Abstract
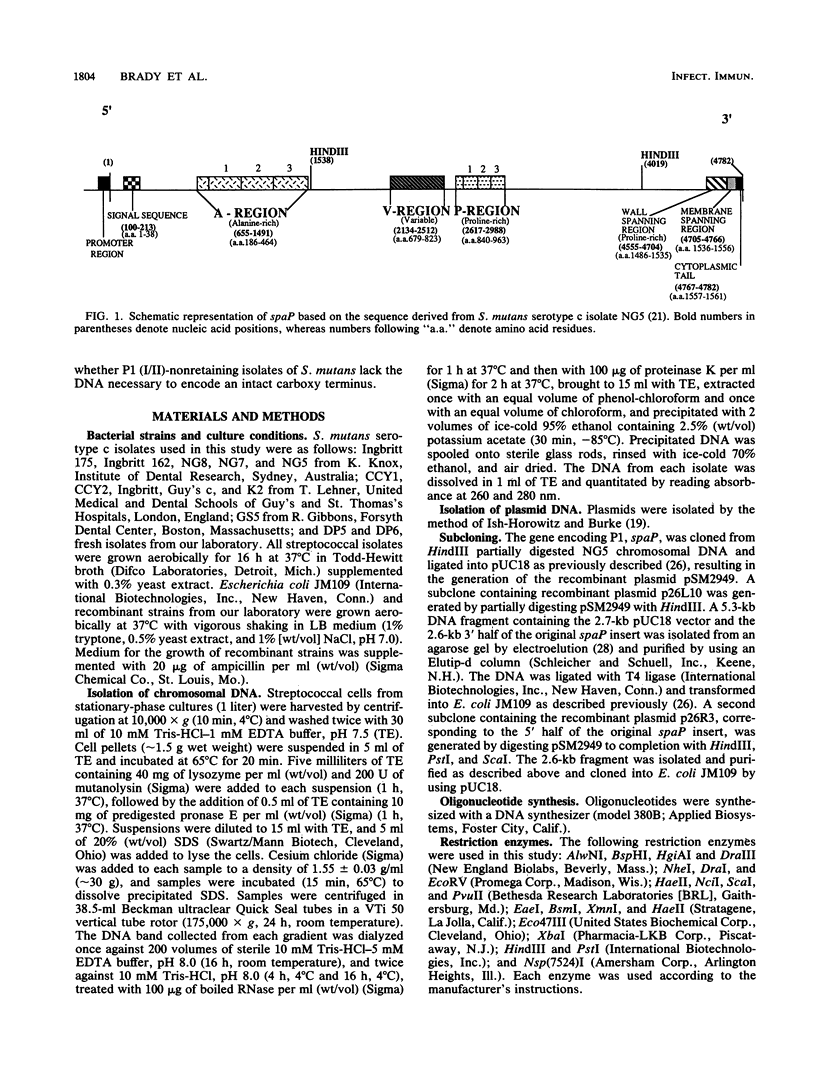
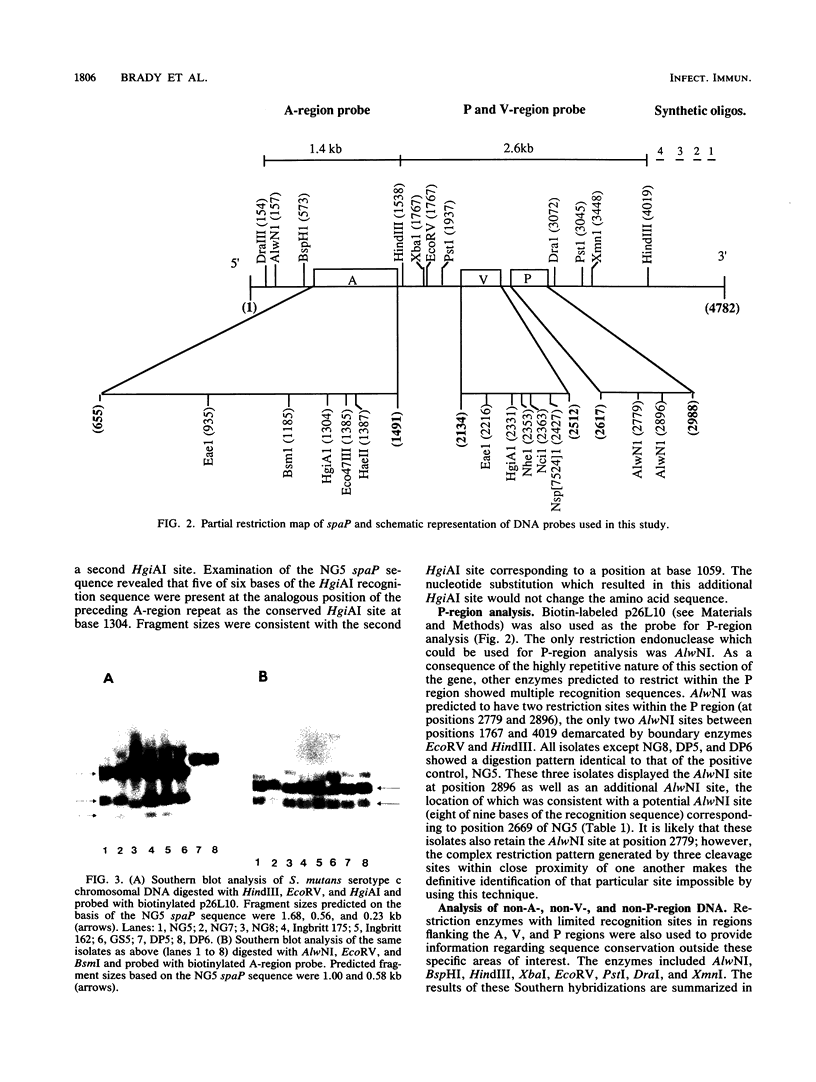
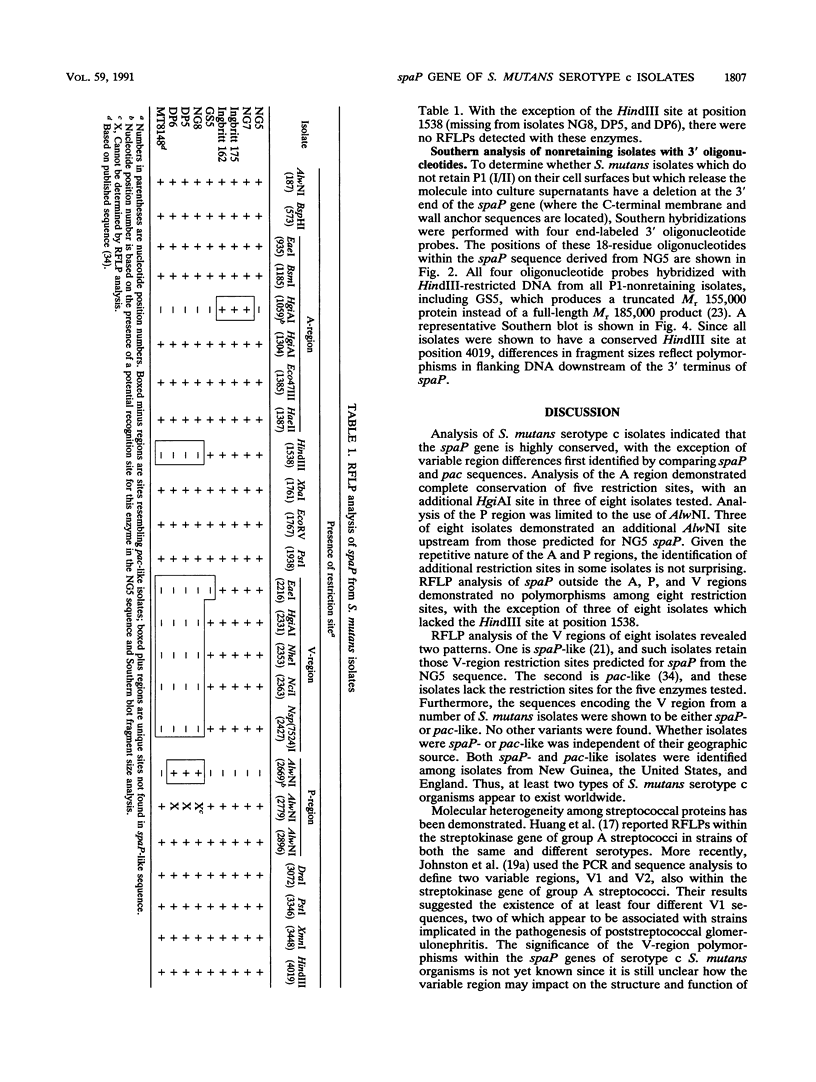
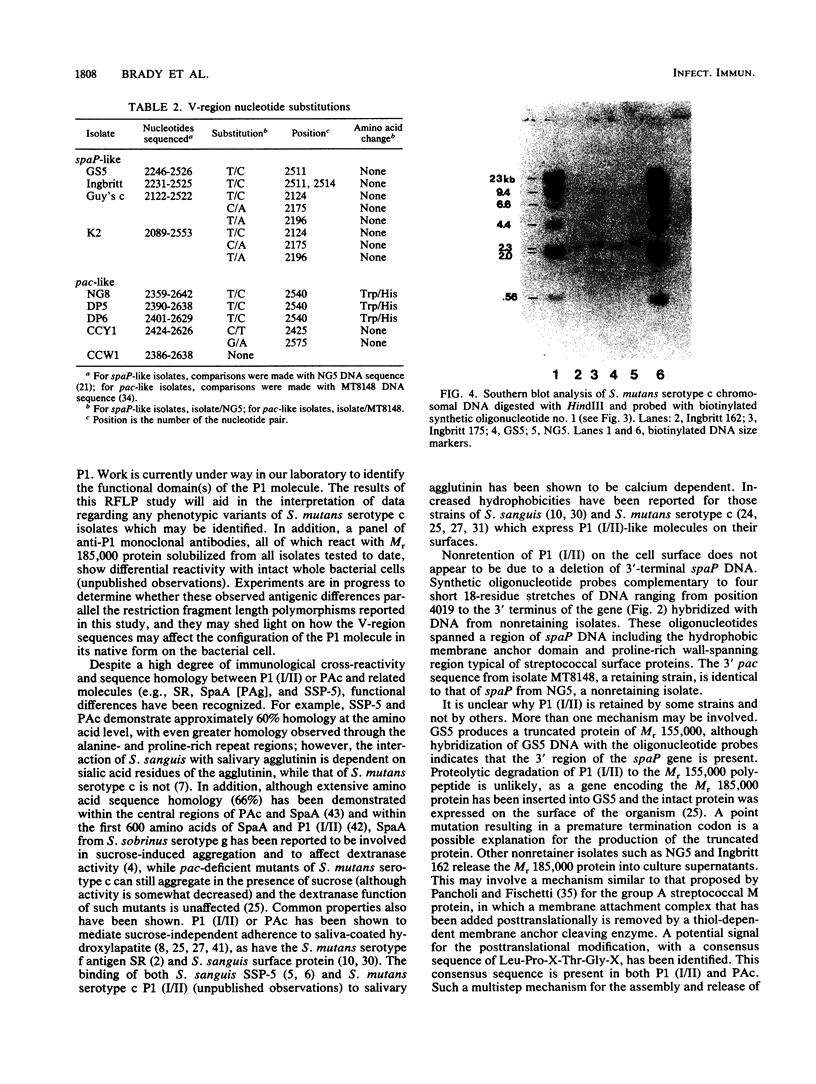
A restriction fragment length polymorphism study was undertaken to determine the extent and location of heterogeneity within spaP encoding the Mr 185,000 cell surface protein P1 (antigen I/II) of Streptococcus mutans serotype c isolates. The gene was found to be highly conserved except for a central variable (V) region predicted to encode less than 150 amino acids. Sequence analysis identified two V-region variants. These differences were independent of the geographic source of the isolates. Southern analysis using synthetic oligonucleotide probes indicated that nonretention of P1 (I/II) by some isolates is not due to a deletion of the 3'-terminal DNA necessary to encode an intact carboxy terminus.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abiko Y., Hayakawa M., Aoki H., Saito S., Takiguchi H. Cloning of the gene for cell-surface protein antigen A from Streptococcus sobrinus (serotype d). Arch Oral Biol. 1989;34(7):571–575. doi: 10.1016/0003-9969(89)90096-4. [DOI] [PubMed] [Google Scholar]
- Ackermans F., Klein J. P., Ogier J., Bazin H., Cormont F., Frank R. M. Purification and characterization of a saliva-interacting cell-wall protein from Streptococcus mutans serotype f by using monoclonal-antibody immunoaffinity chromatography. Biochem J. 1985 May 15;228(1):211–217. doi: 10.1042/bj2280211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayakawa G. Y., Boushell L. W., Crowley P. J., Erdos G. W., McArthur W. P., Bleiweis A. S. Isolation and characterization of monoclonal antibodies specific for antigen P1, a major surface protein of mutans streptococci. Infect Immun. 1987 Nov;55(11):2759–2767. doi: 10.1128/iai.55.11.2759-2767.