Abstract
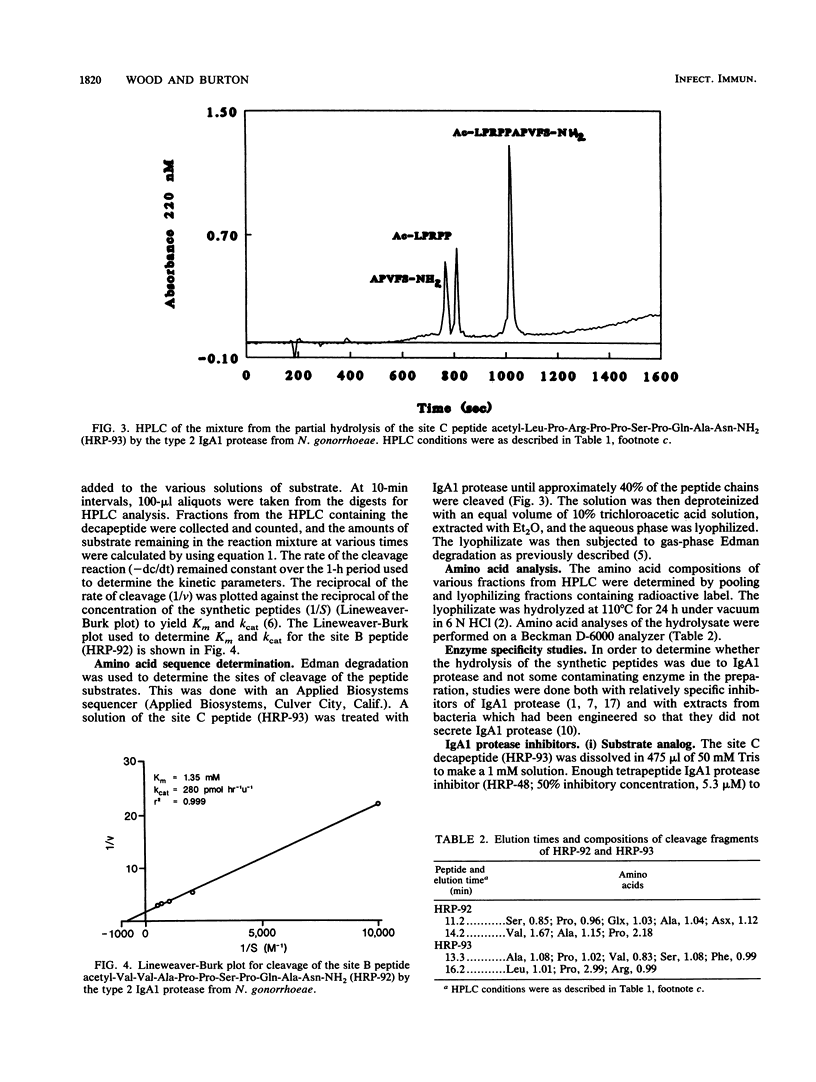
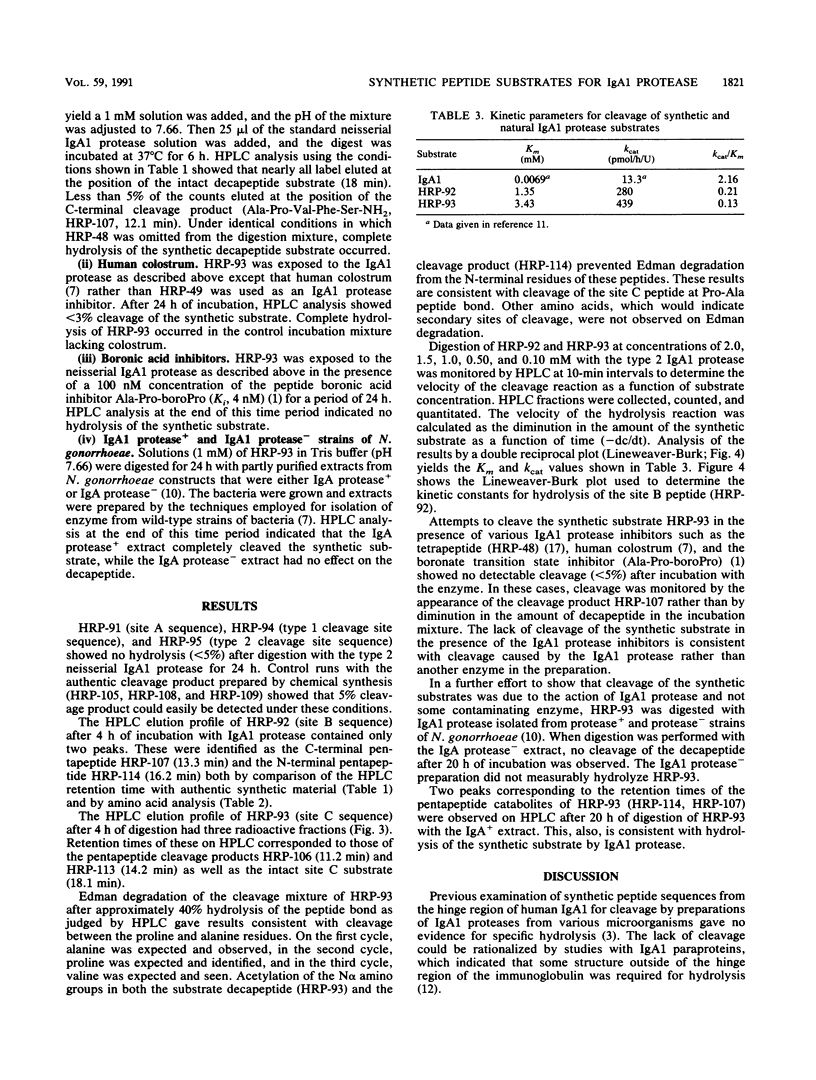
Neisseria gonorrhoeae secretes protease which inactive human immunoglobulin A1 (IgA1) by cleavage of specific peptide bonds in the hinge region. The type 2 IgA1 protease (EC 3.4.24.13) is secreted as a 169-kDa precursor which undergoes autoproteolysis at three sites (A, B, and C) to release the 106-kDa active form of the enzyme (J. Pohlner, R. Halter, K. Beyreuther, and T. F. Meyer. Nature [London] 325:458-462, 1987). Synthetic decapeptides consisting of five residues on each side of the three autoproteolytic cleavage sites and their potential pentapeptide catabolites were prepared by solid-phase synthesis. Cleavage of the decapeptides by the type 2 IgA1 protease from N. gonorrhoeae was monitored by high-performance liquid chromatography. Peptides homologous with the amino acid sequences around the B and C sites are cleaved by the IgA1 protease. Amino acid analysis and Edman degradation show that the cleavage products have both the composition and amino acid sequence which would be expected from cleavage at the predicted sites. Km values of 1.35 mM and 3.43 mM and kcat values of 280 pmol/h/U and 439 pmol/h/U for the site B and site C peptides, respectively, were determined. The catalytic efficiency (kcat/Km) for the synthetic substrates is about 10% of that reported for intact IgA1. Cleavage of the peptides is inhibited by IgA1 protease inhibitors such as the tetrapeptide substrate analog inhibitor HRP-48, human colostrum, and a peptide-boronate transition state inhibitor. An extract from an N. gonorrhoeae construct lacking active IgA1 protease failed to cleave the synthetic substrate, while an extract from the control construct