Abstract
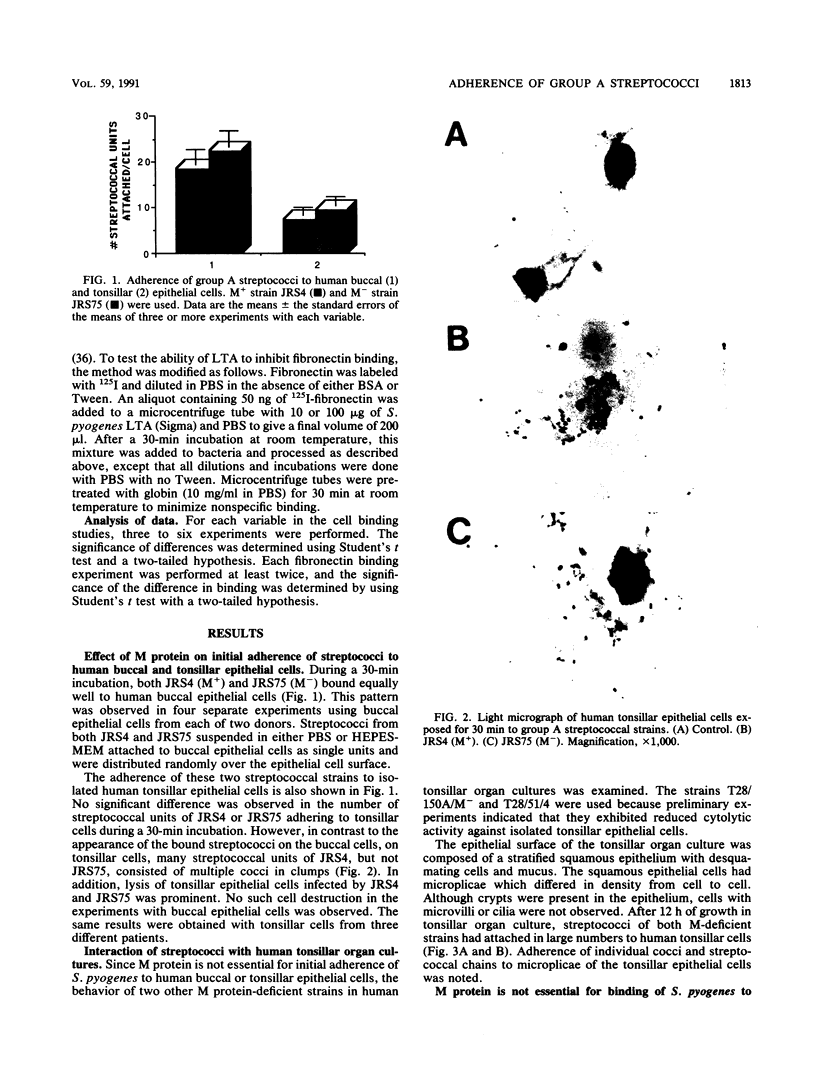
The role of the M protein in adherence of group A streptococci to human epithelial cells was directly tested by using an isogenic pair of M+ and M- strains. There was no difference between these strains in the number of streptococcal units that adhered to buccal or tonsillar epithelial cells, indicating the following: (i) that adhesins that are not dependent upon M protein expression are present on the surface of group A streptococci and (ii) that the M protein is not the primary streptococcal adherence ligand. However, the M+ strain adhered to tonsillar epithelial cells as aggregates. This aggregation was dependent on the presence of the M protein, since the isogenic M- strain did not clump. The coaggregation of streptococci suggests that the M protein plays an important role in promoting the formation of microcolonies after initial attachment. Binding to fibronectin, a potential epithelial cell receptor for group A streptococci, was also the same for the isogenic M+ and M- strains as well as for an isogenic strain with a regulatory mutation that decreases the expression of M protein. In summary, the M protein is not the primary streptococcal adhesin, nor is it required to orient the streptococcal adhesin and/or fibronectin receptor.
