Abstract
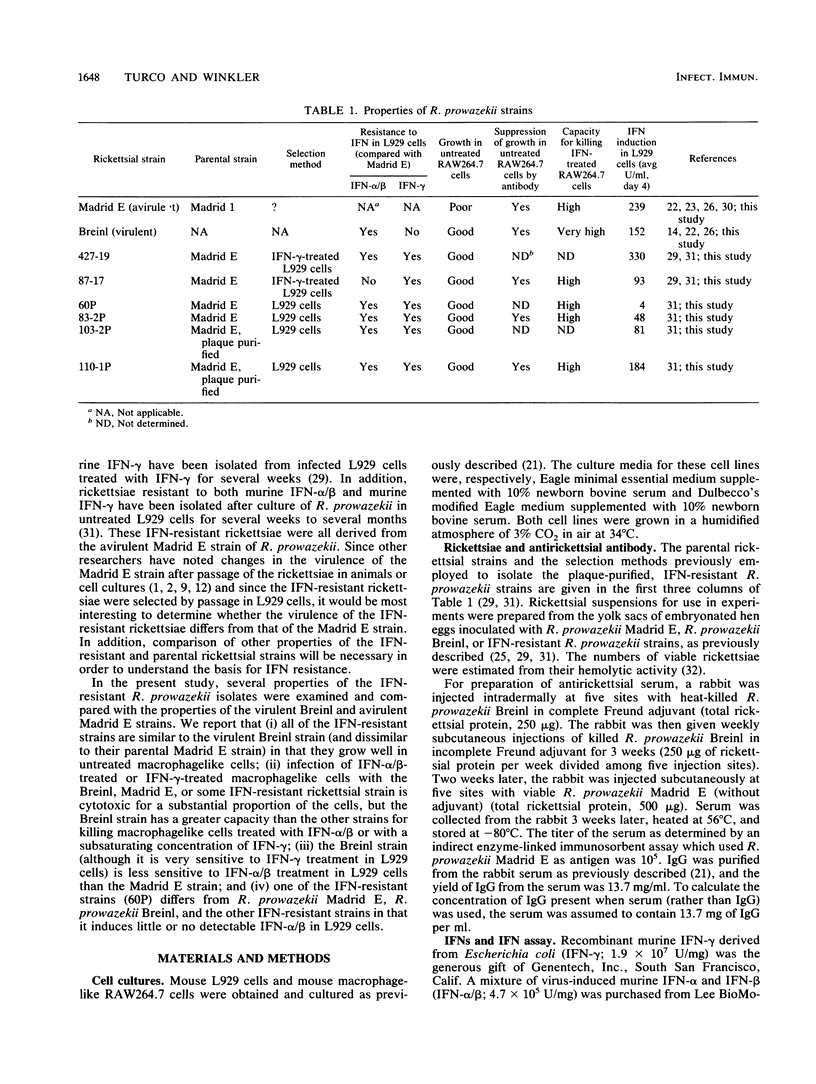
Several properties of virulent, avirulent, and interferon-resistant Rickettsia prowazekii strains were compared. All of the interferon-resistant rickettsial strains (which were derived from the avirulent Madrid E strain) resembled the virulent Breinl strain in that they grew well in untreated mouse macrophagelike RAW264.7 cells. In contrast, the avirulent Madrid E strain grew poorly in untreated RAW264.7 cells. Pretreatment of interferon-resistant rickettsiae or R. prowazekii Breinl with antirickettsial serum or immunoglobulin G suppressed the ability of the rickettsiae to grow in untreated RAW264.7 cells. Interferon-resistant R. prowazekii strains, like the Madrid E and Breinl strains, rapidly killed a substantial proportion of RAW264.7 cells that had been treated with gamma interferon or very high concentrations of alpha/beta interferon. Untreated infected RAW264.7 cells and interferon-treated mock-infected RAW264.7 cells were not killed during the same period. In cultures of RAW264.7 cells treated with either alpha/beta interferon (120 to 1,200 U/ml) or a subsaturating concentration of gamma interferon (0.5 U/ml), R. prowazekii Breinl organisms killed a higher percentage of the cells than did comparable numbers of R. prowazekii Madrid E organisms or interferon-resistant rickettsiae. Although R. prowazekii Breinl (like R. prowazekii Madrid E) was quite sensitive to gamma interferon in mouse L929 cells, the Breinl strain was resistant to murine alpha/beta interferon compared with the Madrid E strain and the two strains selected for resistance to murine gamma interferon. One of the interferon-resistant strains (strain 60P, which was selected for resistance to murine alpha/beta interferon) differed from the other R. prowazekii strains in that it induced little or no detectable interferon in L929 cell cultures.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balayera N. M., Nikdskaya V. N. Enhanced virulence of the vaccine strain E of Rickettsia prowazeke on passaging in white mice and guinea pigs. Acta Virol. 1972 Jun;16(1):80–82. [PubMed] [Google Scholar]
- Beaman L., Wisseman C. L., Jr Mechanisms of immunity in typhus infections. VI. Differential opsonizing and neutralizing action of human typhus rickettsia-specific cytophilic antibodies in cultures of human macrophages. Infect Immun. 1976 Oct;14(4):1071–1076. doi: 10.1128/iai.14.4.1071-1076.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIMENEZ D. F. STAINING RICKETTSIAE IN YOLK-SAC CULTURES. Stain Technol. 1964 May;39:135–140. doi: 10.3109/10520296409061219. [DOI] [PubMed] [Google Scholar]
- Gambrill M. R., Wisseman C. L., Jr Mechanisms of immunity in typhus infections. I. Multiplication of typhus rickettsiae in human macrophage cell cultures in the nonimmune system: influence of virulence of rickettsial strains and of chloramphenicol. Infect Immun. 1973 Oct;8(4):519–527. doi: 10.1128/iai.8.4.519-527.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonder J. C., Kenyon R. H., Pedersen C. E., Jr Epidemic typhus infection in cynomolgus monkeys (Macaca fascicularis). Infect Immun. 1980 Oct;30(1):219–223. doi: 10.1128/iai.30.1.219-223.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignatovich V. F. Enhancement of the antigenic activity and virulence of the vaccine strain E of Rickettsia prow azeki by passages in cell culture. Acta Virol. 1975 Nov;19(6):481–485. [PubMed] [Google Scholar]