which secretes active enzyme completely hydrolyzed the synthetic peptide. Neither the site A peptide nor synthetic decapeptides encompassing cleavage sites in the hinge region of IgA1 are hydrolyzed by IgA1 protease. These are the first synthetic substrates to be reported for any IgA1 protease.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachovchin W. W., Plaut A. G., Flentke G. R., Lynch M., Kettner C. A. Inhibition of IgA1 proteinases from Neisseria gonorrhoeae and Hemophilus influenzae by peptide prolyl boronic acids. J Biol Chem. 1990 Mar 5;265(7):3738–3743. [PubMed] [Google Scholar]
- Burton J., Poulsen K., Haber E. Competitive inhibitors of renin. Inhibitors effective at physiological pH. Biochemistry. 1975 Aug 26;14(17):3892–3898. doi: 10.1021/bi00688a024. [DOI] [PubMed] [Google Scholar]
- Burton J., Wood S. G., Lynch M., Plaut A. G. Substrate analogue inhibitors of the IgA1 proteinases from Neisseria gonorrhoeae. J Med Chem. 1988 Aug;31(8):1647–1651. doi: 10.1021/jm00403a027. [DOI] [PubMed] [Google Scholar]
- Gilbert J. V., Plaut A. G., Longmaid B., Lamm M. E. Inhibition of microbial IgA proteases by human secretory IgA and serum. Mol Immunol. 1983 Sep;20(9):1039–1049. doi: 10.1016/0161-5890(83)90045-7. [DOI] [PubMed] [Google Scholar]
- Halter R., Pohlner J., Meyer T. F. IgA protease of Neisseria gonorrhoeae: isolation and characterization of the gene and its extracellular product. EMBO J. 1984 Jul;3(7):1595–1601. doi: 10.1002/j.1460-2075.1984.tb02016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilian M., Mestecky J., Kulhavy R., Tomana M., Butler W. T. IgA1 proteases from Haemophilus influenzae, Streptococcus pneumoniae, Neisseria meningitidis, and Streptococcus sanguis: comparative immunochemical studies. J Immunol. 1980 Jun;124(6):2596–2600. [PubMed] [Google Scholar]