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demuth D. R., Berthold P., Leboy P. S., Golub E. E., Davis C. A., Malamud D. Saliva-mediated aggregation of Enterococcus faecalis transformed with a Streptococcus sanguis gene encoding the SSP-5 surface antigen. Infect Immun. 1989 May;57(5):1470–1475. doi: 10.1128/iai.57.5.1470-1475.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demuth D. R., Davis C. A., Corner A. M., Lamont R. J., Leboy P. S., Malamud D. Cloning and expression of a Streptococcus sanguis surface antigen that interacts with a human salivary agglutinin. Infect Immun. 1988 Sep;56(9):2484–2490. doi: 10.1128/iai.56.9.2484-2490.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demuth D. R., Golub E. E., Malamud D. Streptococcal-host interactions. Structural and functional analysis of a Streptococcus sanguis receptor for a human salivary glycoprotein. J Biol Chem. 1990 May 5;265(13):7120–7126. [PubMed] [Google Scholar]
- Fischetti V. A. Streptococcal M protein: molecular design and biological behavior. Clin Microbiol Rev. 1989 Jul;2(3):285–314. doi: 10.1128/cmr.2.3.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fives-Taylor P. M., Thompson D. W. Surface properties of Streptococcus sanguis FW213 mutants nonadherent to saliva-coated hydroxyapatite. Infect Immun. 1985 Mar;47(3):752–759. doi: 10.1128/iai.47.3.752-759.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forester H., Hunter N., Knox K. W. Characteristics of a high molecular weight extracellular protein of Streptococcus mutans. J Gen Microbiol. 1983 Sep;129(9):2779–2788. doi: 10.1099/00221287-129-9-2779. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J. Adherent interactions which may affect microbial ecology in the mouth. J Dent Res. 1984 Mar;63(3):378–385. doi: 10.1177/00220345840630030401. [DOI] [PubMed] [Google Scholar]
