Abstract
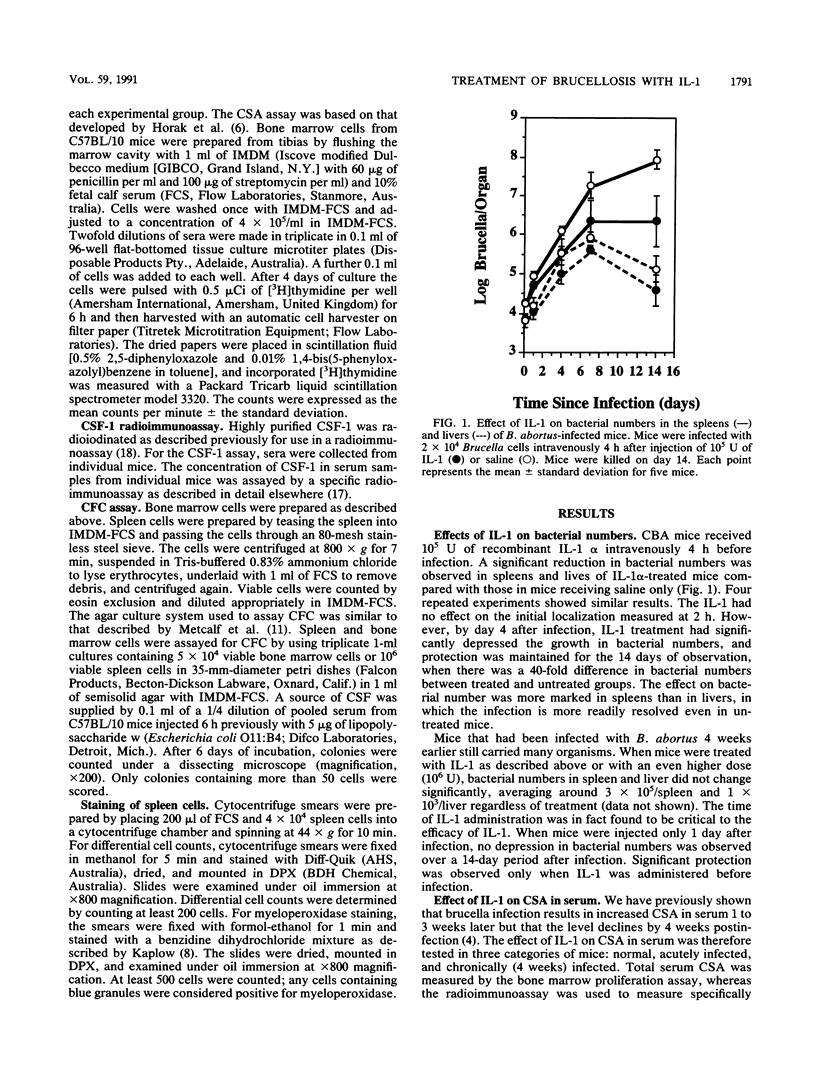
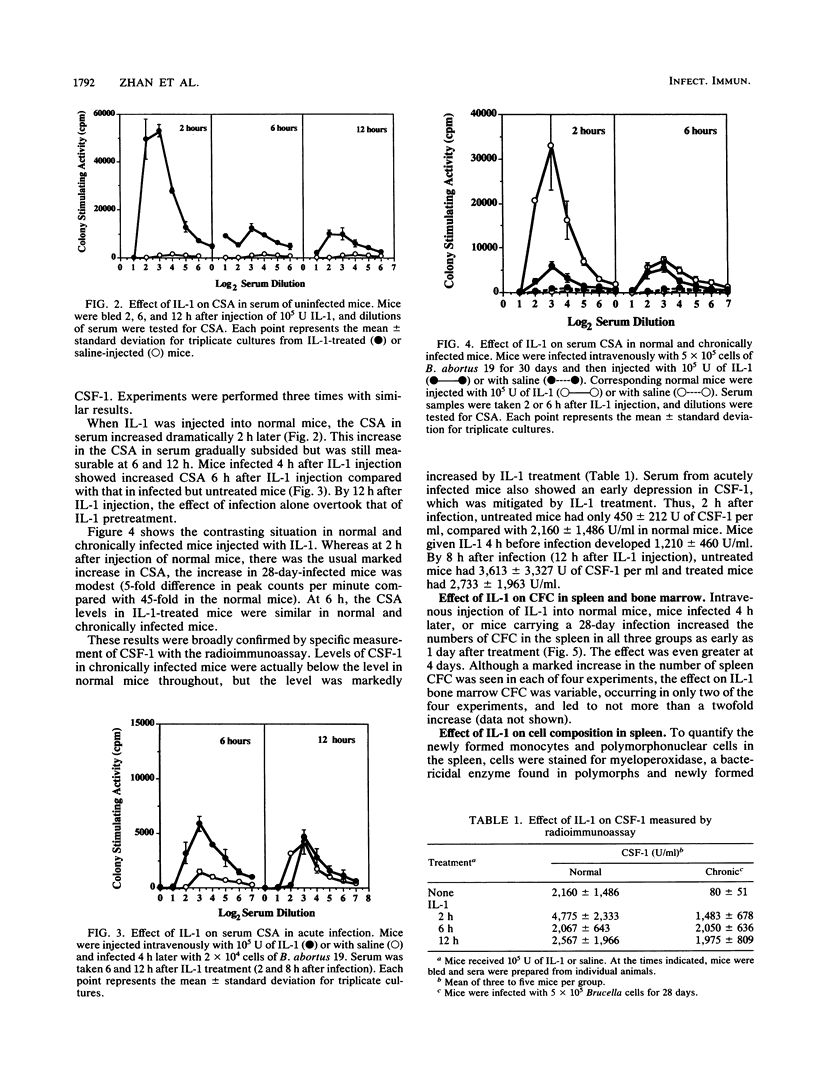
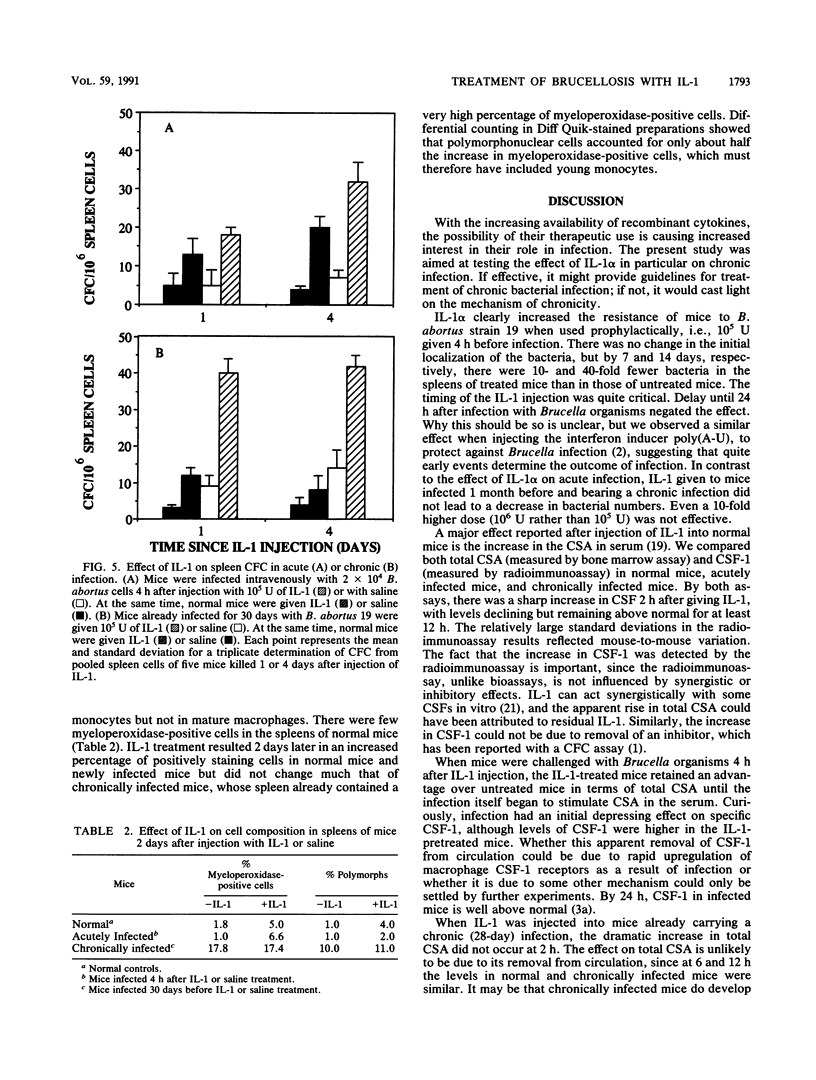
Intravenously injected recombinant human interleukin-1 alpha (IL-1 alpha) given to mice 4 h before infection with Brucella abortus 19 depressed the growth of bacteria in the spleen and liver. However, the same dose (10(5) U) or a 10-fold higher dose was not able to decrease numbers of bacteria when given to chronically infected mice. IL-1 injected into normal mice induced a dramatic increase 2 h later in colony-stimulating activity in serum, measured by bone marrow proliferation, and in colony-stimulating factor 1, measured by radioimmunoassay. Colony-stimulating factor levels declined but remained higher than normal for at least 12 h. The early peak stimulation was not observed in chronically infected mice, but the more prolonged elevation was. As a result of IL-1 treatment, the number of colony-forming cells, especially in the spleen, was increased in normal and acutely or chronically infected mice. Myeloperoxidase staining of newly formed monocytes and polymorphonuclear cells in the spleen revealed an increase in the number of these cells in normal and acutely infected mice as a result of IL-1 treatment, but there was no increase in the already high numbers in chronically infected mice. The relationship between these observations and the basis of chronic infection are discussed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cheers C., Cone R. E. Effect of polyadenine: polyuridine on brucellosis in conventional and congenitally athymic mice. J Immunol. 1974 Apr;112(4):1535–1539. [PubMed] [Google Scholar]
