Abstract
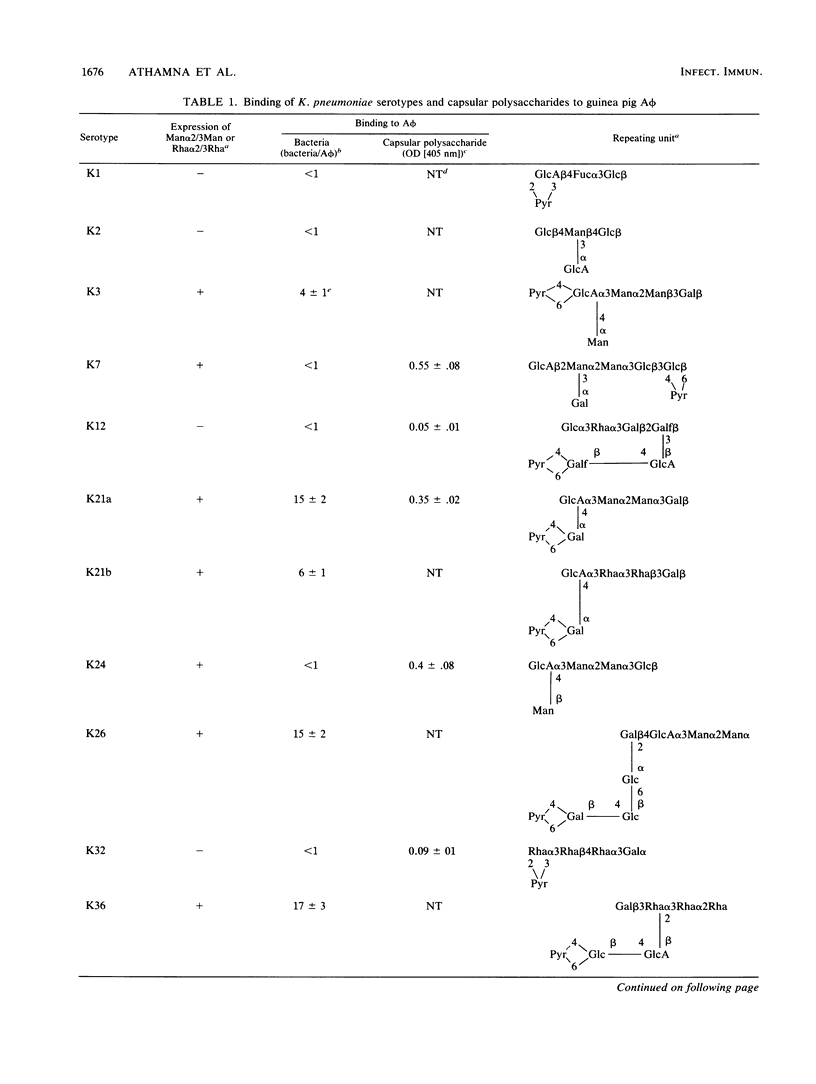
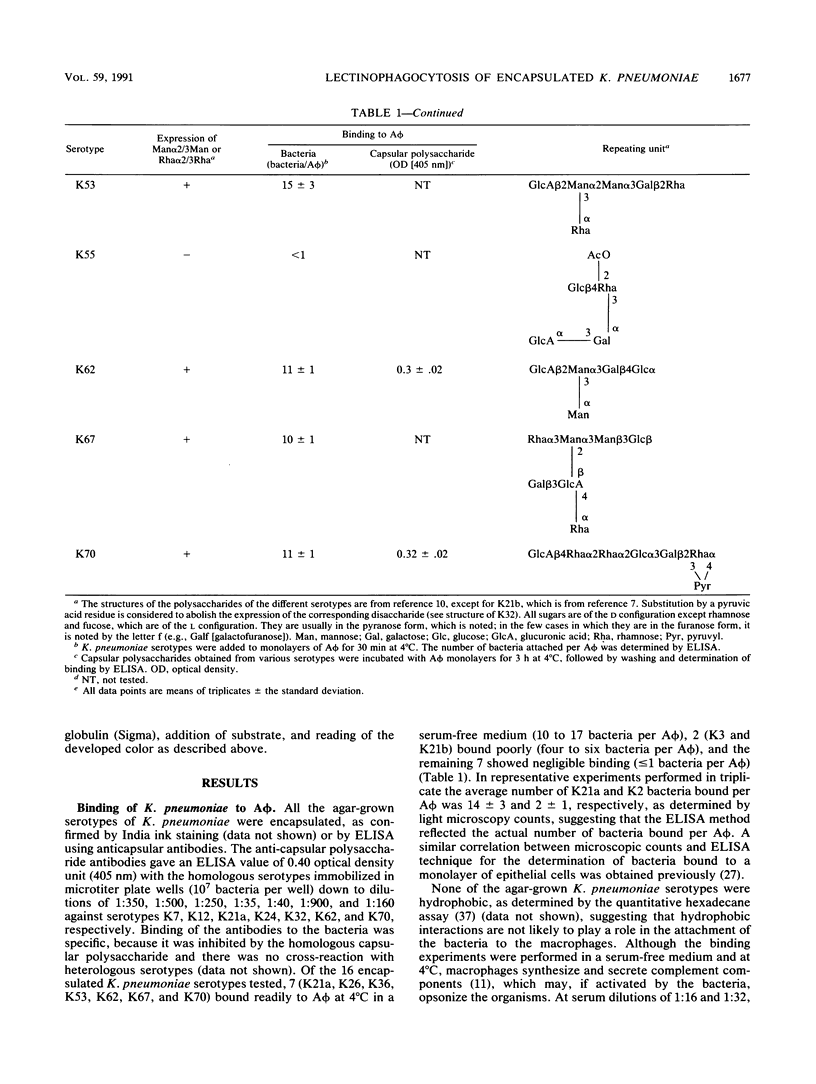
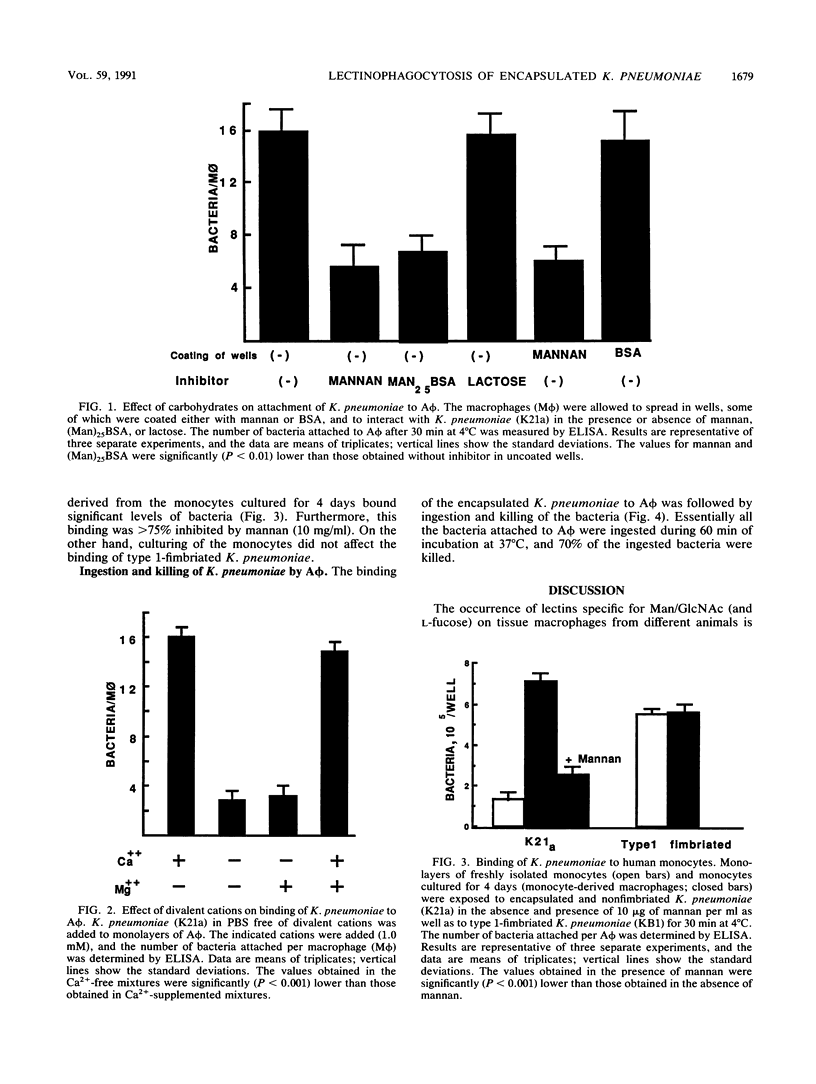
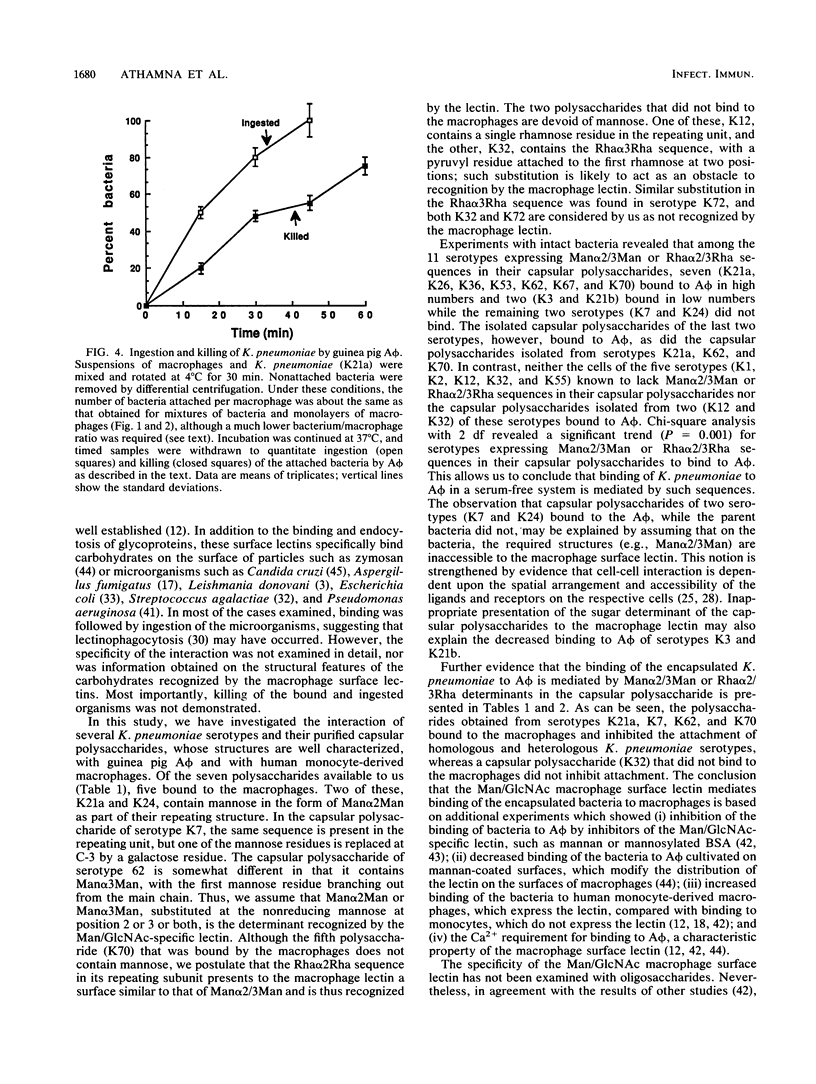
Macrophages express a mannose/N-acetylglucosamine-specific lectin which serves as a receptor for nonopsonic phagocytosis of mannose-coated particles. We have examined the binding to guinea pig alveolar macrophages in a serum-free medium of 16 Klebsiella pneumoniae serotypes and of the capsular polysaccharides isolated from 7 of these serotypes. Only five polysaccharides containing the repeating sequence Man alpha 2/3Man or L-Rha alpha 2/3-L-Rha bound to the macrophages. Of the 11 bacterial serotypes expressing such disaccharides in their capsular polysaccharides, 7 bound efficiently, 2 bound poorly, and 2 did not bind at all. No binding occurred with five serotypes lacking these disaccharides. Binding of the bacteria was inhibited by homologous and heterologous capsular polysaccharides that contain the disaccharide sequences, by mannan, and by (Man)25BSA (where BSA is bovine serum albumin). Man alpha 2/3Man-containing oligosaccharides were potent inhibitors compared with monosaccharides. Binding was dependent on Ca2+, modulated by cultivating the macrophages on mannan-coated surfaces, and increased in human monocyte-derived macrophages compared with monocytes. The bulk of the bacteria bound to the macrophages was internalized and killed. The data taken together suggest that Klebsiella pneumoniae cells undergo lectinophagocytosis mediated by capsular disaccharides recognized by the mannose/N-acetylglucosamine-specific lectin of macrophages. This may enhance clearance of the organisms from the serum-poor environment of the lung.