- Guss B., Eliasson M., Olsson A., Uhlén M., Frej A. K., Jörnvall H., Flock J. I., Lindberg M. Structure of the IgG-binding regions of streptococcal protein G. EMBO J. 1986 Jul;5(7):1567–1575. doi: 10.1002/j.1460-2075.1986.tb04398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada S., Slade H. D. Biology, immunology, and cariogenicity of Streptococcus mutans. Microbiol Rev. 1980 Jun;44(2):331–384. doi: 10.1128/mr.44.2.331-384.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead S. K., Fischetti V. A., Scott J. R. Complete nucleotide sequence of type 6 M protein of the group A Streptococcus. Repetitive structure and membrane anchor. J Biol Chem. 1986 Feb 5;261(4):1677–1686. [PubMed] [Google Scholar]
- Holt R. G., Abiko Y., Saito S., Smorawinska M., Hansen J. B., Curtiss R., 3rd Streptococcus mutans genes that code for extracellular proteins in Escherichia coli K-12. Infect Immun. 1982 Oct;38(1):147–156. doi: 10.1128/iai.38.1.147-156.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang T. T., Malke H., Ferretti J. J. Heterogeneity of the streptokinase gene in group A streptococci. Infect Immun. 1989 Feb;57(2):502–506. doi: 10.1128/iai.57.2.502-506.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes M., Machardy S. M., Sheppard A. J., Woods N. C. Evidence for an immunological relationship between Streptococcus mutans and human cardiac tissue. Infect Immun. 1980 Feb;27(2):576–588. doi: 10.1128/iai.27.2.576-588.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ish-Horowicz D., Burke J. F. Rapid and efficient cosmid cloning. Nucleic Acids Res. 1981 Jul 10;9(13):2989–2998. doi: 10.1093/nar/9.13.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly C., Evans P., Bergmeier L., Lee S. F., Progulske-Fox A., Harris A. C., Aitken A., Bleiweis A. S., Lehner T. Sequence analysis of the cloned streptococcal cell surface antigen I/II. FEBS Lett. 1989 Nov 20;258(1):127–132. doi: 10.1016/0014-5793(89)81632-1. [DOI] [PubMed] [Google Scholar]