Full text
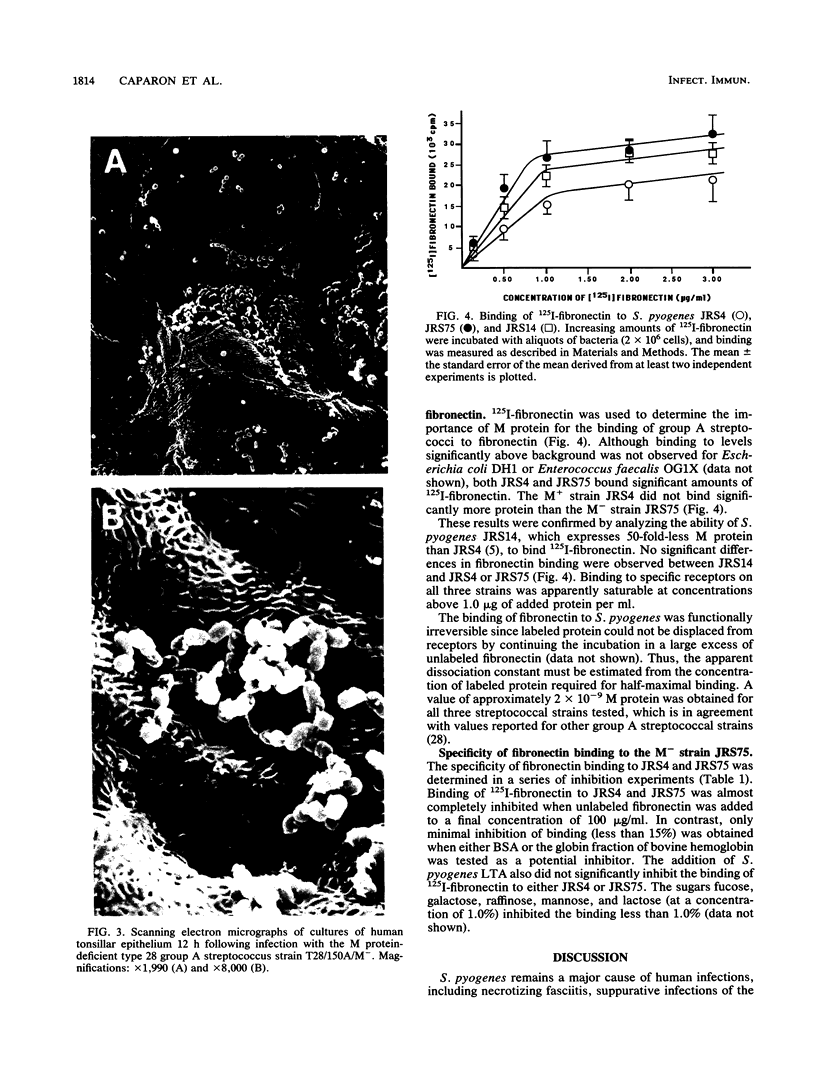
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham S. N., Beachey E. H., Simpson W. A. Adherence of streptococcus pyogenes, Escherichia coli, and Pseudomonas aeruginosa to fibronectin-coated and uncoated epithelial cells. Infect Immun. 1983 Sep;41(3):1261–1268. doi: 10.1128/iai.41.3.1261-1268.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkan M., Ofek I., Beachey E. H. Adherence pharyngeal and skin strains of group A streptococci to human skin and oral epithelial cells. Infect Immun. 1977 Nov;18(2):555–557. doi: 10.1128/iai.18.2.555-557.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babu J. P., Simpson W. A., Courtney H. S., Beachey E. H. Interaction of human plasma fibronectin with cariogenic and non-cariogenic oral streptococci. Infect Immun. 1983 Jul;41(1):162–168. doi: 10.1128/iai.41.1.162-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beachey E. H. Bacterial adherence: adhesin-receptor interactions mediating the attachment of bacteria to mucosal surface. J Infect Dis. 1981 Mar;143(3):325–345. doi: 10.1093/infdis/143.3.325. [DOI] [PubMed] [Google Scholar]
- Beachey E. H., Ofek I. Epithelial cell binding of group A streptococci by lipoteichoic acid on fimbriae denuded of M protein. J Exp Med. 1976 Apr 1;143(4):759–771. doi: 10.1084/jem.143.4.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caparon M. G., Scott J. R. Identification of a gene that regulates expression of M protein, the major virulence determinant of group A streptococci. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8677–8681. doi: 10.1073/pnas.84.23.8677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cone L. A., Woodard D. R., Schlievert P. M., Tomory G. S. Clinical and bacteriologic observations of a toxic shock-like syndrome due to Streptococcus pyogenes. N Engl J Med. 1987 Jul 16;317(3):146–149. doi: 10.1056/NEJM198707163170305. [DOI] [PubMed] [Google Scholar]
- Dale J. B., Beachey E. H. Multiple, heart-cross-reactive epitopes of streptococcal M proteins. J Exp Med. 1985 Jan 1;161(1):113–122. doi: 10.1084/jem.161.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellen R. P., Gibbons R. J. M protein-associated adherence of Streptococcus pyogenes to epithelial surfaces: prerequisite for virulence. Infect Immun. 1972 May;5(5):826–830. doi: 10.1128/iai.5.5.826-830.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischetti V. A., Jones K. F., Scott J. R. Size variation of the M protein in group A streptococci. J Exp Med. 1985 Jun 1;161(6):1384–1401. doi: 10.1084/jem.161.6.1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Ike Y., Craig R. A., White B. A., Yagi Y., Clewell D. B. Modification of Streptococcus faecalis sex pheromones after acquisition of plasmid DNA. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5369–5373. doi: 10.1073/pnas.80.17.5369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K. F., Manjula B. N., Johnston K. H., Hollingshead S. K., Scott J. R., Fischetti V. A. Location of variable and conserved epitopes among the multiple serotypes of streptococcal M protein. J Exp Med. 1985 Mar 1;161(3):623–628. doi: 10.1084/jem.161.3.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANCEFIELD R. C. Current knowledge of type-specific M antigens of group A streptococci. J Immunol. 1962 Sep;89:307–313. [PubMed] [Google Scholar]