- Cheers C., Pagram F. Macrophage activation during experimental murine brucellosis: a basis for chronic infection. Infect Immun. 1979 Feb;23(2):197–205. doi: 10.1128/iai.23.2.197-205.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheers C., Stanley E. R. Macrophage production during murine listeriosis: colony-stimulating factor 1 (CSF-1) and CSF-1-binding cells in genetically resistant and susceptible mice. Infect Immun. 1988 Nov;56(11):2972–2978. doi: 10.1128/iai.56.11.2972-2978.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheers C., Young A. M. Serum colony stimulating activity and colony forming cells in murine brucellosis: relationship to immunopathology. Microb Pathog. 1987 Sep;3(3):185–194. doi: 10.1016/0882-4010(87)90095-7. [DOI] [PubMed] [Google Scholar]
- Czuprynski C. J., Brown J. F., Young K. M., Cooley A. J., Kurtz R. S. Effects of murine recombinant interleukin 1 alpha on the host response to bacterial infection. J Immunol. 1988 Feb 1;140(3):962–968. [PubMed] [Google Scholar]
- Horak H., Turner A. R., Shaw A. R., Yau O. W. Stimulation of [3H]thymidine uptake in mouse marrow by granulocyte-macrophage colony stimulating factor from mouse lung conditioned medium. J Immunol Methods. 1983 Jan 28;56(2):253–260. doi: 10.1016/0022-1759(83)90417-9. [DOI] [PubMed] [Google Scholar]
- Johnson C. S., Keckler D. J., Topper M. I., Braunschweiger P. G., Furmanski P. In vivo hematopoietic effects of recombinant interleukin-1 alpha in mice: stimulation of granulocytic, monocytic, megakaryocytic, and early erythroid progenitors, suppression of late-stage erythropoiesis, and reversal of erythroid suppression with erythropoietin. Blood. 1989 Feb 15;73(3):678–683. [PubMed] [Google Scholar]
