Abstract
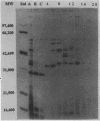
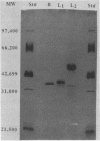
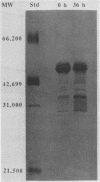
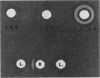
Previously we described the partial purification of a novel hemolysin from Bacillus cereus and showed that hemolytic activity required the combined action of at least two components, called B and L to signify their cell-binding and cell-lytic roles in this activity. On further purification, as described in the present article, a combination of anion-exchange chromatography and polyacrylamide gel electrophoresis separated three proteins, B, L1, and L2 (35, 36, and 45 kDa, respectively). Individually, these proteins were inactive in hemolytic and vascular permeability assays, and combinations of B and L1 or B and L2 were either inactive or slightly active. Combinations of all three moieties produced the unique ring-shaped zone of hemolysis, a previously described characteristic of hemolysin BL, as well as edema and bluing in the vascular permeability assay. Since the vascular permeability assay is known to correlate with enterotoxicity, these results suggest that hemolysin BL is enterotoxigenic. Furthermore, the molecular weights and isoelectric point values of the hemolysin BL components are consistent with those described by others for the multicomponent diarrheal enterotoxin of B. cereus. Immunofluorescent staining of B-treated erythrocytes confirmed that B binds to cells as an initial step required before the L components can act to cause cell lysis.
Full text
PDF

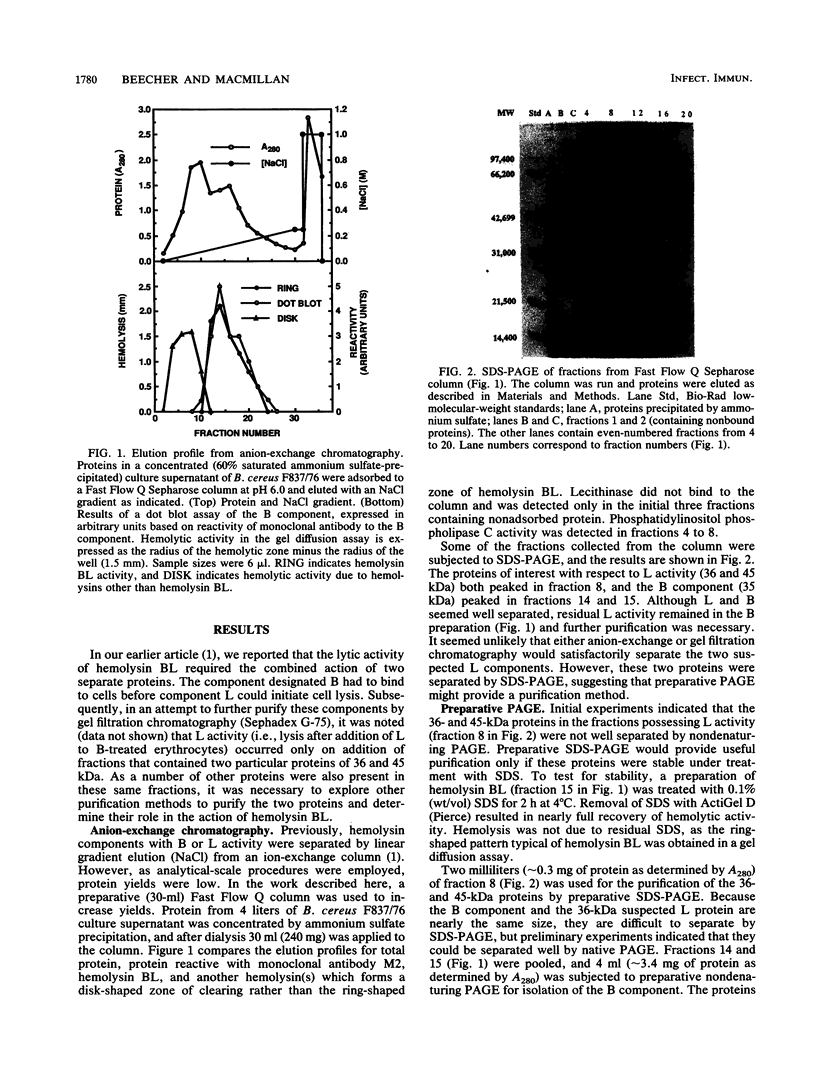

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beecher D. J., MacMillan J. D. A novel bicomponent hemolysin from Bacillus cereus. Infect Immun. 1990 Jul;58(7):2220–2227. doi: 10.1128/iai.58.7.2220-2227.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernheimer A. W. Assay of hemolytic toxins. Methods Enzymol. 1988;165:213–217. doi: 10.1016/s0076-6879(88)65033-6. [DOI] [PubMed] [Google Scholar]