- McGee Z. A., Johnson A. P., Taylor-Robinson D. Human fallopian tubes in organ culture: preparation, maintenance, and quantitation of damage by pathogenic microorganisms. Infect Immun. 1976 Feb;13(2):608–618. doi: 10.1128/iai.13.2.608-618.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mergenhagen S. E., Sandberg A. L., Chassy B. M., Brennan M. J., Yeung M. K., Donkersloot J. A., Cisar J. O. Molecular basis of bacterial adhesion in the oral cavity. Rev Infect Dis. 1987 Sep-Oct;9 (Suppl 5):S467–S474. doi: 10.1093/clinids/9.supplement_5.s467. [DOI] [PubMed] [Google Scholar]
- Myhre E. B., Kuusela P. Binding of human fibronectin to group A, C, and G streptococci. Infect Immun. 1983 Apr;40(1):29–34. doi: 10.1128/iai.40.1.29-34.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norgren M., Caparon M. G., Scott J. R. A method for allelic replacement that uses the conjugative transposon Tn916: deletion of the emm6.1 allele in Streptococcus pyogenes JRS4. Infect Immun. 1989 Dec;57(12):3846–3850. doi: 10.1128/iai.57.12.3846-3850.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofek I., Beachey E. H., Jefferson W., Campbell G. L. Cell membrane-binding properties of group A streptococcal lipoteichoic acid. J Exp Med. 1975 May 1;141(5):990–1003. doi: 10.1084/jem.141.5.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofek I., Simpson W. A., Beachey E. H. Formation of molecular complexes between a structurally defined M protein and acylated or deacylated lipoteichoic acid of Streptococcus pyogenes. J Bacteriol. 1982 Feb;149(2):426–433. doi: 10.1128/jb.149.2.426-433.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips G. N., Jr, Flicker P. F., Cohen C., Manjula B. N., Fischetti V. A. Streptococcal M protein: alpha-helical coiled-coil structure and arrangement on the cell surface. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4689–4693. doi: 10.1073/pnas.78.8.4689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. R., Guenthner P. C., Malone L. M., Fischetti V. A. Conversion of an M- group A streptococcus to M+ by transfer of a plasmid containing an M6 gene. J Exp Med. 1986 Nov 1;164(5):1641–1651. doi: 10.1084/jem.164.5.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. R., Pulliam W. M., Hollingshead S. K., Fischetti V. A. Relationship of M protein genes in group A streptococci. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1822–1826. doi: 10.1073/pnas.82.6.1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson W. A., Beachey E. H. Adherence of group A streptococci to fibronectin on oral epithelial cells. Infect Immun. 1983 Jan;39(1):275–279. doi: 10.1128/iai.39.1.275-279.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson W. A., Courtney H. S., Ofek I. Interactions of fibronectin with streptococci: the role of fibronectin as a receptor for Streptococcus pyogenes. Rev Infect Dis. 1987 Jul-Aug;9 (Suppl 4):S351–S359. doi: 10.1093/clinids/9.supplement_4.s351. [DOI] [PubMed] [Google Scholar]
