Abstract
The acoustical cues for sound location are generated by spatial- and frequency-dependent filtering of propagating sound waves by the head and external ears. Although rats have been a common model system for anatomy, physiology, and psychophysics of localization, there have been few studies of the acoustical cues available to rats. Here, directional transfer functions (DTFs), the directional components of the head-related transfer functions, were measured in six adult rats. The cues to location were computed from the DTFs. In the frontal hemisphere, spectral notches were present for frequencies from ∼16 to 30 kHz; in general, the frequency corresponding to the notch increased with increases in source elevation and in azimuth toward the ipsilateral ear. The maximum high-frequency envelope-based interaural time differences (ITDs) were 130 μs, whereas low-frequency (<3.5 kHz) fine-structure ITDs were 160 μs; both types of ITDs were larger than predicted from spherical head models. Interaural level differences (ILDs) strongly depended on location and frequency. Maximum ILDs were <10 dB for frequencies <8 kHz and were as large as 20–40 dB for frequencies >20 kHz. Removal of the pinna eliminated the spectral notches, reduced the acoustic gain and ILDs, altered the acoustical axis, and reduced the ITDs.
INTRODUCTION
Whether predator or prey, sound localization is critical to the survival of most animals. The acoustical cues for sound source location are generated by the spatial- and frequency-dependent filtering of the propagating sound waves by the head and external ears (Figs. 12). Therefore, the linear dimensions of the head and pinnae are critical factors in determining the magnitude and frequency ranges of the resultant cues to location. There are three main cues: interaural differences in time (ITD) and level (ILD) and monaural spectral shape cues (Fig. 2).
Figure 1.
The dimensions of the animal subjects. Across the six rats, the average head diameter (FG) was 29.6±1.3 mm, the average pinna width (DE) was 11.4±0.5 mm, and the average pinna lengths (AC) and (BC) were 16.8±0.8 and 17.7±1.7 mm, respectively.
Figure 2.
Examples of the three primary acoustical cues to sound location, spectral notches, interaural time differences (ITDs), and interaural level differences (ILDs), for five sources in azimuth along the horizontal plane. At each location, the directional transfer functions (DTFs, top panels) and impulse responses (lower panels) are shown for the left (light gray lines) and right (dark lines) ears. Spectral notches are apparent in the DTFs, ITDs are given by the delay between the left- and right-ear impulse responses, and ILDs are given by the difference in the left- and right-ear DTFs.
Behavioral, anatomical, and physiological studies of the mechanisms of sound localization have suggested that there can be considerable differences (Irving and Harrison, 1967; Kelly, 1980; Heffner, 1997) and similarities (Brand et al., 2002; McAlpine and Grothe, 2003) among different species making cross species generalizations difficult and perhaps even misleading. These differences might be reconciled by taking into consideration the sound localization cues that are actually available for each particular species. In other words, proper interpretation of the data from anatomical, physiological, and behavioral studies of sound localization requires detailed knowledge of the properties of acoustical information available to the species under study. Unfortunately, for some common species, such as the rat, the cues to location have not been measured. Yet in other species, there has been considerable study of the cues, including human (Wightman and Kistler, 1989; Middlebrooks et al., 1989; Middlebrooks and Green, 1990), cat (Wiener et al., 1966; Moore and Irvine, 1979; Roth et al., 1980; Phillips et al., 1982; Irvine, 1987; Musicant et al., 1990; Rice et al., 1992; Xu and Middlebrooks, 2000), monkey (Spezio et al., 2000), ferrets (Carlile, 1990; Schnupp et al., 2003), tammar wallaby (Coles and Guppy, 1986), various species of bat (Jen and Chen, 1988; Obrist et al., 1993; Fuzessery, 1996; Firzlaff and Schuller, 2003; Aytekin et al., 2004), guinea pig (Carlile and Pettigrew, 1987; Sterbing et al., 2003), gerbil (Maki and Furukawa, 2005), mouse (Chen et al., 1995), and barn owl (Moiseff, 1989; Keller et al., 1998).
In this paper, we extend these studies to include the albino rat (Rattus norvegicus). Rats have been a common model system for the behavior (Beecher and Harrison, 1971; Burlile et al., 1985; Heffner and Heffner, 1985; Harrison, 1988; Heffner et al., 1994), anatomy (Beyerl, 1978; Kelly, 1980; Kelly and Kavanagh, 1986; Kelly and Glazier, 1978), physiology (Flammino and Clopton, 1975; Inbody and Feng, 1981; Kelly and Sally, 1988; Finlayson and Caspary, 1991; Kelly and Phillips, 1991; Li and Kelly, 1992; Irvine et al., 1995; Kelly et al., 1998; Irvine et al., 2001), and development (Silverman and Clopton, 1977; Clopton and Silverman, 1977; Kelly and Judge, 1985; Kandler and Gillespie, 2005) of sound localization mechanisms. However, aside from some spatially and spectrally sparse measurements of the ILD cues by Harrison and Downey (1970), little is actually known about the localization cues available to rats. To fill this void, here we measured the directional transfer functions (DTFs) (Middlebrooks and Green, 1990), the directional components of the head-related transfer functions (HRTFs), for adult rats. From the DTFs, the primary acoustical cues to location were computed and examined, including the spectral “notches” (Rice et al., 1992), ITDs, and the spatial and frequency dependence of the ILD cues. The acoustic gains of the DTFs were examined at individual frequencies to determine the “acoustical axis,” the spatial location of the maximum gain at a particular frequency. Finally, the role of the pinna in generating the cues was examined by repeating the acoustical measurements after surgical removal of the pinna. Our measurements confirm that the three typical acoustical cues that are available to most mammals are also available in rats. Moreover, we demonstrate that the pinna substantially contributes to the formation of all three of the primary cues to location.