- Kelly C., Evans P., Ma J. K., Bergmeier L. A., Taylor W., Brady L. J., Lee S. F., Bleiweis A. S., Lehner T. Sequencing and characterization of the 185 kDa cell surface antigen of Streptococcus mutans. Arch Oral Biol. 1990;35 (Suppl):33S–38S. doi: 10.1016/0003-9969(90)90128-w. [DOI] [PubMed] [Google Scholar]
- Knox K. W., Hardy L. N., Wicken A. J. Comparative studies on the protein profiles and hydrophobicity of strains of Streptococcus mutans serotype c. J Gen Microbiol. 1986 Sep;132(9):2541–2548. doi: 10.1099/00221287-132-9-2541. [DOI] [PubMed] [Google Scholar]
- Koga T., Asakawa H., Okahashi N., Takahashi I. Effect of subculturing on expression of a cell-surface protein antigen by Streptococcus mutans. J Gen Microbiol. 1989 Dec;135(12):3199–3207. doi: 10.1099/00221287-135-12-3199. [DOI] [PubMed] [Google Scholar]
- Koga T., Okahashi N., Takahashi I., Kanamoto T., Asakawa H., Iwaki M. Surface hydrophobicity, adherence, and aggregation of cell surface protein antigen mutants of Streptococcus mutans serotype c. Infect Immun. 1990 Feb;58(2):289–296. doi: 10.1128/iai.58.2.289-296.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. F., Progulske-Fox A., Bleiweis A. S. Molecular cloning and expression of a Streptococcus mutans major surface protein antigen, P1 (I/II), in Escherichia coli. Infect Immun. 1988 Aug;56(8):2114–2119. doi: 10.1128/iai.56.8.2114-2119.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. F., Progulske-Fox A., Erdos G. W., Piacentini D. A., Ayakawa G. Y., Crowley P. J., Bleiweis A. S. Construction and characterization of isogenic mutants of Streptococcus mutans deficient in major surface protein antigen P1 (I/II). Infect Immun. 1989 Nov;57(11):3306–3313. doi: 10.1128/iai.57.11.3306-3313.