- KAPLOW L. S. SIMPLIFIED MYELOPEROXIDASE STAIN USING BENZIDINE DIHYDROCHLORIDE. Blood. 1965 Aug;26:215–219. [PubMed] [Google Scholar]
- MACKANESS G. B. THE IMMUNOLOGICAL BASIS OF ACQUIRED CELLULAR RESISTANCE. J Exp Med. 1964 Jul 1;120:105–120. doi: 10.1084/jem.120.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf D., Johnson G. R., Mandel T. E. Colony formation in agar by multipotential hemopoietic cells. J Cell Physiol. 1979 Feb;98(2):401–420. doi: 10.1002/jcp.1040980216. [DOI] [PubMed] [Google Scholar]
- Metcalf D. The molecular biology and functions of the granulocyte-macrophage colony-stimulating factors. Blood. 1986 Feb;67(2):257–267. [PubMed] [Google Scholar]
- Neta R., Sztein M. B., Oppenheim J. J., Gillis S., Douches S. D. The in vivo effects of interleukin 1. I. Bone marrow cells are induced to cycle after administration of interleukin 1. J Immunol. 1987 Sep 15;139(6):1861–1866. [PubMed] [Google Scholar]
- Ozaki Y., Ohashi T., Minami A., Nakamura S. Enhanced resistance of mice to bacterial infection induced by recombinant human interleukin-1a. Infect Immun. 1987 Jun;55(6):1436–1440. doi: 10.1128/iai.55.6.1436-1440.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quesenberry P., Cohen H., Levin J., Sullivan R., Bealmear P., Ryan M. Effects of bacterial infection and irradiation on serum colony-stimulating factor levels in tolerant and nontolerant CF1 mice. Blood. 1978 Feb;51(2):229–244. [PubMed] [Google Scholar]