METHODOLOGY
Animal preparation
Six adult female Sprague Dawley rats were used (mean weight of 287±12 g). All surgical and experimental procedures complied with the guidelines of the University of Colorado Health Science Center Animal Care and Use Committees and the National Institutes of Health. Rats were euthanized with sodium pentobarbital (100 mg∕kg, i.p.) prior to acoustic measurements. We adapted the technique of Obrist et al. (1993) and Wotton et al. (1995) in their study of the acoustical cues in bats in which the euthanized animals were frozen prior to the acoustic measurements. Here, the euthanized rats were frozen before performing the experiments. Care was taken to ensure that the head position relative to the body and the pinna position relative to the head were maintained in a natural posture during freezing. Freezing the tissue facilitated the consistency of the insertion of the probe tube microphones to deeper portions of the ear canal. The weight, head diameter, and pinna height and width [Figs. 1a, 1b] of each animal were measured after freezing. Measurements of four animals before and after freezing showed that these dimensions were not significantly changed by freezing (paired t25=−1.26, p=0.22). The acoustic measurement procedure lasted ∼1 hour during which the animals remained frozen. Similar techniques have been used to measure the acoustical cues in frozen (Obrist et al., 1993; Wotton et al., 1995), formaline-fixed (Fuzessery, 1996; Aytekin et al., 2004; Firzlaff and Schuller, 2003; Koay et al., 1998), alcohol-fixed (Obrist et al., 1993), and cadaver (Harrison and Downey, 1970; Moore and Irvine, 1979; Middlebrooks and Pettigrew, 1981; Coles and Guppy, 1986; Martin and Webster, 1989; Chen et al., 1995; Maki and Furukawa, 2005) animals.
After the linear measurements of the head and pinna, a small hole was made in the wall of posterior aspect of pinna with a 14-gauge needle. A 50-mm-long flexible probe tube (Bruel and Kjaer, part no. AF-0555, 1.65 mm outer diameter) was inserted into the hole so that the tip of the tube was just within the ear canal and fixed in place with super glue. The animal was then placed in the center of the sound-attenuating room (see below), with its interaural axis aligned in the arc of loudspeakers by using three lasers, two at the poles and one at (0°,0°). To examine the role of the pinna, after taking the first set of acoustic measurements, the pinnae of three animals were removed and the measurements were repeated. Removal of the pinna did not alter the position of the probe tube microphone in the ear canal and animals remained centered in the loudspeaker arc.
Experimental setup
All experiments were performed in an ∼3×3×3 m3 (interior dimensions) double-walled, sound-attenuating room (IAC, Bronx, NY), where the walls and equipment were lined with 4-in.-thick reticulated wedged acoustic foam (Sonex Classic). Stimuli were presented from 25 loudspeakers (Morel MDT-20) attached to a custom-built horizontally oriented [i.e., “single-pole” coordinate system (Middlebrooks and Pettigrew, 1981; Leong and Carlile, 1998)] semicircular boom. The 25 loudspeakers were spaced in azimuth along the arc at 7.5° spacing, from −90° (left) to +90° (right). The imaginary axis of rotation of the arc was aligned with the interaural axis of the animal (i.e., through the ears). The radius of the arc was 1 m. The 25 loudspeakers were selected from a larger set (∼100) on the basis of best-matching frequency responses. A stepper motor (Advanced Micro Systems AMH34-1303-3) and motor controller∕power supply (Advanced Micro Systems CMAX-810) under computer control could position the arc in elevation with a precision of <1°. The semicircular arc was moved in steps of 7.5° along the elevation by using the stepper motor controlled via personal computer by custom written software in MATLAB (Mathworks, Natick, MA). Stimuli were presented from a total of 625 different locations, covering azimuth and elevation. The elevation spanned −45° to +225°.
The measurement stimuli consisted of 11th order maximum length sequences (MLSs) (Rife and Vanderkooy, 1989) repeated without interruption 128 times from each loudspeaker. The MLS sequence was presented at full 24-bit resolution at a rate of 97 656.25 Hz (Tucker-Davis Technologies, TDT RP2.1, Alachua, FL). A single sequence of the 11th order MLS is comprised of 2047 samples (211–1) and is 20.96 ms in duration. Loudspeakers for stimulus presentation were selected via two daisy-chained TDT multiplexers (TDT PM2R) and the stimulus was amplified (TDT SA1) before being presented to the loudspeaker. The resulting acoustic waveforms in the ear canals of the left and right ears were simultaneously recorded through two probe tube microphones (Bruel and Kjaer, Type-4182), amplified (TDT MA2), and collected by using two analog to digital converter channels at 97 656.25 Hz (TDT RP2.1). Stimulus presentation, acquisition, analysis, and movement of the speaker arc were controlled by custom software in MATLAB. In all experiments, a calibration measurement was also made for each of the 25 loudspeakers in the absence of the animal by placing the tips of the probe tubes so that they corresponded to the location where the center of the head of the rats would be located. The calibration measurements capture the spectral characteristics of the loudspeakers and microphones for later processing.
Data processing and data analysis
The acoustic impulse response for each ear and each location was calculated by circular cross correlation of the original 11th order MLS stimulus and the in-ear recording from the probe tube microphone (Rife and Vanderkooy, 1989). The impulse responses were then truncated to 512 points (5.12 ms duration) by a 512-point Hanning window where the center of the window was set to approximately coincide with the point of maximum amplitude in the impulse response. This windowing procedure removes the small-amplitude reflections that may be contained in the impulse response. Next, the HRTFs were derived by dividing the frequency response of the in-ear recording by that of appropriate loudspeaker calibration measurement. This procedure removes the loudspeaker and microphone frequency response from each in-ear measurement. The resulting function is referred to as the HRTF, as it represents the acoustical gain and delay introduced by the head and the pinna. However, the resulting HRTF can be highly dependent on the exact placement of the tip of the probe tube microphone in the ear canal relative to the tympanic membrane (Middlebrooks et al., 1989; Chan and Geisler, 1990). To reduce the confounding effects of the probe tube placement in the ear canal, for each ear the DTFs were then calculated from the HRTFs by dividing the HRTF made at each spatial location by the geometrical mean of all the measured HRTFs across all measurement locations for that ear. The spectral features resulting from the exact placement of the probe tube microphone in the ear canal are expected to be similar for all measurement locations (i.e., they are not dependent on spatial location), so this “common” spectral feature is removed from the HRTFs, resulting in the DTFs (Middlebrooks et al., 1989). In essence, the DTFs are the sound source direction-dependent components of HRTFs.