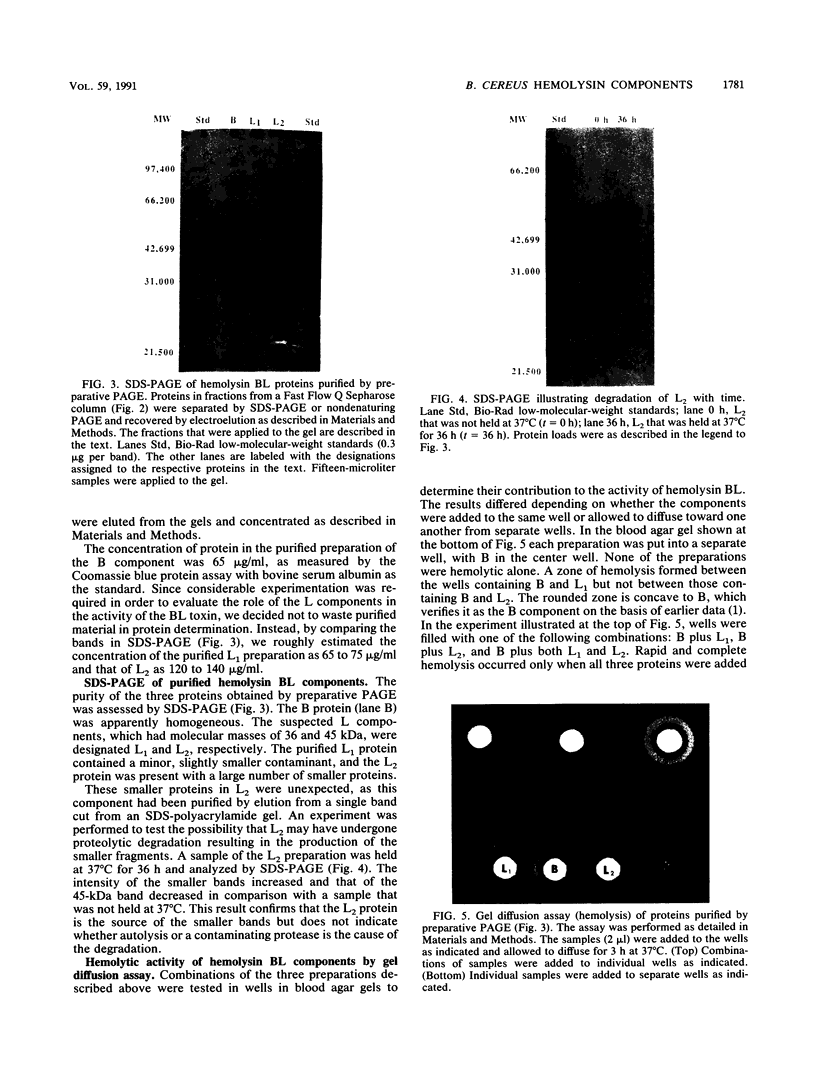
- Bernheimer A. W., Grushoff P. Cereolysin: production, purification and partial characterization. J Gen Microbiol. 1967 Jan;46(1):143–150. doi: 10.1099/00221287-46-1-143. [DOI] [PubMed] [Google Scholar]
- Bitsaev A. R., Ezepchuk Iu V. Molekuliarnaia priroda patogennogo deistviia, vyzyvaemogo B. cereus. Mol Gen Mikrobiol Virusol. 1987 Jul;(7):18–23. [PubMed] [Google Scholar]
- FOSSUM K. SEPARATION OF HEMOLYSIN AND EGG YOLK TURBIDITY FACTOR IN CELL-FREE EXTRACTS OF BACILLUS CEREUS. Acta Pathol Microbiol Scand. 1963;59:400–406. doi: 10.1111/j.1699-0463.1963.tb01810.x. [DOI] [PubMed] [Google Scholar]
- Gilmore M. S., Cruz-Rodz A. L., Leimeister-Wächter M., Kreft J., Goebel W. A Bacillus cereus cytolytic determinant, cereolysin AB, which comprises the phospholipase C and sphingomyelinase genes: nucleotide sequence and genetic linkage. J Bacteriol. 1989 Feb;171(2):744–753. doi: 10.1128/jb.171.2.744-753.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glatz B. A., Spira W. M., Goepfert J. M. Alteration of vascular permeability in rabbits by culture filtrates of Bacillus cereus and related species. Infect Immun. 1974 Aug;10(2):299–303. doi: 10.1128/iai.10.2.299-303.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikezawa H., Mori M., Ohyabu T., Taguchi R. Studies on sphingomyelinase of Bacillus cereus. I. Purification and properties. Biochim Biophys Acta. 1978 Feb 27;528(2):247–256. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leppla S. H. Production and purification of anthrax toxin. Methods Enzymol. 1988;165:103–116. doi: 10.1016/s0076-6879(88)65019-1. [DOI] [PubMed] [Google Scholar]
- Linder R. Alteration of mammalian membranes by the cooperative and antagonistic actions of bacterial proteins. Biochim Biophys Acta. 1984 Dec 4;779(4):423–435. doi: 10.1016/0304-4157(84)90019-4. [DOI] [PubMed] [Google Scholar]
