Abstract
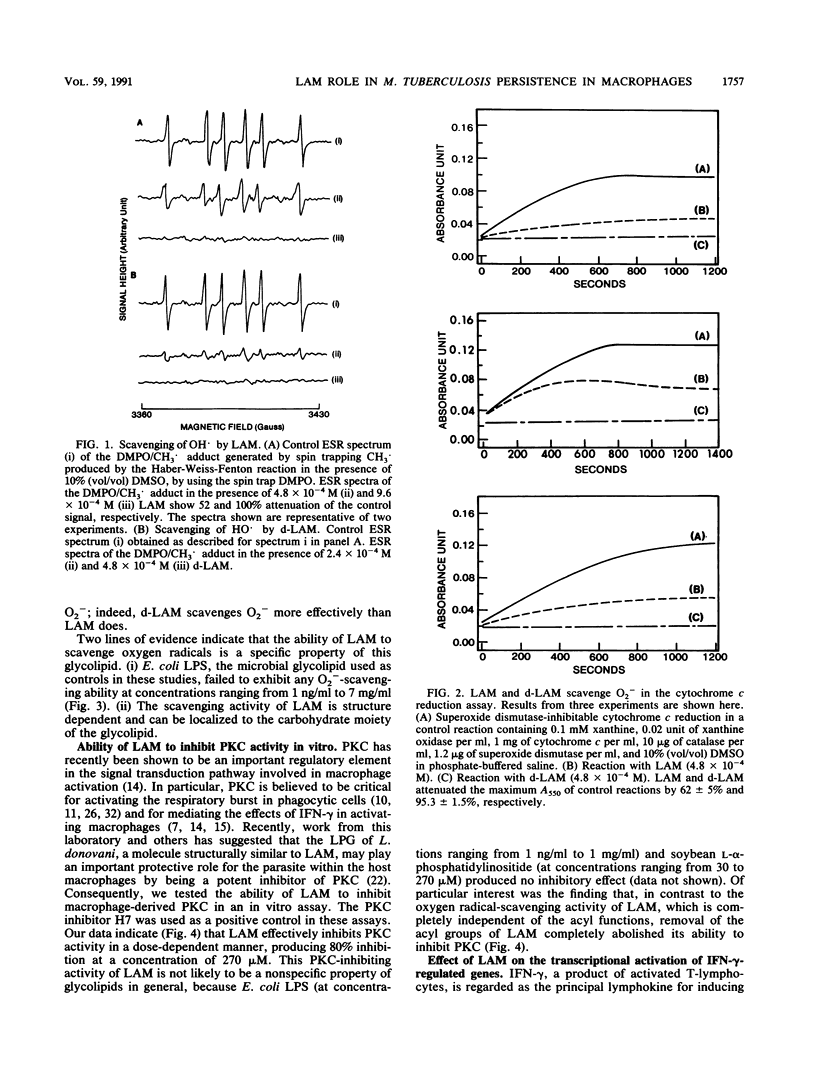
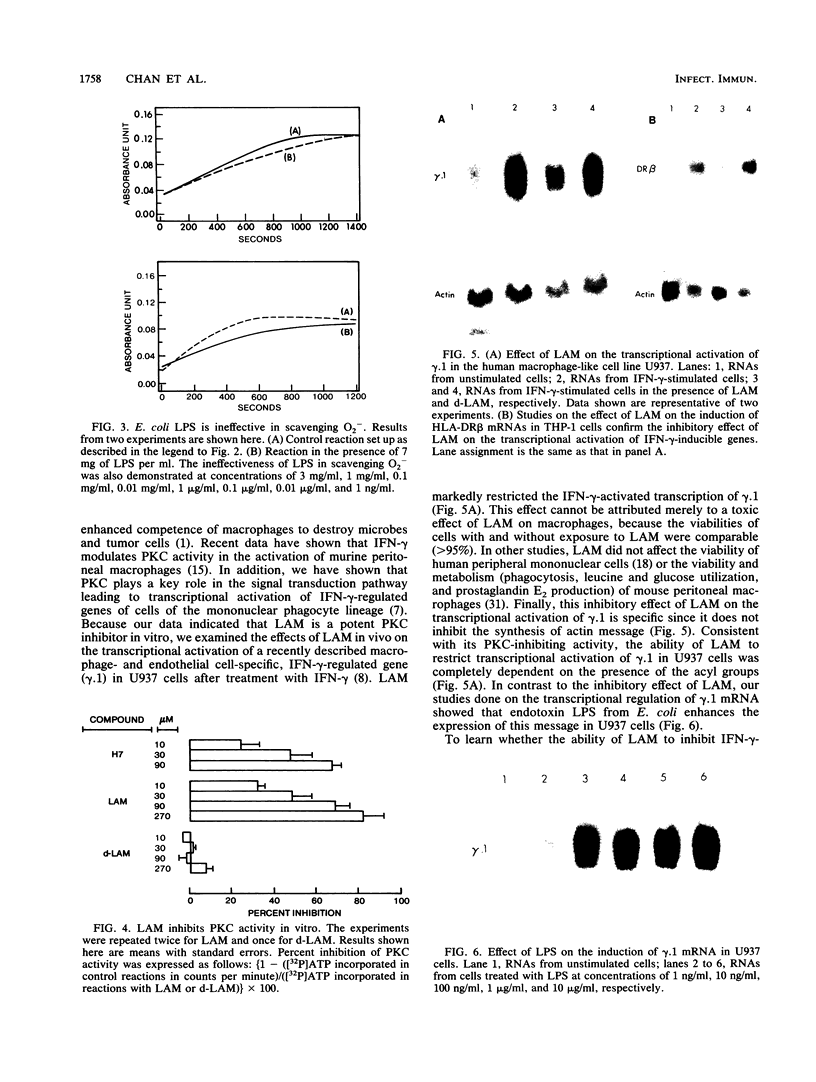
Mycobacterium tuberculosis and Mycobacterium leprae, the causative agents of tuberculosis and leprosy, respectively, produce large quantities of lipoarabinomannan (LAM), a highly immunogenic, cell wall-associated glycolipid. This molecule has been previously reported to be a potent inhibitor of gamma interferon-mediated activation of murine macrophages. Studies of the mechanism by which this mycobacterial glycolipid down-regulates macrophage effector functions provide evidence that LAM acts at several levels and that it can (i) scavenge potentially cytotoxic oxygen free radicals, (ii) inhibit protein kinase C activity, and (iii) block the transcriptional activation of gamma interferon-inducible genes in human macrophage-like cell lines. These results suggest that LAM can inhibit macrophage activation and triggering and cytocidal activity and that it may represent a chemically defined virulence factor contributing to the persistence of mycobacteria within mononuclear phagocytes.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. O., Hamilton T. A. Molecular transductional mechanisms by which IFN gamma and other signals regulate macrophage development. Immunol Rev. 1987 Jun;97:5–27. doi: 10.1111/j.1600-065x.1987.tb00514.x. [DOI] [PubMed] [Google Scholar]
- Brennan P. J. Structure of mycobacteria: recent developments in defining cell wall carbohydrates and proteins. Rev Infect Dis. 1989 Mar-Apr;11 (Suppl 2):S420–S430. doi: 10.1093/clinids/11.supplement_2.s420. [DOI] [PubMed] [Google Scholar]
- Chan J., Fujiwara T., Brennan P., McNeil M., Turco S. J., Sibille J. C., Snapper M., Aisen P., Bloom B. R. Microbial glycolipids: possible virulence factors that scavenge oxygen radicals. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2453–2457. doi: 10.1073/pnas.86.7.2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Daniel T. M. Robert Koch, tuberculosis, and the subsequent history of medicine. Am Rev Respir Dis. 1982 Mar;125(3 Pt 2):1–3. doi: 10.1164/arrd.1982.125.3P2.1. [DOI] [PubMed] [Google Scholar]
- Fan X. D., Goldberg M., Bloom B. R. Interferon-gamma-induced transcriptional activation is mediated by protein kinase C. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5122–5125. doi: 10.1073/pnas.85.14.5122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X. D., Stark G. R., Bloom B. R. Molecular cloning of a gene selectively induced by gamma interferon from human macrophage cell line U937. Mol Cell Biol. 1989 May;9(5):1922–1928. doi: 10.1128/mcb.9.5.1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flesch I., Kaufmann S. H. Mycobacterial growth inhibition by interferon-gamma-activated bone marrow macrophages and differential susceptibility among strains of Mycobacterium tuberculosis. J Immunol. 1987 Jun 15;138(12):4408–4413. [PubMed] [Google Scholar]
