence of Candida albicans to human epithelial cells. Infect Immun. 1989 Jul;57(7):1894–1900. doi: 10.1128/iai.57.7.1894-1900.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez-Lucho V., Ginsburg V., Krivan H. C. Cryptococcus neoformans, Candida albicans, and other fungi bind specifically to the glycosphingolipid lactosylceramide (Gal beta 1-4Glc beta 1-1Cer), a possible adhesion receptor for yeasts. Infect Immun. 1990 Jul;58(7):2085–2090. doi: 10.1128/iai.58.7.2085-2090.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalo A., Segal E., Sahar E., Dayan D. Interaction of Candida albicans with genital mucosal surfaces: involvement of fibronectin in adherence. J Infect Dis. 1988 Jun;157(6):1253–1256. doi: 10.1093/infdis/157.6.1253. [DOI] [PubMed] [Google Scholar]
- Karlsson K. A. Animal glycosphingolipids as membrane attachment sites for bacteria. Annu Rev Biochem. 1989;58:309–350. doi: 10.1146/annurev.bi.58.070189.001521. [DOI] [PubMed] [Google Scholar]
- Kennedy M. J. Adhesion and association mechanisms of Candida albicans. Curr Top Med Mycol. 1988;2:73–169. doi: 10.1007/978-1-4612-3730-3_4. [DOI] [PubMed] [Google Scholar]
- Kennedy M. J., Sandin R. L. Influence of growth conditions on Candida albicans adhesion, hydrophobicity and cell wall ultrastructure. J Med Vet Mycol. 1988 Apr;26(2):79–92. [PubMed] [Google Scholar]
- Kennedy M. J., Volz P. A., Edwards C. A., Yancey R. J. Mechanisms of association of Candida albicans with intestinal mucosa. J Med Microbiol. 1987 Dec;24(4):333–341. doi: 10.1099/00222615-24-4-333. [DOI] [PubMed] [Google Scholar]
- Kimura L. H., Pearsall N. N. Adherence of Candida albicans to human buccal epithelial cells. Infect Immun. 1978 Jul;21(1):64–68. doi: 10.1128/iai.21.1.64-68.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King R. D., Lee J. C., Morris A. L. Adherence of Candida albicans and other Candida species to mucosal epithelial cells. Infect Immun. 1980 Feb;27(2):667–674. doi: 10.1128/iai.27.2.667-674.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klotz S. A. Adherence of Candida albicans to components of the subendothelial extracellular matrix. FEMS Microbiol Lett. 1990 Mar 15;56(3):249–254. doi: 10.1016/s0378-1097(05)80049-7. [DOI] [PubMed] [Google Scholar]
- Kobata A., Ginsburg V., Tsuda M. Oligosaccharides of human milk. I. Isolation and characterization. Arch Biochem Biophys. 1969 Mar;130(1):509–513. doi: 10.1016/0003-9861(69)90063-0. [DOI] [PubMed] [Google Scholar]
- Kobata A., Yamashita K., Tachibana Y. Oligosaccharides from human milk. Methods Enzymol. 1978;50:216–220. doi: 10.1016/0076-6879(78)50023-2. [DOI] [PubMed] [Google Scholar]
- Krivan H. C., Olson L. D., Barile M. F., Ginsburg V., Roberts D. D. Adhesion of Mycoplasma pneumoniae to sulfated glycolipids and inhibition by dextran sulfate. J Biol Chem. 1989 Jun 5;264(16):9283–9288. [PubMed] [Google Scholar]
- Krivan H. C., Roberts D. D., Ginsburg V. Many pulmonary pathogenic bacteria bind specifically to the carbohydrate sequence GalNAc beta 1-4Gal found in some glycolipids. Proc Natl Acad Sci U S A. 1988 Aug;85(16):6157–6161. doi: 10.1073/pnas.85.16.6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. Y., Scocca J. R. A common structural unit in asparagine-oligosaccharides of several glycoproteins from different sources. J Biol Chem. 1972 Sep 25;247(18):5753–5758. [PubMed] [Google Scholar]
- McCourtie J., Douglas L. J. Extracellular polymer of Candida albicans: isolation, analysis and role in adhesion. J Gen Microbiol. 1985 Mar;131(3):495–503. doi: 10.1099/00221287-131-3-495. [DOI] [PubMed] [Google Scholar]
- Mehentee J. F., Hay R. J. In vitro adherence of Candida albicans strains to murine gastrointestinal mucosal cells and explants and the role of environmental pH. J Gen Microbiol. 1989 Aug;135(8):2181–2188. doi: 10.1099/00221287-135-8-2181. [DOI] [PubMed] [Google Scholar]