The amplitude spectra of the DTFs were calculated after the spectra were passed through a bank of 300 bandpass filters, the center frequencies of which were spaced at intervals of 0.0143 octave spanning from 2 to 40 kHz. The 3 dB bandwidth of filters was held constant across all frequencies at 0.12 octaves, and the upper and lower slopes of the filters fell off at ∼105 dB∕octave. These filters have properties similar to the bank of bandpass filters that have been used elsewhere to filter DTFs (Middlebrooks, 1999; Xu and Middlebrooks, 2000; Schnupp et al., 2003). The filter bandwidths used here were comparable to neural frequency tuning curves found in various auditory nuclei in the rat (Hernandez et al., 2005).
Two binaural cues to sound location were studied here. The ILD spectrum was derived by computing the differences (in decibels) in the DTFs, frequency by frequency between right and left ears at all elevations, and for all azimuth angles. Positive ILD indicates that the decibel level at the left ear was higher than the decibel level at the right ear. The ILDs for particular frequencies and locations were extracted from the ILD spectra. The ITDs in this paper were measured in two ways. First, ITDs based on the envelopes of the left- and right-ear impulse responses were computed. We choose to focus on envelope ITDs because the rat audiograms reveal that they have poor hearing for frequencies <2 kHz (Heffner et al., 1994) where ongoing ITDs in the fine structure would be useful. And a recent study has suggested that rats might not be able to use ongoing ITDs in the fine structure for sound localization of low-frequency pure tones (Wesolek et al., 2007). For each sound location, the envelopes of the left and right ear impulse responses were extracted by computing the magnitudes of their respective Hilbert transforms. These envelopes were then cross correlated and the delay corresponding to the maximum point was taken as the ITD for that particular location (Middlebrooks and Green, 1990). This process was repeated for each location. We also computed low-frequency ongoing ITDs in the fine structure by first low-pass filtering the impulse responses at 3.5 kHz, cross correlating the left and right impulse responses for each spatial location, and then defining the ITD at that location as the delay corresponding to the maximum in the correlation function.
For spatial plotting purposes, the data were displayed as Aitov projections. In this projection the nose of the animal is considered to be projecting out of the page at 0° azimuth and 0° elevation, as if the animals were looking at the reader. The spatial plots were plotted for frontal data for elevations from −45° to +90° and azimuth from +90° to −90°.
RESULTS
The results are based on DTF measurements from six adult female rats (Sprague Dawley). The mean weight was 287±12 g (n=6). Measurements of several linear dimensions of the head and pinna were made (Fig. 1). Across the six animals, the average head diameter FG was 29.6±1.3 mm, the average pinna width DE was 11.4±0.5 mm, and the average pinna lengths AC and BC were 16.8±0.8 and 17.7±1.7 mm, respectively (both the left and right pinna were measured, yielding a total of 12 measurements of AC, BC, and DE for the six animals). The six rats were remarkably similar in size and weight and the acoustical properties were also very similar. For this reason, we display the results from only three of the animals. Average cue values are based on all six animals, unless otherwise noted. Figure 2 shows the left and right ear impulse responses and their corresponding DTFs from one animal at five azimuthal locations at 0° elevation. Figure 2 illustrates the three main acoustical cues to location that are of particular interest in this paper; the ITDs, ILDs, and spectral notches.
Monaural aspects of the DTFs
Frequency range and spatial-location dependence of spectral notches
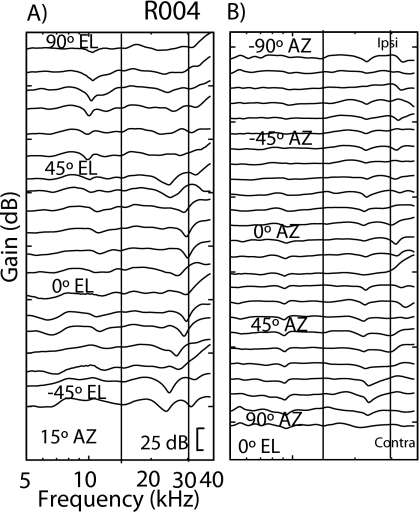
We observed a systematic change in the frequency of the first (lowest frequency) spectral notch with changes in source location (e.g., Rice et al., 1992). Figure 3 shows DTFs for the left ear of one animal (R016) for elevations ranging from −45° to 90° in 7.5° steps for 15° azimuth (panel A) and for azimuths ranging from −90° (ipsilateral ear) to 90° in 7.5° steps at 0° elevation (panel B). Similar results are shown for a different animal (R004, panels C and D). Spectral notches were observed for frequencies above 15 kHz in all animals. At a given sound source azimuth, the frequencies of the first spectral notch generally increased with increasing source elevation. The first notch frequency was easily detectable and systematically moved with elevation for sources in the frontal hemisphere (e.g., Fig. 3). However, the notch was difficult to detect or erratically moved with source locations above and behind the animals.
Figure 3.
The DTF gains for the left ear of two animals (R016 and R004) for sources at 15° azimuth and varying in elevation from −45° to +90° (panels A and C) and at 0° elevation varying from −90° (ipsilateral) to +90° (contralateral) azimuth (panels B and D). The two vertical bars in each figure bound the approximate frequency range of the first spectral notches, 16–30 kHz.
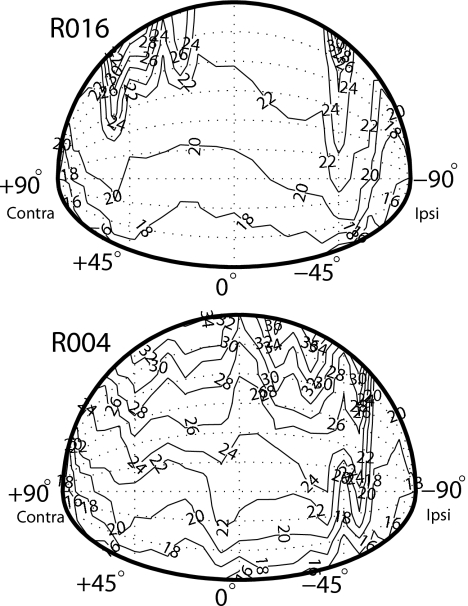
The first notch frequency also tended to increase with changing source azimuth toward the ipsilateral ear. This is evident for R016 [Fig. 3b] for changing source azimuth from the contralateral hemisphere toward the ipsilateral ear (−90°). As was the case for source elevation, the first notch frequency movements with source azimuth occurred for sources in the frontal hemisphere, but were difficult to detect or did not move in an orderly manner for sources behind the animal. Across the six rats, the notches tended to increase from ∼16 to 30 kHz as the source increased in elevation from −45° to 90° and from 17 to 23 kHz as the source azimuth went from ∼+30° to −90° (i.e., toward the ipsilateral ear).