- Gavioli R., Spisani S., Giuliani A., Traniello S. Protein kinase C mediates human neutrophil cytotoxicity. Biochem Biophys Res Commun. 1987 Nov 13;148(3):1290–1294. doi: 10.1016/s0006-291x(87)80273-5. [DOI] [PubMed] [Google Scholar]
- Gennaro R., Florio C., Romeo D. Activation of protein kinase C in neutrophil cytoplasts. Localization of protein substrates and possible relationship with stimulus-response coupling. FEBS Lett. 1985 Jan 28;180(2):185–190. doi: 10.1016/0014-5793(85)81068-1. [DOI] [PubMed] [Google Scholar]
- Hahn H., Kaufmann S. H. The role of cell-mediated immunity in bacterial infections. Rev Infect Dis. 1981 Nov-Dec;3(6):1221–1250. doi: 10.1093/clinids/3.6.1221. [DOI] [PubMed] [Google Scholar]
- Hamilton T. A., Becton D. L., Somers S. D., Gray P. W., Adams D. O. Interferon-gamma modulates protein kinase C activity in murine peritoneal macrophages. J Biol Chem. 1985 Feb 10;260(3):1378–1381. [PubMed] [Google Scholar]
- Hunter S. W., Gaylord H., Brennan P. J. Structure and antigenicity of the phosphorylated lipopolysaccharide antigens from the leprosy and tubercle bacilli. J Biol Chem. 1986 Sep 15;261(26):12345–12351. [PubMed] [Google Scholar]
- Kaplan G., Gandhi R. R., Weinstein D. E., Levis W. R., Patarroyo M. E., Brennan P. J., Cohn Z. A. Mycobacterium leprae antigen-induced suppression of T cell proliferation in vitro. J Immunol. 1987 May 1;138(9):3028–3034. [PubMed] [Google Scholar]
- Le Peuch C. J., Ballester R., Rosen O. M. Purified rat brain calcium- and phospholipid-dependent protein kinase phosphorylates ribosomal protein S6. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6858–6862. doi: 10.1073/pnas.80.22.6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeely T. B., Turco S. J. Inhibition of protein kinase C activity by the Leishmania donovani lipophosphoglycan. Biochem Biophys Res Commun. 1987 Oct 29;148(2):653–657. doi: 10.1016/0006-291x(87)90926-0. [DOI] [PubMed] [Google Scholar]
- Molloy A., Gaudernack G., Levis W. R., Cohn Z. A., Kaplan G. Suppression of T-cell proliferation by Mycobacterium leprae and its products: the role of lipopolysaccharide. Proc Natl Acad Sci U S A. 1990 Feb;87(3):973–977. doi: 10.1073/pnas.87.3.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulder J. W. Comparative biology of intracellular parasitism. Microbiol Rev. 1985 Sep;49(3):298–337. doi: 10.1128/mr.49.3.298-337.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontremoli S., Melloni E., Salamino F., Sparatore B., Michetti M., Sacco O., Horecker B. L. Activation of NADPH oxidase and phosphorylation of membrane proteins in human neutrophils: coordinate inhibition by a surface antigen-directed monoclonal antibody. Biochem Biophys Res Commun. 1986 Nov 14;140(3):1121–1126. doi: 10.1016/0006-291x(86)90751-5. [DOI] [PubMed] [Google Scholar]
