Abstract
Serotype-specific fimbriae of Bordetella pertussis are considered potential components of new-generation vaccines against whooping cough. Attempts to characterize fimbriae, and indeed other virulence determinants, produced by B. pertussis have been frustrated on one hand by low yields from B. pertussis itself and on the other by an inability to produce native recombinant products in Escherichia coli. In order to try to circumvent this problem, we have examined the expression of B. pertussis serotype 2 fimbriae in Bordetella parapertussis and Bordetella bronchiseptica from native as well as E. coli expression signals. These studies revealed that the fimbrial gene product was expressed from the original B. pertussis promoter and Shine-Dalgarno sequence in both B. parapertussis and B. bronchiseptica. The transcriptional start site of the gene was located 146 nucleotides upstream of its ATG start codon. A recombinant fimbrial subunit gene containing PLAC and the atpE translation initiation region of E. coli was also expressed in B. bronchiseptica. In all cases in which gene expression was detected the gene product was expressed as serotype 2-specific fimbriae as determined by enzyme-linked immunosorbent assay (ELISA) and immunoelectron microscopic investigation of the bacterial cell surface.
Full text
PDF

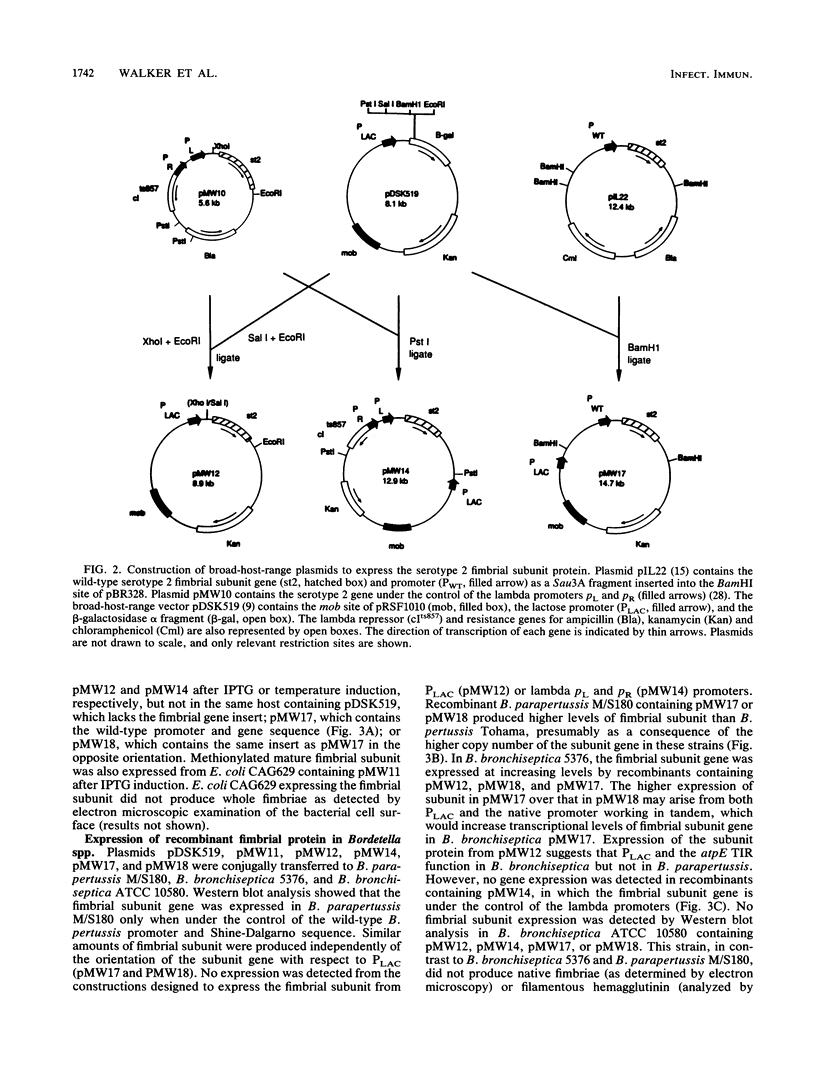
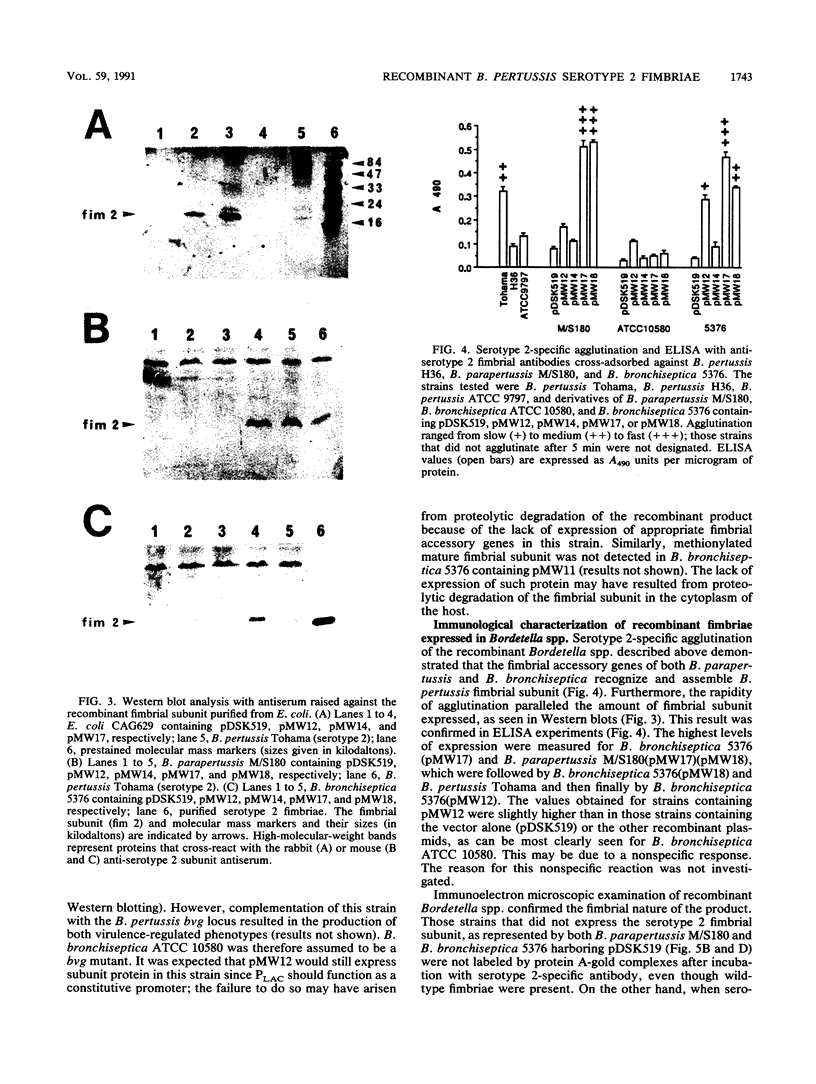


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- De Lorenzo V., Herrero M., Giovannini F., Neilands J. B. Fur (ferric uptake regulation) protein and CAP (catabolite-activator protein) modulate transcription of fur gene in Escherichia coli. Eur J Biochem. 1988 May 2;173(3):537–546. doi: 10.1111/j.1432-1033.1988.tb14032.x. [DOI] [PubMed] [Google Scholar]
- Finan T. M., Kunkel B., De Vos G. F., Signer E. R. Second symbiotic megaplasmid in Rhizobium meliloti carrying exopolysaccharide and thiamine synthesis genes. J Bacteriol. 1986 Jul;167(1):66–72. doi: 10.1128/jb.167.1.66-72.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross R., Rappuoli R. Positive regulation of pertussis toxin expression. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3913–3917. doi: 10.1073/pnas.85.11.3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irons L. I., Ashworth L. A., Robinson A. Release and purification of fimbriae from Bordetella pertussis. Dev Biol Stand. 1985;61:153–163. [PubMed] [Google Scholar]