- Neuhoff V., Arold N., Taube D., Ehrhardt W. Improved staining of proteins in polyacrylamide gels including isoelectric focusing gels with clear background at nanogram sensitivity using Coomassie Brilliant Blue G-250 and R-250. Electrophoresis. 1988 Jun;9(6):255–262. doi: 10.1002/elps.1150090603. [DOI] [PubMed] [Google Scholar]
- Pendleton I. R., Bernheimer A. W., Grushoff P. Purification and partial characterization of hemolysins from Bacillus thuringiensis. J Invertebr Pathol. 1973 Mar;21(2):131–135. doi: 10.1016/0022-2011(73)90192-4. [DOI] [PubMed] [Google Scholar]
- SLEIN M. W., LOGAN G. F., Jr Partial purification and properties of two phospholipases of Bacillus cereus. J Bacteriol. 1963 Feb;85:369–381. doi: 10.1128/jb.85.2.369-381.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
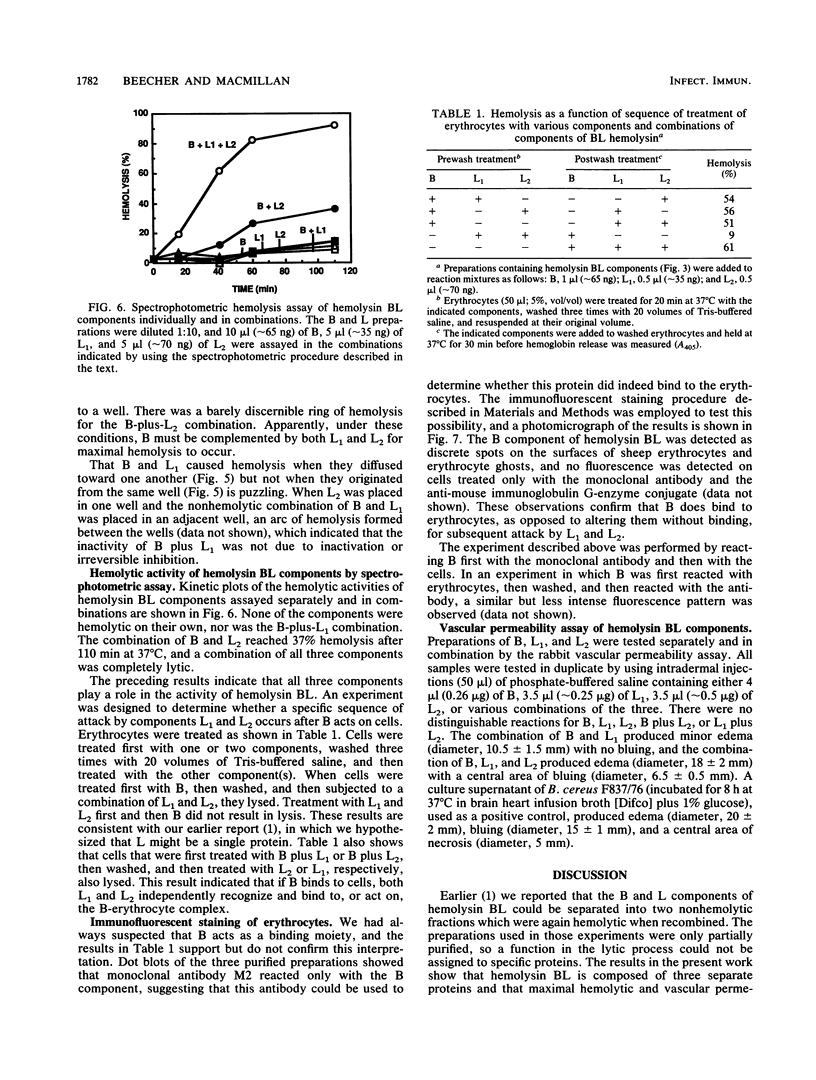
- Stoscheck C. M. Quantitation of protein. Methods Enzymol. 1990;182:50–68. doi: 10.1016/0076-6879(90)82008-p. [DOI] [PubMed] [Google Scholar]
- Tasheva B., Dessev G. Artifacts in sodium dodecyl sulfate-polyacrylamide gel electrophoresis due to 2-mercaptoethanol. Anal Biochem. 1983 Feb 15;129(1):98–102. doi: 10.1016/0003-2697(83)90057-x. [DOI] [PubMed] [Google Scholar]
- Thompson N. E., Ketterhagen M. J., Bergdoll M. S., Schantz E. J. Isolation and some properties of an enterotoxin produced by Bacillus cereus. Infect Immun. 1984 Mar;43(3):887–894. doi: 10.1128/iai.43.3.887-894.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbull P. C. Bacillus cereus toxins. Pharmacol Ther. 1981;13(3):453–505. doi: 10.1016/0163-7258(81)90026-7. [DOI] [PubMed] [Google Scholar]
- Turnbull P. C., Kramer J. M., Jørgensen K., Gilbert R. J., Melling J. Properties and production characteristics of vomiting, diarrheal, and necrotizing toxins of Bacillus cereus. Am J Clin Nutr. 1979 Jan;32(1):219–228. doi: 10.1093/ajcn/32.1.219. [DOI] [PubMed] [Google Scholar]