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen P. M., Fisher D., Saunders J. R., Hart C. A. The role of capsular polysaccharide K21b of Klebsiella and of the structurally related colanic-acid polysaccharide of Escherichia coli in resistance to phagocytosis and serum killing. J Med Microbiol. 1987 Dec;24(4):363–370. doi: 10.1099/00222615-24-4-363. [DOI] [PubMed] [Google Scholar]
- Athamna A., Ofek I. Enzyme-linked immunosorbent assay for quantitation of attachment and ingestion stages of bacterial phagocytosis. J Clin Microbiol. 1988 Jan;26(1):62–66. doi: 10.1128/jcm.26.1.62-66.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell J. M. Receptors and recognition mechanisms of Leishmania species. Trans R Soc Trop Med Hyg. 1985;79(5):606–612. doi: 10.1016/0035-9203(85)90166-x. [DOI] [PubMed] [Google Scholar]
- Burger R., Deubel U., Hadding U., Bitter-Suermann D. Identification of functionally relevant determinants on the complement component C3 with monoclonal antibodies. J Immunol. 1982 Nov;129(5):2042–2050. [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Chowdhury T. A., Jansson P. E., Lindberg B., Lindquist U. Structural studies of an extracellular polysaccharide (K21b) elaborated by Klebsiella type 21b. Carbohydr Res. 1989 Jul 1;190(1):145–151. doi: 10.1016/0008-6215(89)84154-0. [DOI] [PubMed] [Google Scholar]
- Cryz S. J., Jr, Fürer E., Germanier R. Purification and vaccine potential of Klebsiella capsular polysaccharides. Infect Immun. 1985 Oct;50(1):225–230. doi: 10.1128/iai.50.1.225-230.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryz S. J., Jr, Mortimer P. M., Mansfield V., Germanier R. Seroepidemiology of Klebsiella bacteremic isolates and implications for vaccine development. J Clin Microbiol. 1986 Apr;23(4):687–690. doi: 10.1128/jcm.23.4.687-690.