- Keen N. T., Tamaki S., Kobayashi D., Trollinger D. Improved broad-host-range plasmids for DNA cloning in gram-negative bacteria. Gene. 1988 Oct 15;70(1):191–197. doi: 10.1016/0378-1119(88)90117-5. [DOI] [PubMed] [Google Scholar]
- Kimura A., Mountzouros K. T., Schad P. A., Cieplak W., Cowell J. L. Pertussis toxin analog with reduced enzymatic and biological activities is a protective immunogen. Infect Immun. 1990 Oct;58(10):3337–3347. doi: 10.1128/iai.58.10.3337-3347.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp S., Mekalanos J. J. Two trans-acting regulatory genes (vir and mod) control antigenic modulation in Bordetella pertussis. J Bacteriol. 1988 Nov;170(11):5059–5066. doi: 10.1128/jb.170.11.5059-5066.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee C. K., Roberts A., Perrin S. Expression of pertussis toxin in Bordetella bronchiseptica and Bordetella parapertussis carrying recombinant plasmids. Infect Immun. 1989 May;57(5):1413–1418. doi: 10.1128/iai.57.5.1413-1418.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z. M., Brennan M. J., David J. L., Carter P. H., Cowell J. L., Manclark C. R. Comparison of type 2 and type 6 fimbriae of Bordetella pertussis by using agglutinating monoclonal antibodies. Infect Immun. 1988 Dec;56(12):3184–3188. doi: 10.1128/iai.56.12.3184-3188.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livey I., Duggleby C. J., Robinson A. Cloning and nucleotide sequence analysis of the serotype 2 fimbrial subunit gene of Bordetella pertussis. Mol Microbiol. 1987 Sep;1(2):203–209. doi: 10.1111/j.1365-2958.1987.tb00513.x. [DOI] [PubMed] [Google Scholar]
- Mooi F. R., van der Heide H. G., ter Avest A. R., Welinder K. G., Livey I., van der Zeijst B. A., Gaastra W. Characterization of fimbrial subunits from Bordetella species. Microb Pathog. 1987 Jun;2(6):473–484. doi: 10.1016/0882-4010(87)90054-4. [DOI] [PubMed] [Google Scholar]
- Nagai K., Thøgersen H. C. Synthesis and sequence-specific proteolysis of hybrid proteins produced in Escherichia coli. Methods Enzymol. 1987;153:461–481. doi: 10.1016/0076-6879(87)53072-5. [DOI] [PubMed] [Google Scholar]