Figure 4 shows the iso-first-notch frequency contours for the left ear (−90° is ipsilateral) for two animals (R0016 and R004) for sound sources in the frontal hemisphere. An automated procedure based on that described by Maki and Furukawa (2005) was used to find the frequency corresponding to the spectral notch for each ear and each spatial location. As detailed above, the first notch frequencies were either often difficult to detect or erratically moved with source location for extreme lateral azimuths and for elevations above and behind the animal. Yet in the middle part of the frontal hemisphere, the notch frequency contours were largely mirror symmetrical about the midline between right and left ears. The notches with lower frequencies were distributed on the contralateral azimuths and lower elevations, and the notch frequencies generally increased as the sources were ipsilaterally moved in azimuth and toward higher elevations. Although data are only shown in Figs. 34 for two animals, similar results were observed in the other animals.
Figure 4.
A plot of the isofrequency contours of the first notch frequencies for sources in the frontal hemisphere for the left ears of two animals (R016 and R004). First, notch frequencies increase with source elevation and azimuth toward the ipsilateral ear (−90° in this figure).
The role of the pinna in generating the spectral notches
Many studies have speculated that the spectral features, such as the notch, are generated by the pinna. To test this hypothesis, the pinna on both sides were surgically removed and the acoustic measurements were repeated in three animals (R001, R002, and R004). Removal of the pinna did not alter the position of the probe tube in the ear canal and care was taken to ensure that the animal remained centered in the loudspeaker arc (see Methods). Figure 5 shows DTFs for left ear of one animal [R004, Figs. 3c, 3d] after pinna removal; the DTFs are plotted in the same way as Fig. 3. The spectral notches apparent when the pinna were intact [Figs. 3c, 3d] were no longer apparent when the pinna were removed [Figs. 5a, 5b]. Removal of the pinna also eliminated the spectral notch cue in the other two animals (e.g., see the “head only” data in Fig. 6). This experiment supports the hypothesis that the spectral notches occurring from ∼16 to 30 kHz in rats are exclusively produced by the pinna.
Figure 5.
The DTFs for the left ear of one animal (R004) after the pinna were removed for sources at 15° azimuth and varying in elevation from −45° to +90° (panel A) and at 0° elevation varying from −90° (ipsilateral) to +90° (contralateral) azimuth (panel B). The spectral notches apparent with the pinna (Figs. 3c, 3d) were no longer present after pinna removal. The vertical bars bound the approximate frequency range of the first spectral notches, 16–30 kHz (see Fig. 3).
Figure 6.
Spatial distribution of DTF gains for seven frequencies for the right ear (+90° is ipsilateral) of one animal (R002) for three different conditions; intact animal with head and pinna (“head+pinna”), head only after the pinna were removed (“head only”), and the contribution of the pinna (“pinna only”) which was computed as the difference of the “head+pinna” and “head only” measurements. The color bar indicates the gain in decibels.
Spatial distribution of DTF amplitude gain
The acoustical gain of the DTF varied with source direction and frequency. Figure 6 (left column, “head+pinna”) shows the distribution of DTF gains for seven frequencies for sources in the frontal hemisphere for the right ear (+90° is ipsilateral) of one intact animal (R002). DTF gain was plotted only for the right ear as it was almost mirror symmetric with that of the left ear in most of the animals (this will be apparent when the ILD cue to location is discussed). The amplitude gain contained nonsystematic peaks and dips for sources behind the animal, so these sources were not considered further. Moreover, even in the frontal hemisphere, the DTF gains were also complicated for the frequencies corresponding to the first spectral notch (∼16–30 kHz); the spectral notches in Fig. 6 are particularly apparent for this animal from 20 to 30 kHz. The maximum DTF gains observed for the group of six animals tended to increase as a function of frequency; the gains were ∼4–5 dB at 5 kHz, 6 dB at 10 kHz, 12 dB at 15 kHz, and approached 15–20 dB for frequencies higher than 15 kHz. For frequencies higher than ∼20 kHz, there were often two or more distinct regions of high gain. For frequencies <5 kHz, the gains were <4 dB.
Since DTFs were computed, any acoustical gains that were nondirectional were removed from the data. For example, there is a large nondirectional gain associated with the resonance frequency of the ear canal. The gain of the canal resonance was estimated from the common components of the HRTFs (see Methods). The common component of the HRTFs averaged across the six rats revealed a peak in the gain of 24.1±3.3 dB occurring at 17.2 kHz.
Acoustical axis
The direction of maximum acoustical gain at a given frequency in the DTF gain spectra is known as the acoustical axis (Middlebrooks and Pettigrew, 1981; Phillips et al., 1982). Figure 6 (left column, “head+pinna”) shows the acoustical gain for one animal (R002) from which the acoustical axis could be found. The acoustical axis for each animal did not systematically vary as a function of frequency, but rather often made sudden and spatially distant jumps from one location to another. In all animals, the spatial directivity and the acoustical axis were not well defined for frequencies below ∼10 kHz. For the group of six animals, the acoustical axis for each frequency was observed after the spatial DTF gains were averaged across the animals. On average, for 10 kHz the acoustical axis occurred at (45°, −30°), for 15 kHz (22.5°, 15°), for 20 kHz (45°, 30°), for 25 kHz (37.5°, 37.5°), for 30 kHz (45°, 60°), and for 35 kHz the acoustical axis was (7.5°, 22.5°) (for these data, positive azimuths correspond to the ipsilateral hemisphere). The results based on the across-animal average DTF gains are in agreement with the individual results shown in Fig. 6 (left column).