- Rook G. A., Steele J., Ainsworth M., Champion B. R. Activation of macrophages to inhibit proliferation of Mycobacterium tuberculosis: comparison of the effects of recombinant gamma-interferon on human monocytes and murine peritoneal macrophages. Immunology. 1986 Nov;59(3):333–338. [PMC free article] [PubMed] [Google Scholar]
- SBARRA A. J., KARNOVSKY M. L. The biochemical basis of phagocytosis. I. Metabolic changes during the ingestion of particles by polymorphonuclear leukocytes. J Biol Chem. 1959 Jun;234(6):1355–1362. [PubMed] [Google Scholar]
- Samuni A., Carmichael A. J., Russo A., Mitchell J. B., Riesz P. On the spin trapping and ESR detection of oxygen-derived radicals generated inside cells. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7593–7597. doi: 10.1073/pnas.83.20.7593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibille J. C., Doi K., Aisen P. Hydroxyl radical formation and iron-binding proteins. Stimulation by the purple acid phosphatases. J Biol Chem. 1987 Jan 5;262(1):59–62. [PubMed] [Google Scholar]
- Sibley L. D., Hunter S. W., Brennan P. J., Krahenbuhl J. L. Mycobacterial lipoarabinomannan inhibits gamma interferon-mediated activation of macrophages. Infect Immun. 1988 May;56(5):1232–1236. doi: 10.1128/iai.56.5.1232-1236.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley L. D., Krahenbuhl J. L. Induction of unresponsiveness to gamma interferon in macrophages infected with Mycobacterium leprae. Infect Immun. 1988 Aug;56(8):1912–1919. doi: 10.1128/iai.56.8.1912-1919.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundström C., Nilsson K. Establishment and characterization of a human histiocytic lymphoma cell line (U-937). Int J Cancer. 1976 May 15;17(5):565–577. doi: 10.1002/ijc.2910170504. [DOI] [PubMed] [Google Scholar]
- Tsuchiya S., Yamabe M., Yamaguchi Y., Kobayashi Y., Konno T., Tada K. Establishment and characterization of a human acute monocytic leukemia cell line (THP-1). Int J Cancer. 1980 Aug;26(2):171–176. doi: 10.1002/ijc.2910260208. [DOI] [PubMed] [Google Scholar]
- Wilson E., Olcott M. C., Bell R. M., Merrill A. H., Jr, Lambeth J. D. Inhibition of the oxidative burst in human neutrophils by sphingoid long-chain bases. Role of protein kinase C in activation of the burst. J Biol Chem. 1986 Sep 25;261(27):12616–12623. [PubMed] [Google Scholar]