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezekowitz R. A., Sim R. B., Hill M., Gordon S. Local opsonization by secreted macrophage complement components. Role of receptors for complement in uptake of zymosan. J Exp Med. 1984 Jan 1;159(1):244–260. doi: 10.1084/jem.159.1.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezekowitz R. A., Stahl P. D. The structure and function of vertebrate mannose lectin-like proteins. J Cell Sci Suppl. 1988;9:121–133. doi: 10.1242/jcs.1988.supplement_9.6. [DOI] [PubMed] [Google Scholar]
- Finlay G. J., Baguley B. C., Wilson W. R. A semiautomated microculture method for investigating growth inhibitory effects of cytotoxic compounds on exponentially growing carcinoma cells. Anal Biochem. 1984 Jun;139(2):272–277. doi: 10.1016/0003-2697(84)90002-2. [DOI] [PubMed] [Google Scholar]
- Goldstein E., Lippert W., Warshauer D. Pulmonary alveolar macrophage. Defender against bacterial infection of the lung. J Clin Invest. 1974 Sep;54(3):519–528. doi: 10.1172/JCI107788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotschlich E. C. Thoughts on the evolution of strategies used by bacteria for evasion of host defenses. Rev Infect Dis. 1983 Sep-Oct;5 (Suppl 4):S778–S783. doi: 10.1093/clinids/5.supplement_4.s778. [DOI] [PubMed] [Google Scholar]
- Kan V. L., Bennett J. E. Lectin-like attachment sites on murine pulmonary alveolar macrophages bind Aspergillus fumigatus conidia. J Infect Dis. 1988 Aug;158(2):407–414. doi: 10.1093/infdis/158.2.407. [DOI] [PubMed] [Google Scholar]
- Kataoka M., Tavassoli M. Development of specific surface receptors recognizing mannose-terminal glycoconjugates in cultured monocytes: a possible early marker for differentiation of monocyte into macrophage. Exp Hematol. 1985 Jan;13(1):44–50. [PubMed] [Google Scholar]