- Jerrells T. R., Turco J., Winkler H. H., Spitalny G. L. Neutralization of lymphokine-mediated antirickettsial activity of fibroblasts and macrophages with monoclonal antibody specific for murine interferon gamma. Infect Immun. 1986 Jan;51(1):355–359. doi: 10.1128/iai.51.1.355-359.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazar J., Krautwurst P. A., Gordon F. B. Effect of Interferon and Interferon Inducers on Infections with a Nonviral Intracellular Microorganism, Rickettsia akari. Infect Immun. 1971 Jun;3(6):819–824. doi: 10.1128/iai.3.6.819-824.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazár J., Brezina R., Urvölgyi J. Studies on the E strain of Rickettsia prowazeki. Bull World Health Organ. 1973;49(3):257–265. [PMC free article] [PubMed] [Google Scholar]
- Kazár J. Interferon-like inhibitor in mouse sera induced by rickettsiae. Acta Virol. 1966 May;10(3):277–277. [PubMed] [Google Scholar]
- Keysary A., McCaul T. F., Winkler H. H. Roles of the Fc receptor and respiratory burst in killing of Rickettsia prowazekii by macrophagelike cell lines. Infect Immun. 1989 Aug;57(8):2390–2396. doi: 10.1128/iai.57.8.2390-2396.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Jerrells T. R., Spitalny G. L., Walker D. H. Gamma interferon as a crucial host defense against Rickettsia conorii in vivo. Infect Immun. 1987 May;55(5):1252–1255. doi: 10.1128/iai.55.5.1252-1255.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace J. L., Russell S. W., LeBlanc P. A., Murasko D. M. Comparative effects of various classes of mouse interferons on macrophage activation for tumor cell killing. J Immunol. 1985 Feb;134(2):977–981. [PubMed] [Google Scholar]
- Snyder J. C., Anderson C. R. THE SUSCEPTIBILITY OF THE EASTERN COTTON RAT, SIGMODON HISPIDUS HISPIDUS, TO EUROPEAN TYPHUS. Science. 1942 Jan 2;95(2453):23–23. doi: 10.1126/science.95.2453.23. [DOI] [PubMed] [Google Scholar]
- Sonenshine D. E., Bozeman F. M., Williams M. S., Masiello S. A., Chadwick D. P., Stocks N. I., Lauer D. M., Elisberg B. L. Epizootiology of epidemic typhus (Rickettsia prowazekii) in flying squirrels. Am J Trop Med Hyg. 1978 Mar;27(2 Pt 1):339–349. doi: 10.4269/ajtmh.1978.27.339. [DOI] [PubMed] [Google Scholar]
- Turco J., Keysary A., Winkler H. H. Interferon-gamma- and rickettsia-induced killing of macrophage-like cells is inhibited by anti-rickettsial antibodies and does not require the respiratory burst. J Interferon Res. 1989 Oct;9(5):615–629. doi: 10.1089/jir.1989.9.615. [DOI] [PubMed] [Google Scholar]