The contribution of the pinna to acoustical gain and the acoustical axis
Figure 6 shows the DTF gain of the right ear (+90° is ipsilateral) for one animal (R002) at seven frequencies for three conditions; the original intact measurements (head+pinna), measurements after pinna removal (head only), and the difference between the intact and pinna-removed measurements (“pinna only”). The pinna only data show that the pinna significantly contributed to the DTF gain, but only at high frequencies above 20 kHz. The pinna by themselves generate up to 5–12 dB of gain for frequencies from 20 to 35 kHz. Below 20 kHz, the gain produced by the pinna is generally small, <2 dB. The pinna also produced substantial attenuation, some of which was shown in Fig. 5 where pinna removal eliminated the spectral notch cues. The spectral notches (i.e., regions of low gain surrounded by relatively higher gain) are particularly evident for frequencies of 20, 25, and 30 kHz in the head+pinna and pinna only acoustical gains (Fig. 6, left and right columns, respectively). The notches are not apparent in the head only condition (Fig. 6, middle column).
The pinna also contributed to the location of the acoustic axis. Examination of the head only condition shows that the gain produced by the head is largely symmetrical about the midsagittal plane and tends to monotonically increase as source location moves in azimuth from contralateral to ipsilateral. The head only acoustical axis tends to occur in the area from ∼45° to 90° at the ipsilateral ear and around 0° elevation. The acoustic axis with the pinna present does not obey this symmetry, suggesting that the directivity of the pinna at high frequencies determines the acoustic axis.
ILDs
Variation of ILD with frequency
The difference between left and right ear DTF gains (e.g., Fig. 6) results in the ILD spectra. ILD cues varied with frequency and source location. Positive and negative ILDs indicate higher DTF gain for left and right ears, respectively. Figure 7 shows for two animals (left column, R016; right column, R004) the ILDs at five different elevations when the azimuth was fixed at 0° (top row) and for five azimuths when the elevation was fixed at 0° (bottom row). ILDs varied with changes in source azimuth and frequency, but not with changes in source elevation. The distinctive positive and negative peaks in the ILDs between 15 and 30 kHz for sources varying in elevation are due to slight asymmetries in the spectral notch frequencies at the left and right ears (Figs. 346). These positive and negative peaks systematically moved to higher frequencies with changing azimuth angle and constant elevation consistent with the movements of the first notch frequencies (Figs. 346) Also, as a function of azimuth, ILDs are small, on the order of a few decibels, for low frequencies, and become systematically larger for frequencies up to ∼20 kHz. For frequencies from ∼20 to 30 kHz, the spectral notches that are primarily present in the contralateral ear (farthest from the source) help to create very large ILDs approaching 40 dB for some frequencies. Note also that for some source azimuths and frequencies, ILD was negative due to the first spectral notch at the ipsilateral ear.
Figure 7.
The ILD spectrum for two animals (R016 and R004). ILD spectrum is the frequency-by-frequency difference between left- and right-ear DTFs at a given location. Positive ILD indicates higher gain at the left ear than the right. ILDs do not change as with source elevation along the midsagittal plane (top row) but do substantially change with source azimuth along the horizontal plane (bottom row). ILDs for some frequencies and some locations may be as large at 40 dB.
Variation of ILD with azimuth and frequency
Figure 8a illustrates the way that the ILD cues for different frequencies vary with azimuth along the horizontal plane (i.e., at a constant elevation of 0°). The data are the mean (± standard error of the mean) ILDs computed across the six animals as a function of azimuth at six different frequencies. In general, ILDs symmetrically varied about the midline. At 5 kHz, the ILD steadily increased from −7 to +7 dB as the azimuth changed from −90° to +90°. For 10, 15, and 20 kHz, the ILDs varied from ∼±10, ∼±17, and ∼±26 dB, respectively, for azimuths between ∼±70°, but then tended to slightly decrease for larger azimuths. For 25 and 30 kHz, the ILDs varied from ∼±30 dB as the azimuth changed from −90° to +90°. Finally, the ILDs at 35 kHz were ±15 dB. At 35 kHz, the ILD cues were quite variable from animal to animal, leading to smaller average ILDs at each azimuth. The maximum mean ILDs across the six animals for sources along the horizontal plane in the frontal hemisphere were 7 dB at 5 kHz, 10 dB at 10 kHz, 17 dB at 15 kHz, 26 dB at 20 kHz, 28 dB at 25 kHz, 27 dB at 30 kHz, and 15 dB at 35 kHz.
Figure 8.
(A) The mean ILD cue (◻, +pin) computed across the six intact animals varies as a function of azimuth along the horizontal plane and frequency. Error bars indicated ±1 standard error of the mean (SEM). The mean ILDs are shown for six frequencies from 5 to 30 kHz. The mean ILD cue for three animals after removal of the pinna is also shown (●, −pin). Removal of the pinna reduced the ILDs for frequencies >10 kHz. (B) Rate of change of the ILD cue (i.e., ILD slope) with changes in source azimuth along the horizontal plane between ±30°. The ILD slope was obtained from the mean ILD cues computed across the six animals (panel A). Error bars plot ±1 SEM.
For most frequencies examined, the ILD cue was largely symmetric in azimuth about the midline (at 0° elevation) and also varied approximately linearly for sources between ±30°. We noticed that as the frequency increased, the rate of change of the ILD cue with changes in source azimuth, the ILD slope, also increased. Figure 8b plots the slope of the ILD cue (dB∕deg) for sources between ±30°. The slope steadily increased from ∼0.03 dB∕deg for frequencies <1.5 kHz up to 0.42 dB∕deg by 18.5 kHz. For higher frequencies, particularly between 18 and 30 kHz where the spectral notches occur, the ILD slope considerably varied from animal to animal. This can also be appreciated in the across-animal variability in ILD in Fig. 8a.