- Simpson W. J., LaPenta D., Chen C., Cleary P. P. Coregulation of type 12 M protein and streptococcal C5a peptidase genes in group A streptococci: evidence for a virulence regulon controlled by the virR locus. J Bacteriol. 1990 Feb;172(2):696–700. doi: 10.1128/jb.172.2.696-700.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speziale P., Hök M., Switalski L. M., Wadström T. Fibronectin binding to a Streptococcus pyogenes strain. J Bacteriol. 1984 Feb;157(2):420–427. doi: 10.1128/jb.157.2.420-427.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanislawski L., Simpson W. A., Hasty D., Sharon N., Beachey E. H., Ofek I. Role of fibronectin in attachment of Streptococcus pyogenes and Escherichia coli to human cell lines and isolated oral epithelial cells. Infect Immun. 1985 Apr;48(1):257–259. doi: 10.1128/iai.48.1.257-259.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens D. S., Hoffman L. H., McGee Z. A. Interaction of Neisseria meningitidis with human nasopharyngeal mucosa: attachment and entry into columnar epithelial cells. J Infect Dis. 1983 Sep;148(3):369–376. doi: 10.1093/infdis/148.3.369. [DOI] [PubMed] [Google Scholar]
- Stephens D. S., McGee Z. A. Attachment of Neisseria meningitidis to human mucosal surfaces: influence of pili and type of receptor cell. J Infect Dis. 1981 Apr;143(4):525–532. doi: 10.1093/infdis/143.4.525. [DOI] [PubMed] [Google Scholar]
- Sting R., Schmitt M., Blobel H. Isolierung eines Fibronektin-Rezeptors von Streptococcus pyogenes durch Affinitätschromatographie und anschliessender Hochleistungflüssigkeitschromatographie. J Chromatogr. 1989 Dec 29;497:258–262. [PubMed] [Google Scholar]
- Swanson J. Gonococcal adherence: selected topics. Rev Infect Dis. 1983 Sep-Oct;5 (Suppl 4):S678–S684. doi: 10.1093/clinids/5.supplement_4.s678. [DOI] [PubMed] [Google Scholar]
- Swanson J., Hsu K. C., Gotschlich E. C. Electron microscopic studies on streptococci. I. M antigen. J Exp Med. 1969 Nov 1;130(5):1063–1091. doi: 10.1084/jem.130.5.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss E. I., London J., Kolenbrander P. E., Hand A. R., Siraganian R. Localization and enumeration of fimbria-associated adhesins of Bacteroides loescheii. J Bacteriol. 1988 Mar;170(3):1123–1128. doi: 10.1128/jb.170.3.1123-1128.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zetter B. R., Daniels T. E., Quadra-White C., Greenspan J. S. LETS protein in normal and pathological human oral epithelium. J Dent Res. 1979 Jan;58(1):484–488. doi: 10.1177/00220345790580010401. [DOI] [PubMed] [Google Scholar]