- Koomey J. M., Gill R. E., Falkow S. Genetic and biochemical analysis of gonococcal IgA1 protease: cloning in Escherichia coli and construction of mutants of gonococci that fail to produce the activity. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7881–7885. doi: 10.1073/pnas.79.24.7881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornfeld S. J., Plaut A. G. Secretory immunity and the bacterial IgA proteases. Rev Infect Dis. 1981 May-Jun;3(3):521–534. doi: 10.1093/clinids/3.3.521. [DOI] [PubMed] [Google Scholar]
- Plaut A. G., Gilbert J. V., Artenstein M. S., Capra J. D. Neisseria gonorrhoeae and neisseria meningitidis: extracellular enzyme cleaves human immunoglobulin A. Science. 1975 Dec 12;190(4219):1103–1105. doi: 10.1126/science.810892. [DOI] [PubMed] [Google Scholar]
- Plaut A. G., Gilbert J. V., Heller I. Assay and properties of IgA protease of Streptococcus sanguis. Adv Exp Med Biol. 1978;107:489–495. doi: 10.1007/978-1-4684-3369-2_55. [DOI] [PubMed] [Google Scholar]
- Pohlner J., Halter R., Beyreuther K., Meyer T. F. Gene structure and extracellular secretion of Neisseria gonorrhoeae IgA protease. 1987 Jan 29-Feb 4Nature. 325(6103):458–462. doi: 10.1038/325458a0. [DOI] [PubMed] [Google Scholar]
- Schechter I., Berger A. On the size of the active site in proteases. I. Papain. Biochem Biophys Res Commun. 1967 Apr 20;27(2):157–162. doi: 10.1016/s0006-291x(67)80055-x. [DOI] [PubMed] [Google Scholar]
- Wood S. G., Lynch M., Plaut A. G., Burton J. Tetrapeptide inhibitors of the IgA1 proteinases from type I Neisseria gonorrhoeae. J Med Chem. 1989 Oct;32(10):2407–2411. doi: 10.1021/jm00130a030. [DOI] [PubMed] [Google Scholar]