Spatial distribution of ILD at various frequencies
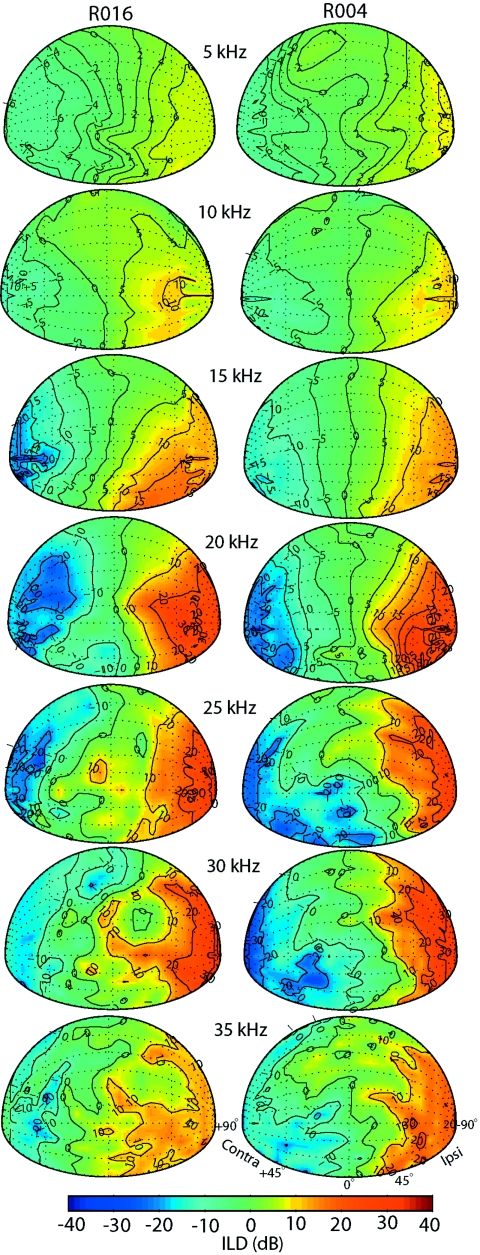
The data presented above indicate how ILD varies as a function of azimuth and frequency along the cardinal azimuthal dimension. Similar to the DTF gains, however, the ILD cue is actually a complex function of azimuth, elevation, and frequency. The spatial distributions of ILDs at various frequencies were studied by similarly plotting them to the monaural DTF gains (e.g., Fig. 6). Figure 9 shows the ILDs calculated at seven frequencies for two animals (R016 and R004). As was seen in Fig. 8a for the across-animal average ILDs, the ILDs were, in general, symmetrical between the right and left hemispheres for frequencies up to ∼15–20 kHz, but were more complex for higher frequencies. This is due, in part, to the role of the pinna, which generate the spectral notches (Figs. 346) and contribute to the DTF gain and location of the acoustic axis (Fig. 6). In particular, for the frequency ranges of the prominent first notches (∼15–30 kHz), the spatial distribution of ILDs can be quite complex.
Figure 9.
Spatial distribution of ILDs at seven different frequencies for two animals (R016 and R004) for locations in the frontal hemisphere. Positive ILDs indicate higher gain at the left ear (−90°) than the right ear (+90°). Color bar indicates ILD magnitude in dB.
Spatial distribution of directions with maximum and minimum ILDs
Like the monaural acoustical axis, the spatial location of the maximum ILD was dependent on frequency. Figure 9 shows the directions of maxima and minima in the ILD cue for different frequencies for animals R016 and R004. We defined the minimum of the ILD cue at each frequency as the binaural acoustical axis (using the maximum ILD yielded comparable results, as expected given the symmetry of the ILD computation). The binaural acoustic axes for R016 for 10 kHz were ∼(45°,0°), for 15 kHz ∼(52.5°,−7.5°), and ∼(52.5°,30°) for 20 and 25 kHz. Due to the complexity of the ILD cue for higher frequencies, the determination of the binaural acoustic axis was not always clear. As was done for Fig. 6, the spatial distributions of the ILD spectra were averaged across the six animals. On average, the binaural axes were (90°, 0°) for 5 kHz, (75°, 0°) for 10 kHz, (75°,−22.5°) for 15 kHz, (75°, 0°) for 20 kHz, (75°, 30°) for 25 kHz, (90°, 0°) for 30 kHz, and (45°,−15°) for 35 kHz.
The contributions of the pinna to the ILD cues
The importance of pinna in generating acoustical gain and the resulting ILD cues was examined by computing the spatial distributions of ILD before and after pinna removal. Figure 8a shows plots of the average ILDs as a function frequency and azimuth along the horizontal plane recorded in three animals (R001, R002, and R004) in two conditions: original measurements in the intact animals (+pin) and measurements after pinna removal (−pin). The contribution of the pinna themselves to ILD (−pin) can be seen where the two functions differ. This generally occurred for frequencies of 10 kHz and above; for frequencies <10 kHz, the two functions were nearly identical. For the higher frequencies, particularly those where the spectral notches were observed (16–30 kHz, Figs. 346) and where the pinna themselves introduce considerable acoustical gain (e.g., Fig. 6, “pinna only”), the pinna substantially contributed to the ILD. From the difference of the functions in Fig. 8a, the maximum contributions of the pinna to ILD were 2.2, 7.4, 7.8, 17.5, 10.9, 15.4, and 8.4 dB for frequencies of 5, 10, 15, 20, 25, 30, and 35 kHz, respectively.
ITDs
Spatial distribution of ITDs
The ITD cue was dependent mostly on sound source azimuth. Positive and negative ITD values indicate that sound was leading at left and right ears respectively. The detailed frequency dependence of ongoing ITDs in the fine structure (or equivalent interaural phase differences) was not examined in detail in this paper. Rather, we focus here on the interaural envelope delays conveyed by the impulse responses of the DTFs (e.g., Middlebrooks and Green, 1990). Figure 10a shows spatial distribution of envelope ITDs in the frontal hemisphere for two animals (R016 and R004). For both animals, the ITD was symmetric about the midline. Although not shown, comparable results were observed for ITDs in the rear hemisphere. The same trends were observed for the other four animals. Across the six animals in this study, the maximum ITD averaged 127±14 μs. The maximum ITDs based on five of six animals (one animal had empirical ITDs that were anomalously larger than expected based on its head diameter) were positively correlated (r=0.79) with the linear head diameter. Maximum ITDs in all animals tended to occur within ±10° of the most lateral azimuth (90°).