- Levi M. H., Pasculle A. W., Dowling J. N. Role of the alveolar macrophage in host defense and immunity to Legionella micdadei pneumonia in the guinea pig. Microb Pathog. 1987 Apr;2(4):269–282. doi: 10.1016/0882-4010(87)90125-2. [DOI] [PubMed] [Google Scholar]
- Maayan M. C., Ofek I., Medalia O., Aronson M. Population shift in mannose-specific fimbriated phase of Klebsiella pneumoniae during experimental urinary tract infection in mice. Infect Immun. 1985 Sep;49(3):785–789. doi: 10.1128/iai.49.3.785-789.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michl J., Pieczonka M. M., Unkeless J. C., Silverstein S. C. Effects of immobilized immune complexes on Fc- and complement-receptor function in resident and thioglycollate-elicited mouse peritoneal macrophages. J Exp Med. 1979 Sep 19;150(3):607–621. doi: 10.1084/jem.150.3.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofek I., Beachey E. H., Bisno A. L. Resistance of Neisseria gonorrhoeae to phagocytosis: relationship to colonial morphology and surface pili. J Infect Dis. 1974 Mar;129(3):310–316. doi: 10.1093/infdis/129.3.310. [DOI] [PubMed] [Google Scholar]
- Ofek I., Beachey E. H. Mannose binding and epithelial cell adherence of Escherichia coli. Infect Immun. 1978 Oct;22(1):247–254. doi: 10.1128/iai.22.1.247-254.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofek I., Courtney H. S., Schifferli D. M., Beachey E. H. Enzyme-linked immunosorbent assay for adherence of bacteria to animal cells. J Clin Microbiol. 1986 Oct;24(4):512–516. doi: 10.1128/jcm.24.4.512-516.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofek I., Goldhar J., Eshdat Y., Sharon N. The importance of mannose specific adhesins (lectins) in infections caused by Escherichia coli. Scand J Infect Dis Suppl. 1982;33:61–67. [PubMed] [Google Scholar]
