Abstract
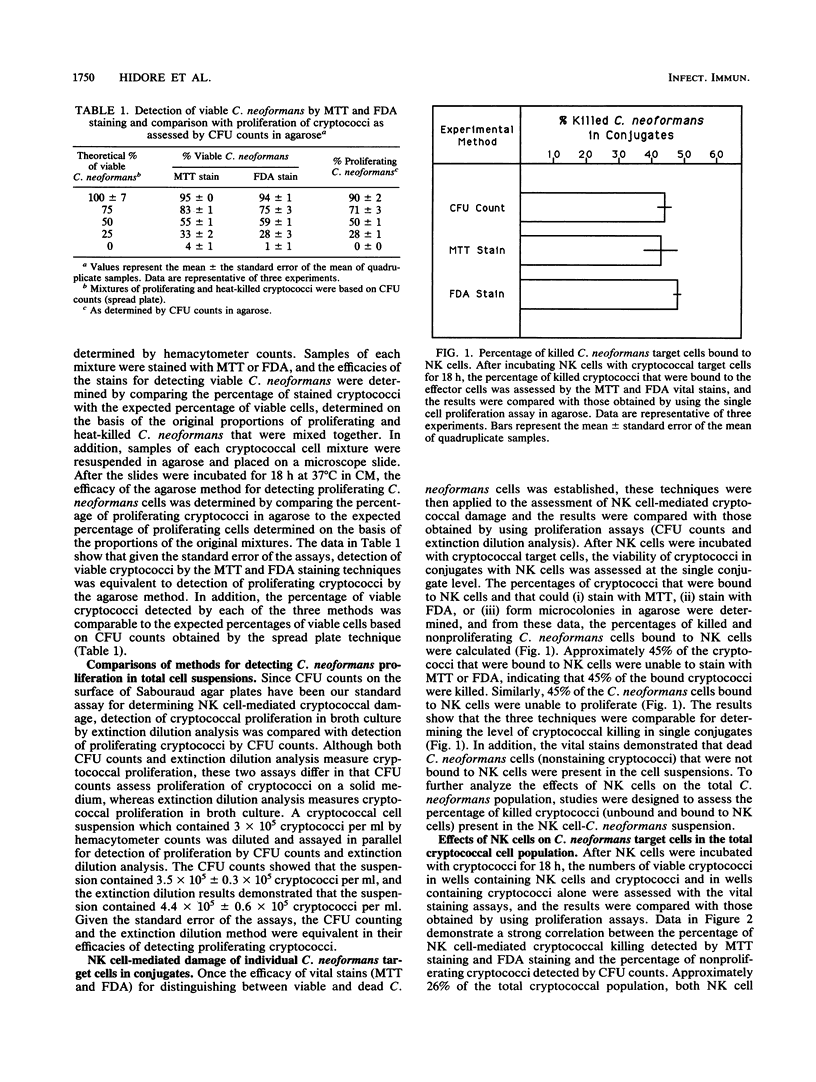
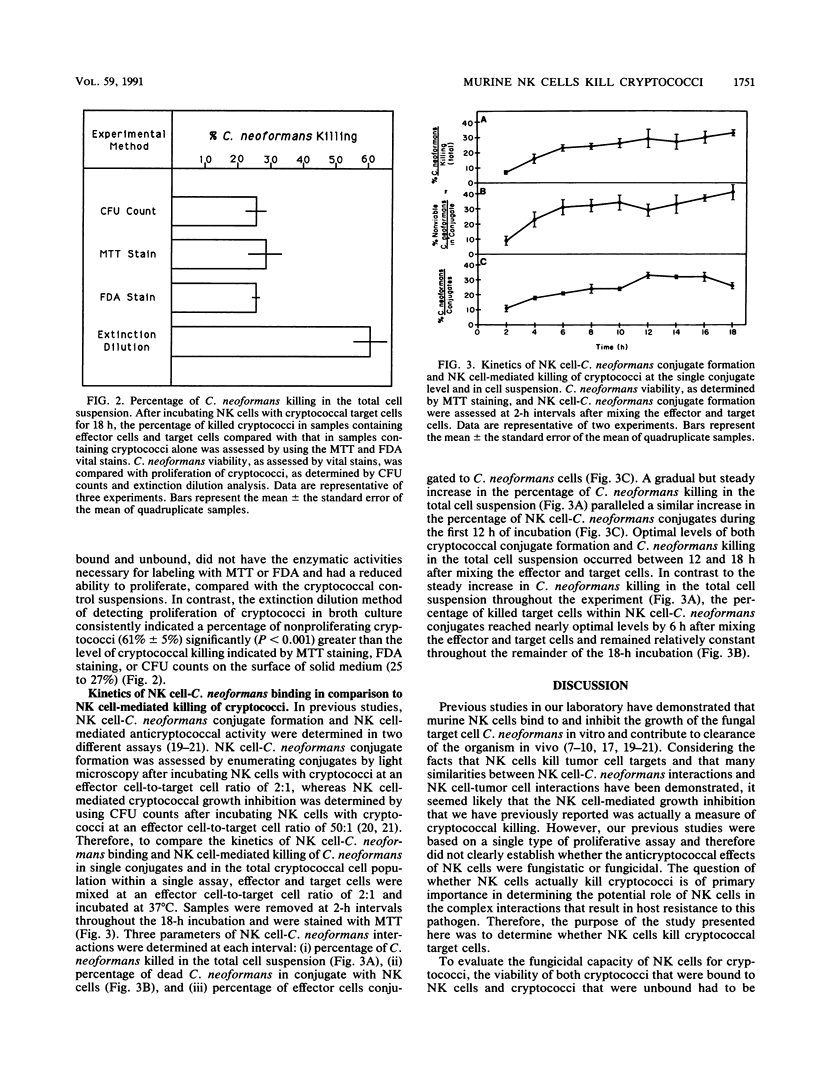
Murine natural killer (NK) cells have been shown to bind to and inhibit the growth of Cryptococcus neoformans in vitro and to contribute to clearance of the organism in vivo. However, it is unclear whether NK cells actually kill cryptococci or simply inhibit proliferation of the fungal target. Therefore, the studies presented here were designed to determine whether NK cells are fungicidal to C. neoformans targets. C. neoformans viability was determined on the basis of the metabolic function of two different enzyme systems, as measured by the two vital stains MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] and fluorescein diacetate. Cryptococcal viability, as determined by vital stains, was compared with cryptococcal proliferation, as measured by microcolony formation in agarose at the individual cell level and by CFU counts or extinction dilution analysis in the total cell suspension. Initial comparisons of the vital stains and proliferation assays indicated that these methods effectively distinguished between live and heat-killed cryptococci at the individual cell level and in the total cell suspensions. After cryptococci were incubated with murine NK cells for 18 h, vital stains demonstrated that at the single conjugate level and in the total cell suspension, NK cells kill bound C. neoformans target cells. In addition, the numbers of dead cryptococci in the NK cell-C. neoformans suspensions as determined by the vital stains were comparable to the numbers of cryptococci that were unable to proliferate. Kinetics of NK cell-mediated C. neoformans binding and killing at the single conjugate level and in the total cell suspension were assessed by MTT staining at 2-h intervals after mixing effector and target cells, and the data support the concept that NK cell-C. neoformans binding precedes cryptococcal death. Furthermore, unbound, dead fungal cells were observed in the NK cell-C. neoformans suspensions after 18 h, suggesting that NK cell-C. neoformans interactions may involve both effector cell recycling and killing of unbound cryptococci by soluble cytotoxic factors. In conclusion, the results of these studies firmly establish that NK cells kill C. neoformans.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beuchat L. R. Injury and repair of yeasts and moulds. Soc Appl Bacteriol Symp Ser. 1984;(12):293–308. [PubMed] [Google Scholar]