Figure 10.
(A) Spatial distribution of envelope ITDs for two animals (R016 and R004) for locations in the frontal hemisphere. Positive ITDs indicate that the signal leads to the left ear. Color bar and countour lines indicate the ITD in microseconds. (B) The envelope ITDs in three animals (R001, R002, and R004) as a function of azimuth along the horizontal plane in two conditions: intact (filled symbols, +pin) and after removal of the pinna (open symbols, −pin). Removal of the pinna reduced the ITDs at all azimuthal locations.
Examination of the spatial dependence of ITD in Fig. 10a shows that envelope-based ITDs essentially follow the contours of azimuth and show little to no dependence on elevation. Results such as this would be expected from a spherical head model of ITDs. As such, similar results on maximum envelope ITD were obtained by first collapsing the ITD measurements from all elevations onto a single dimension of azimuth and then fitting the Woodworth (1938) spherical head model to these data. The Woodworth model is given by
| (1) |
where r is the radius of the head (in meters), c is the speed of sound (340 m∕s), and θ is the lateral angle of the source relative to the median plane. In the model, the radius r is the only free parameter. For all animals, the fitted model produced correlation coefficients >0.95. Finding the maximum ITD by using this procedure allows the ITD data from all spatial locations to contribute to the estimate.
By using this same procedure, low-frequency ongoing ITDs in the fine structure were also calculated as described in the Methods. In short, the left and right ear impulse responses at each location were low-pass filtered (3.5 kHz), cross correlated, and the ITD was taken as the delay corresponding to the maximum in the correlation function. ITDs measured in this way were larger than the envelope-based high-frequency ITDs. As before, the Woodworth model fits all produced correlation coefficients >0.95. Across the six rats, the maximum low-frequency ITDs estimated by using the fitting procedure described above was 158±8 μs. This value is 1.25 times larger than the high-frequency envelope ITDs.
Pinna contribution to high-frequency envelope-based ITDs
It is often believed that the pinna do not play a large part in determining the ITD cue to sound location, particularly for low-frequency ongoing ITDs (e.g., Roth et al., 1980). For the high-frequency envelope-based ITD cues measured here, however, we hypothesized that the pinna does play a role. For high-frequency sounds, whose wavelengths are on the order of, or smaller, than the linear dimensions of the pinna, the pinna may present a significant obstacle for the sound. Thus, we expected the pinna to increase the magnitude of the envelope-based ITDs. To test this hypothesis, for three animals (R001, R002, and R004), the pinna were completely removed and ITDs were remeasured.
Figure 10b shows the envelope ITDs measured with (filled symbols) and without the pinna (open symbols) for the three animals for sources varying in azimuth at 0° elevation. The maximum ITDs for these three animals were determined in both conditions by using the fitting procedure described in the previous section. The mean maximum ITDs were significantly reduced from 120 to 77 μs after removing the pinna. The position of the probe tube microphone was not altered in any way by removing the pinna due to its deep placement in the ear canal. Thus, removal of the pinna in rats reduced the maximal high-frequency envelope-based ITDs by 36%. The ITDs in the two conditions were also computed at each azimuth for the three animals, and the average reduction after pinna removal (excluding 0° azimuth) was 27±0.13%. The pinna in the rat appear to alter the ITDs more for lateral angles near the poles than for source locations in front of the animal. The removal of the pinna also reduced the low-frequency ITDs by 32% to an average of 108±3 μs. Thus, for both low- and high-frequency envelope-based ITD cues, the pinna effectively increases the functional diameter of the head, thereby increasing the magnitude of the ITD.
DISCUSSION
Comparison of present study with other animal studies
Monaural aspects of the acoustical cues: spectral notches, gain, and acoustic axis
The spectral notch cues were found in the rat primarily for source locations in the frontal hemisphere. First, notch frequencies increased from ∼16 to 30 kHz as the source elevation increased from −45° to +90° and from ∼17 to 23 kHz as the source azimuth moved from −30° to +90° toward the ipsilateral ear. In general, the first notch frequencies increased as location varied from low elevations and contralateral azimuths to high elevations and ipsilateral azimuths. Similar patterns of first notch frequency changes with source azimuth and elevation changes have been observed in other species, albeit at different rates and over different frequency ranges (Rhesus monkey, 5–15 kHz: Spezio et al., 2000; human, 6–12: Middlebrooks, 1999; bat 30–50 kHz: Wotton et al., 1995; Fuzessery, 1996; Firzlaff and Schuller, 2003; Aytekin et al., 2004). In the cat, where spectral cues have been studied in great detail, first, notch frequencies have been found to vary from ∼8 to 18 kHz (Musicant et al., 1990; Rice et al., 1992; Young et al., 1996; Xu and Middlebrooks, 2000; Tollin, 2004).
In the gerbil, which has slightly smaller pinna and head size than the rat, spectral notch cues have also been observed to move in a similar fashion with azimuth and elevation as the rat, but over a frequency range of ∼25–45 kHz (Maki and Furukawa, 2005). It is likely that the linear dimensions of the pinna determine the frequency range for spectral notches. Maki and Furukawa reported that the pinna height [e.g., (A)–(C), Fig. 1] for their adult gerbils was ∼12 mm, which is 67% of the rat pinna dimensions measured here. If we assume a linear scaling of first notch frequency with pinna size (e.g., Middlebrooks, 1999), then scaling the observed range of gerbil spectral notch cues (25–45 kHz) by 67% yields a predicted spectral notch cue range of 16–30 kHz for the rats, which is what we found here. As expected, species with larger pinna (human, cat, and monkey cited above) produce notches at lower frequencies.