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride B. C., Song M., Krasse B., Olsson J. Biochemical and immunological differences between hydrophobic and hydrophilic strains of Streptococcus mutans. Infect Immun. 1984 Apr;44(1):68–75. doi: 10.1128/iai.44.1.68-75.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris E. J., Ganeshkumar N., McBride B. C. Cell surface components of Streptococcus sanguis: relationship to aggregation, adherence, and hydrophobicity. J Bacteriol. 1985 Oct;164(1):255–262. doi: 10.1128/jb.164.1.255-262.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta H., Kato H., Okahashi N., Takahashi I., Hamada S., Koga T. Characterization of a cell-surface protein antigen of hydrophilic Streptococcus mutans strain GS-5. J Gen Microbiol. 1989 Apr;135(4):981–988. doi: 10.1099/00221287-135-4-981. [DOI] [PubMed] [Google Scholar]
- Okahashi N., Koga T., Hamada S. Purification and immunochemical properties of a protein antigen from serotype g Streptococcus mutans. Microbiol Immunol. 1986;30(1):35–47. doi: 10.1111/j.1348-0421.1986.tb00919.x. [DOI] [PubMed] [Google Scholar]
- Okahashi N., Sasakawa C., Yoshikawa M., Hamada S., Koga T. Cloning of a surface protein antigen gene from serotype c Streptococcus mutans. Mol Microbiol. 1989 Feb;3(2):221–228. doi: 10.1111/j.1365-2958.1989.tb01811.x. [DOI] [PubMed] [Google Scholar]
- Pancholi V., Fischetti V. A. Identification of an endogenous membrane anchor-cleaving enzyme for group A streptococcal M protein. Its implication for the attachment of surface proteins in gram-positive bacteria. J Exp Med. 1989 Dec 1;170(6):2119–2133. doi: 10.1084/jem.170.6.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell M. W., Bergmeier L. A., Zanders E. D., Lehner T. Protein antigens of Streptococcus mutans: purification and properties of a double antigen and its protease-resistant component. Infect Immun. 1980 May;28(2):486–493. doi: 10.1128/iai.28.2.486-493.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell R. R. Distribution of cross-reactive antigens A and B in Streptococcus mutans and other oral streptococci. J Gen Microbiol. 1980 Jun;118(2):383–388. doi: 10.1099/00221287-118-2-383. [DOI] [PubMed] [Google Scholar]