- Shadduck R. K., Waheed A., Wing E. J. Demonstration of a blood-bone marrow barrier to macrophage colony-stimulating factor. Blood. 1989 Jan;73(1):68–73. [PubMed] [Google Scholar]
- Staber F. G., Johnson G. R. The responses of hemopoietic precursor cells in mice to bacterial cell-wall components. J Cell Physiol. 1980 Oct;105(1):143–152. doi: 10.1002/jcp.1041050116. [DOI] [PubMed] [Google Scholar]
- Stanley E. R. Colony-stimulating factor (CSF) radioimmunoassay: detection of a CSF subclass stimulating macrophage production. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2969–2973. doi: 10.1073/pnas.76.6.2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley E. R. The macrophage colony-stimulating factor, CSF-1. Methods Enzymol. 1985;116:564–587. doi: 10.1016/s0076-6879(85)16044-1. [DOI] [PubMed] [Google Scholar]
- Vogel S. N., Douches S. D., Kaufman E. N., Neta R. Induction of colony stimulating factor in vivo by recombinant interleukin 1 alpha and recombinant tumor necrosis factor alpha 1. J Immunol. 1987 Apr 1;138(7):2143–2148. [PubMed] [Google Scholar]
- Young E. J., Borchert M., Kretzer F. L., Musher D. M. Phagocytosis and killing of Brucella by human polymorphonuclear leukocytes. J Infect Dis. 1985 Apr;151(4):682–690. doi: 10.1093/infdis/151.4.682. [DOI] [PubMed] [Google Scholar]
- Zhou Y. Q., Stanley E. R., Clark S. C., Hatzfeld J. A., Levesque J. P., Federici C., Watt S. M., Hatzfeld A. Interleukin-3 and interleukin-1 alpha allow earlier bone marrow progenitors to respond to human colony-stimulating factor 1. Blood. 1988 Dec;72(6):1870–1874. [PubMed] [Google Scholar]