- Ofek I., Sharon N. Lectinophagocytosis: a molecular mechanism of recognition between cell surface sugars and lectins in the phagocytosis of bacteria. Infect Immun. 1988 Mar;56(3):539–547. doi: 10.1128/iai.56.3.539-547.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okutani K., Dutton G. G. Structural investigation of Klebsiella serotype K46 polysaccharide. Carbohydr Res. 1980 Nov 15;86(2):259–271. doi: 10.1016/s0008-6215(00)85903-0. [DOI] [PubMed] [Google Scholar]
- Perry A., Ofek I. Inhibition of blood clearance and hepatic tissue binding of Escherichia coli by liver lectin-specific sugars and glycoproteins. Infect Immun. 1984 Jan;43(1):257–262. doi: 10.1128/iai.43.1.257-262.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds H. Y., Newball H. H. Analysis of proteins and respiratory cells obtained from human lungs by bronchial lavage. J Lab Clin Med. 1974 Oct;84(4):559–573. [PubMed] [Google Scholar]
- Robbins J. B., Austrian R., Lee C. J., Rastogi S. C., Schiffman G., Henrichsen J., Mäkelä P. H., Broome C. V., Facklam R. R., Tiesjema R. H. Considerations for formulating the second-generation pneumococcal capsular polysaccharide vaccine with emphasis on the cross-reactive types within groups. J Infect Dis. 1983 Dec;148(6):1136–1159. doi: 10.1093/infdis/148.6.1136. [DOI] [PubMed] [Google Scholar]
- Robertson J., Caldwell J. R., Castle J. R., Waldman R. H. Evidence for the presence of components of the alternative (properdin) pathway of complement activation in respiratory secretions. J Immunol. 1976 Sep;117(3):900–903. [PubMed] [Google Scholar]
- Silverblatt F. J., Dreyer J. S., Schauer S. Effect of pili on susceptibility of Escherichia coli to phagocytosis. Infect Immun. 1979 Apr;24(1):218–223. doi: 10.1128/iai.24.1.218-223.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverblatt F. J., Ofek I. Interaction of bacterial pili and leukocytes. Infection. 1983 Jul-Aug;11(4):235–238. doi: 10.1007/BF01641208. [DOI] [PubMed] [Google Scholar]