- Neeser J. R., Chambaz A., Golliard M., Link-Amster H., Fryder V., Kolodziejczyk E. Adhesion of colonization factor antigen II-positive enterotoxigenic Escherichia coli strains to human enterocytelike differentiated HT-29 cells: a basis for host-pathogen interactions in the gut. Infect Immun. 1989 Dec;57(12):3727–3734. doi: 10.1128/iai.57.12.3727-3734.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neeser J. R., Koellreutter B., Wuersch P. Oligomannoside-type glycopeptides inhibiting adhesion of Escherichia coli strains mediated by type 1 pili: preparation of potent inhibitors from plant glycoproteins. Infect Immun. 1986 May;52(2):428–436. doi: 10.1128/iai.52.2.428-436.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neeser J. R., Schweizer T. F. A quantitative determination by capillary gas-liquid chromatography of neutral and amino sugars (as O-methyloxime acetates), and a study on hydrolytic conditions for glycoproteins and polysaccharides in order to increase sugar recoveries. Anal Biochem. 1984 Oct;142(1):58–67. doi: 10.1016/0003-2697(84)90516-5. [DOI] [PubMed] [Google Scholar]
- Ofek I., Mirelman D., Sharon N. Adherence of Escherichia coli to human mucosal cells mediated by mannose receptors. Nature. 1977 Feb 17;265(5595):623–625. doi: 10.1038/265623a0. [DOI] [PubMed] [Google Scholar]
- Persi M. A., Burnham J. C., Duhring J. L. Effects of carbon dioxide and pH on adhesion of Candida albicans to vaginal epithelial cells. Infect Immun. 1985 Oct;50(1):82–90. doi: 10.1128/iai.50.1.82-90.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierschbacher M. D., Ruoslahti E. Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature. 1984 May 3;309(5963):30–33. doi: 10.1038/309030a0. [DOI] [PubMed] [Google Scholar]
- Ray T. L., Payne C. D. Scanning electron microscopy of epidermal adherence and cavitation in murine candidiasis: a role for Candida acid proteinase. Infect Immun. 1988 Aug;56(8):1942–1949. doi: 10.1128/iai.56.8.1942-1949.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts D. D., Olson L. D., Barile M. F., Ginsburg V., Krivan H. C. Sialic acid-dependent adhesion of Mycoplasma pneumoniae to purified glycoproteins. J Biol Chem. 1989 Jun 5;264(16):9289–9293. [PubMed] [Google Scholar]
- Rotrosen D., Calderone R. A., Edwards J. E., Jr Adherence of Candida species to host tissues and plastic surfaces. Rev Infect Dis. 1986 Jan-Feb;8(1):73–85. doi: 10.1093/clinids/8.1.73. [DOI] [PubMed] [Google Scholar]
- Rüchel R. Properties of a purified proteinase from the yeast Candida albicans. Biochim Biophys Acta. 1981 May 14;659(1):99–113. doi: 10.1016/0005-2744(81)90274-6. [DOI] [PubMed] [Google Scholar]
- Samaranayake L. P., MacFarlane T. W. Factors affecting the in-vitro adherence of the fungal oral pathogen Candida albicans to epithelial cells of human origin. Arch Oral Biol. 1982;27(10):869–873. doi: 10.1016/0003-9969(82)90043-7. [DOI] [PubMed] [Google Scholar]
- Sandin R. L., Rogers A. L., Patterson R. J., Beneke E. S. Evidence for mannose-mediated adherence of Candida albicans to human buccal cells in vitro. Infect Immun. 1982 Jan;35(1):79–85. doi: 10.1128/iai.35.1.79-85.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal E., Lehrer N., Ofek I. Adherence of Candida albicans to human vaginal epithelial cells: inhibition by amino sugars. Exp Cell Biol. 1982;50(1):13–17. doi: 10.1159/000163121. [DOI] [PubMed] [Google Scholar]
- Segal E., Savage D. C. Adhesion of Candida albicans to mouse intestinal mucosa in vitro: development of the assay and test of inhibitors. J Med Vet Mycol. 1986 Dec;24(6):477–479. [PubMed] [Google Scholar]
- Sharon N., Lis H. Lectins as cell recognition molecules. Science. 1989 Oct 13;246(4927):227–234. doi: 10.1126/science.2552581. [DOI] [PubMed] [Google Scholar]
- Skerl K. G., Calderone R. A., Segal E., Sreevalsan T., Scheld W. M. In vitro binding of Candida albicans yeast cells to human fibronectin. Can J Microbiol. 1984 Feb;30(2):221–227. doi: 10.1139/m84-033. [DOI] [PubMed] [Google Scholar]
- Sobel J. D., Myers P. G., Kaye D., Levison M. E. Adherence of Candida albicans to human vaginal and buccal epithelial cells. J Infect Dis. 1981 Jan;143(1):76–82. doi: 10.1093/infdis/143.1.76. [DOI] [PubMed] [Google Scholar]
- Sobel J. D., Myers P., Levison M. E., Kaye D. Comparison of bacterial and fungal adherence to vaginal exfoliated epithelial cells and human vaginal epithelial tissue culture cells. Infect Immun. 1982 Feb;35(2):697–701. doi: 10.1128/iai.35.2.697-701.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. D., Baseman J. B., Alderete J. F. Fibronectin tetrapeptide is target for syphilis spirochete cytadherence. J Exp Med. 1985 Nov 1;162(5):1715–1719. doi: 10.1084/jem.162.5.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]