- Russell R. R. Wall-associated protein antigens of Streptococcus mutans. J Gen Microbiol. 1979 Sep;114(1):109–115. doi: 10.1099/00221287-114-1-109. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staat R. H., Langley S. D., Doyle R. J. Streptococcus mutans adherence: presumptive evidence for protein-mediated attachment followed by glucan-dependent cellular accumulation. Infect Immun. 1980 Feb;27(2):675–681. doi: 10.1128/iai.27.2.675-681.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staffileno L. K., Hendricks M., LaPolla R., Bohart C., Van Hook P., Rosen J. I., Warner J., Hoey K., Wegemer D., Naso R. B. Cloning of the amino terminal nucleotides of the antigen I/II of Streptococcus sobrinus and the immune responses to the corresponding synthetic peptides. Arch Oral Biol. 1990;35 (Suppl):47S–52S. doi: 10.1016/0003-9969(90)90130-3. [DOI] [PubMed] [Google Scholar]
- Takahashi I., Okahashi N., Sasakawa C., Yoshikawa M., Hamada S., Koga T. Homology between surface protein antigen genes of Streptococcus sobrinus and Streptococcus mutans. FEBS Lett. 1989 Jun 5;249(2):383–388. doi: 10.1016/0014-5793(89)80664-7. [DOI] [PubMed] [Google Scholar]
- Winship P. R. An improved method for directly sequencing PCR amplified material using dimethyl sulphoxide. Nucleic Acids Res. 1989 Feb 11;17(3):1266–1266. doi: 10.1093/nar/17.3.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanders E. D., Lehner T. Separation and characterization of a protein antigen from cells of Streptococcus mutans. J Gen Microbiol. 1981 Feb;122(2):217–225. doi: 10.1099/00221287-122-2-217. [DOI] [PubMed] [Google Scholar]