- COCHRAN W. G. Estimation of bacterial densities by means of the "most probable number". Biometrics. 1950 Jun;6(2):105–116. [PubMed] [Google Scholar]
- Calich V. L., Purchio A., Paula C. R. A new fluorescent viability test for fungi cells. Mycopathologia. 1979 Feb 28;66(3):175–177. doi: 10.1007/BF00683967. [DOI] [PubMed] [Google Scholar]
- Correa B., Purchio A., Paula C. R., Gambale W. Evaluation of a fluorescent method (fluorescein diacetate and ethidium bromide solution) in the study of the viability Cryptococcus neoformans strains. Mycopathologia. 1986 Nov;96(2):91–96. doi: 10.1007/BF00436666. [DOI] [PubMed] [Google Scholar]
- Grimm E. A., Thoma J. A., Bonavida B. Mechanism of cell-mediated cytotoxicity at the single cell level. II. Evidence for first-order kinetics of T cell-mediated cytolysis and for heterogeneity of lytic rate. J Immunol. 1979 Dec;123(6):2870–2877. [PubMed] [Google Scholar]
- Herberman R. B., Reynolds C. W., Ortaldo J. R. Mechanism of cytotoxicity by natural killer (NK) cells. Annu Rev Immunol. 1986;4:651–680. doi: 10.1146/annurev.iy.04.040186.003251. [DOI] [PubMed] [Google Scholar]
- Hidore M. R., Murphy J. W. Correlation of natural killer cell activity and clearance of Cryptococcus neoformans from mice after adoptive transfer of splenic nylon wool-nonadherent cells. Infect Immun. 1986 Feb;51(2):547–555. doi: 10.1128/iai.51.2.547-555.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidore M. R., Murphy J. W. Murine natural killer cell interactions with a fungal target, Cryptococcus neoformans. Infect Immun. 1989 Jul;57(7):1990–1997. doi: 10.1128/iai.57.7.1990-1997.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidore M. R., Murphy J. W. Natural cellular resistance of beige mice against Cryptococcus neoformans. J Immunol. 1986 Dec 1;137(11):3624–3631. [PubMed] [Google Scholar]
- Hidore M. R., Nabavi N., Reynolds C. W., Henkart P. A., Murphy J. W. Cytoplasmic components of natural killer cells limit the growth of Cryptococcus neoformans. J Leukoc Biol. 1990 Jul;48(1):15–26. doi: 10.1002/jlb.48.1.15. [DOI] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Levitz S. M., Diamond R. D. A rapid colorimetric assay of fungal viability with the tetrazolium salt MTT. J Infect Dis. 1985 Nov;152(5):938–945. doi: 10.1093/infdis/152.5.938. [DOI] [PubMed] [Google Scholar]