- Turco J., Winkler H. H. Cloned mouse interferon-gamma inhibits the growth of Rickettsia prowazekii in cultured mouse fibroblasts. J Exp Med. 1983 Dec 1;158(6):2159–2164. doi: 10.1084/jem.158.6.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turco J., Winkler H. H. Comparison of the properties of antirickettsial activity and interferon in mouse lymphokines. Infect Immun. 1983 Oct;42(1):27–32. doi: 10.1128/iai.42.1.27-32.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turco J., Winkler H. H. Differentiation between virulent and avirulent strains of Rickettsia prowazekii by macrophage-like cell lines. Infect Immun. 1982 Mar;35(3):783–791. doi: 10.1128/iai.35.3.783-791.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turco J., Winkler H. H. Effect of mouse lymphokines and cloned mouse interferon-gamma on the interaction of Rickettsia prowazekii with mouse macrophage-like RAW264.7 cells. Infect Immun. 1984 Aug;45(2):303–308. doi: 10.1128/iai.45.2.303-308.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turco J., Winkler H. H. Gamma-interferon-induced inhibition of the growth of Rickettsia prowazekii in fibroblasts cannot be explained by the degradation of tryptophan or other amino acids. Infect Immun. 1986 Jul;53(1):38–46. doi: 10.1128/iai.53.1.38-46.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turco J., Winkler H. H. Inhibition of the growth of Rickettsia prowazekii in cultured fibroblasts by lymphokines. J Exp Med. 1983 Mar 1;157(3):974–986. doi: 10.1084/jem.157.3.974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turco J., Winkler H. H. Interferon-alpha/beta and Rickettsia prowazekii: induction and sensitivity. Ann N Y Acad Sci. 1990;590:168–186. doi: 10.1111/j.1749-6632.1990.tb42219.x. [DOI] [PubMed] [Google Scholar]
- Turco J., Winkler H. H. Isolation of Rickettsia prowazekii with reduced sensitivity to gamma interferon. Infect Immun. 1989 Jun;57(6):1765–1772. doi: 10.1128/iai.57.6.1765-1772.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turco J., Winkler H. H. Selection of alpha/beta interferon- and gamma interferon-resistant rickettsiae by passage of Rickettsia prowazekii in L929 cells. Infect Immun. 1990 Oct;58(10):3279–3285. doi: 10.1128/iai.58.10.3279-3285.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker T. S., Winkler H. H. Rickettsial hemolysis: rapid method for enumeration of metabolically active typhus rickettsiae. J Clin Microbiol. 1979 May;9(5):645–647. doi: 10.1128/jcm.9.5.645-647.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler H. H., Daugherty R. M. Cytoplasmic distinction of avirulent and virulent Rickettsia prowazekii: fusion of infected fibroblasts with macrophage-like cells. Infect Immun. 1983 Jun;40(3):1245–1247. doi: 10.1128/iai.40.3.1245-1247.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler H. H., Turco J. Rickettsia prowazekii and the host cell: entry, growth and control of the parasite. Curr Top Microbiol Immunol. 1988;138:81–107. [PubMed] [Google Scholar]
- Wisseman C. L., Jr, Waddell A. Interferonlike factors from antigen- and mitogen-stimulated human leukocytes with antirickettsial and cytolytic actions on Rickettsia prowazekii. Infected human endothelial cells, fibroblasts, and macrophages. J Exp Med. 1983 Jun 1;157(6):1780–1793. doi: 10.1084/jem.157.6.1780. [DOI] [PMC free article] [PubMed] [Google Scholar]