- Nencioni L., Pizza M., Bugnoli M., De Magistris T., Di Tommaso A., Giovannoni F., Manetti R., Marsili I., Matteucci G., Nucci D. Characterization of genetically inactivated pertussis toxin mutants: candidates for a new vaccine against whooping cough. Infect Immun. 1990 May;58(5):1308–1315. doi: 10.1128/iai.58.5.1308-1315.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicosia A., Rappuoli R. Promoter of the pertussis toxin operon and production of pertussis toxin. J Bacteriol. 1987 Jun;169(6):2843–2846. doi: 10.1128/jb.169.6.2843-2846.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston N. W. Technical problems in the laboratory diagnosis and prevention of whooping-cough. Lab Pract. 1970 May;19(5):482–486. [PubMed] [Google Scholar]
- Robinson A., Irons L. I., Ashworth L. A. Pertussis vaccine: present status and future prospects. Vaccine. 1985 Mar;3(1):11–22. doi: 10.1016/0264-410x(85)90004-0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y., Arai H. Leucocytosis-promoting factor of Bordetella pertussis. I. Purification and characterization. Infect Immun. 1972 Dec;6(6):899–904. doi: 10.1128/iai.6.6.899-904.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauder B., Blöcker H., Frank R., McCarthy J. E. Inducible expression vectors incorporating the Escherichia coli atpE translational initiation region. Gene. 1987;52(2-3):279–283. doi: 10.1016/0378-1119(87)90054-0. [DOI] [PubMed] [Google Scholar]
- Stainer D. W., Scholte M. J. A simple chemically defined medium for the production of phase I Bordetella pertussis. J Gen Microbiol. 1970 Oct;63(2):211–220. doi: 10.1099/00221287-63-2-211. [DOI] [PubMed] [Google Scholar]
- Walker M. J., Birch R. G., Pemberton J. M. Cloning and characterization of an albicidin resistance gene from Klebsiella oxytoca. Mol Microbiol. 1988 Jul;2(4):443–454. doi: 10.1111/j.1365-2958.1988.tb00050.x. [DOI] [PubMed] [Google Scholar]
- Walker M. J., Rohde M., Brownlie R. M., Timmis K. N. Engineering upstream transcriptional and translational signals of Bordetella pertussis serotype 2 fimbrial subunit protein for efficient expression in Escherichia coli: in vitro autoassembly of the expressed product into filamentous structures. Mol Microbiol. 1990 Jan;4(1):39–47. doi: 10.1111/j.1365-2958.1990.tb02013.x. [DOI] [PubMed] [Google Scholar]
- Weiss A. A., Falkow S. Genetic analysis of phase change in Bordetella pertussis. Infect Immun. 1984 Jan;43(1):263–269. doi: 10.1128/iai.43.1.263-269.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willems R., Paul A., van der Heide H. G., ter Avest A. R., Mooi F. R. Fimbrial phase variation in Bordetella pertussis: a novel mechanism for transcriptional regulation. EMBO J. 1990 Sep;9(9):2803–2809. doi: 10.1002/j.1460-2075.1990.tb07468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]