- Lipscomb M. F., Alvarellos T., Toews G. B., Tompkins R., Evans Z., Koo G., Kumar V. Role of natural killer cells in resistance to Cryptococcus neoformans infections in mice. Am J Pathol. 1987 Aug;128(2):354–361. [PMC free article] [PubMed] [Google Scholar]
- Luini W., Boraschi D., Alberti S., Aleotti A., Tagliabue A. Morphological characterization of a cell population responsible for natural killer activity. Immunology. 1981 Aug;43(4):663–668. [PMC free article] [PubMed] [Google Scholar]
- Mackey B. M. Lethal and sublethal effects of refrigeration, freezing and freeze-drying on micro-organisms. Soc Appl Bacteriol Symp Ser. 1984;(12):45–75. [PubMed] [Google Scholar]
- Murphy J. W., Cozad G. C. Immunological unresponsiveness induced by cryptococcal capsular polysaccharide assayed by the hemolytic plaque technique. Infect Immun. 1972 Jun;5(6):896–901. doi: 10.1128/iai.5.6.896-901.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy J. W., Hidore M. R., Nabavi N. Binding interactions of murine natural killer cells with the fungal target Cryptococcus neoformans. Infect Immun. 1991 Apr;59(4):1476–1488. doi: 10.1128/iai.59.4.1476-1488.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy J. W., McDaniel D. O. In vitro reactivity of natural killer (NK) cells against Cryptococcus neoformans. J Immunol. 1982 Apr;128(4):1577–1583. [PubMed] [Google Scholar]
- Nabavi N., Murphy J. W. In vitro binding of natural killer cells to Cryptococcus neoformans targets. Infect Immun. 1985 Oct;50(1):50–57. doi: 10.1128/iai.50.1.50-57.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russek E., Colwell R. R. Computation of most probable numbers. Appl Environ Microbiol. 1983 May;45(5):1646–1650. doi: 10.1128/aem.45.5.1646-1650.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinchieri G. Biology of natural killer cells. Adv Immunol. 1989;47:187–376. doi: 10.1016/S0065-2776(08)60664-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullberg M., Jondal M. Recycling and target binding capacity of human natural killer cells. J Exp Med. 1981 Mar 1;153(3):615–628. doi: 10.1084/jem.153.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]