- Speert D. P., Silverstein S. C. Phagocytosis of unopsonized zymosan by human monocyte-derived macrophages: maturation and inhibition by mannan. J Leukoc Biol. 1985 Nov;38(5):655–658. doi: 10.1002/jlb.38.5.655. [DOI] [PubMed] [Google Scholar]
- Speert D. P., Wright S. D., Silverstein S. C., Mah B. Functional characterization of macrophage receptors for in vitro phagocytosis of unopsonized Pseudomonas aeruginosa. J Clin Invest. 1988 Sep;82(3):872–879. doi: 10.1172/JCI113692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl P. D., Rodman J. S., Miller M. J., Schlesinger P. H. Evidence for receptor-mediated binding of glycoproteins, glycoconjugates, and lysosomal glycosidases by alveolar macrophages. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1399–1403. doi: 10.1073/pnas.75.3.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung S. S., Nelson R. S., Silverstein S. C. Yeast mannans inhibit binding and phagocytosis of zymosan by mouse peritoneal macrophages. J Cell Biol. 1983 Jan;96(1):160–166. doi: 10.1083/jcb.96.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warr G. A. A macrophage receptor for (mannose/glucosamine)-glycoproteins of potential importance in phagocytic activity. Biochem Biophys Res Commun. 1980 Apr 14;93(3):737–745. doi: 10.1016/0006-291x(80)91139-0. [DOI] [PubMed] [Google Scholar]
- Wilson M. E., Pearson R. D. Roles of CR3 and mannose receptors in the attachment and ingestion of Leishmania donovani by human mononuclear phagocytes. Infect Immun. 1988 Feb;56(2):363–369. doi: 10.1128/iai.56.2.363-369.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]