We showed here that the pinna of the rat were essential for generating the spectral notch cues to sound location. Similar observations have been made in other species where the spectral notch cues have also been observed and then eliminated after pinna removal (bat: Wotton et al., 1995; Aytekin et al., 2004; cat: Musicant et al., 1990; ferret: Parsons et al., 1999). Further evidence that the spectral notches are created by the pinna comes from examination of the changes in first notch frequencies with changes in the positions of the pinna on the head (Young et al., 1996; Xu and Middlebrooks, 2000). In these latter studies, systematic rotations of the pinna were accompanied by predictable shifts in the spatial locations of the first notch frequencies. Finally, examination of the audiograms of rats reveals a prominent increase in thresholds of up to 16 dB, relative to neighboring frequencies, for frequencies between 16 and 32 kHz (Kelly and Masterton, 1977; Heffner et al., 1994). This frequency range overlaps that for which we found spectral notches for sources in the frontal hemisphere.
Studies in a variety of species have shown that the acoustic axis changes for different frequencies, but often in complicated and nonsystematic ways. In the rat, the acoustical axis generally changed from lower to higher elevations as the source frequency increased from 10 to 30 kHz. For frequencies >35 kHz, the acoustic axis decreased somewhat in elevation. In some animals, for frequencies >∼20 kHz, there were sometimes two or more distinct spatial locations associated with high gain. This observation is consistent with the splitting of the acoustic axis for frequencies above 8 kHz in humans (Middlebrooks et al., 1989) and above 55 kHz in bats (Firzlaff and Schuller, 2003). Comparable observations on the complexities of the acoustic axis have been reported in other species (cat: Musicant et al., 1990; Phillips et al., 1982; Middlebrooks and Knudsen, 1987; Martin and Webster, 1989; Calford and Pettigrew, 1984; Tammar wallaby: Coles and Guppy, 1986; bat: Obrist et al., 1993; Firzlaff and Schuller, 2003; Fuzessery, 1996; Aytekin et al., 2004; gerbil: Maki and Furukawa, 2005; Rhesus monkey: Spezio et al., 2000; owl: Keller et al., 1998; ferret: Carlile, 1990).
There have been some models of localization based on a cue similar to the acoustical axis (e.g., Musicant and Butler, 1984). However, in general, the acoustical axis in the rat, like that in the cat (Phillips et al., 1982) and gerbil (Maki and Furukawa, 2005), changes in such complex ways with frequency, and often with multiple peaks, that we believe it to be a poor cue for sound source localization. Rather, as suggested by Middlebrooks and Pettigrew (1981), Coles and Guppy (1986), and Young et al. (1996), the frequency dependence of the acoustic axis along with the ability of some animals with mobile pinna to independently manipulate the left and right pinna positions might allow the pinna to operate like an acoustical antenna, increasing the signal-to-noise ratio for sounds of interest, at the expense of other sounds. The increased acoustical gain due to the pinna might then be more useful for source detection than for localization. The pinna of the rat are indeed independently mobile (Li and Frost, 1996).
Finally, we studied the role of the pinna in the monaural DTF gain and the acoustical axis by making acoustic measurements before and after removing the pinna. Similar experiments have been done in other species (cat: Wiener et al., 1966; Phillips et al., 1982; Musciant et al., 1990; guinea pig: Palmer and King, 1985; Carlile and Pettigrew, 1987; Tammar wallaby: Coles and Guppy, 1986; bat: Orbist et al., 1993; Fuzessery, 1996; Aytekin et al., 2004; ferret: Carlile and King, 1994). In agreement with Carlile and Pettigrew (1987) and Carlile and King (1994), after pinna removal the acoustical axis, for virtually all frequencies, was oriented along the ipsilateral interaural axis, supporting the hypothesis that the pinna play a critical role in establishing the acoustic axis for high frequencies. Moreover, the overall gains of the DTFs were reduced for most high frequencies, confirming that the pinna is responsible for part of the gain at high frequencies. In the rat, the pinna themselves generated ∼5–12 dB of gain for frequencies from 20 to 35 kHz. Below 15 kHz, the gain due to the pinna was small. Pinna-only gains of 3–15 dB, with the higher gains occurring at higher frequencies, have been reported in other species (Coles and Guppy, 1986; Carlile and King, 1994; Schnupp et al., 1998).
Finally, since DTFs were computed in this paper, any acoustical gains that were largely nondirectional, such as the gain associated with the resonance frequency of the ear canal, were removed from the data. Examination of the common component of the HRTFs averaged across the six rats revealed a peak in the gain of 24.1±3.3 dB occurring at 17.2 kHz, which is comparable to the recent estimates by Gratton et al. (2008) of 19.1 kHz and ∼27 dB.
Binaural aspects of the acoustical cues
ILDs were highly dependent on both frequency and spatial location. ILDs were small for low frequencies <5 kHz, but rapidly increased for frequencies up to 20 kHz. Up to ∼20 kHz, the ILDs systematically varied with azimuth and would likely provide a very stable cue for localization. Figure 8b shows that the rate of change of the ILD cue with changes in azimuth (i.e., the ILD slope) systematically increased with frequency up to ∼20 kHz. For frequencies from ∼20 to 35 kHz, the ILD cues were complicated due to the presence of the spectral notches. The deep notches resulted in large ILDs that for some frequencies and locations could approach 35 dB or more. For frequencies >35 kHz, the spatial distribution of ILDs was quite variable.
In the cat, the ILD slope has been shown to monotonically increase from ∼0.17 dB∕deg from 2 kHz to ∼0.6 dB∕deg by 8 kHz (Irvine, 1987; Martin and Webster, 1989). These range and magnitude of ILD slope values are comparable to that found here in the rat. In the cat, above 8 kHz, the ILD slopes were also large, but did not systematically vary from frequency to frequency. Thus, as was the case here in the rat, there appears to be a range of frequencies (<8 kHz) in the cat where the ILD cue might also provide a stable cue for localization. Rice et al. (1992) refered to this region as the ΔL region. Interestingly, in the cat, the spectral notches begin to occur first for frequencies at ∼8 kHz. In the rat, the notches first begin at about 16 kHz. In both species, it appears that ILDs systematically vary with azimuth and also increase their slope for frequencies up to where the spectral notches first occur.
We showed here that the pinna play an important role in the formation and spatial distribution of the ILD cue in rats. Removal of both pinna changed the acoustical gain and the acoustic axis for frequencies >10 kHz, so it should not be surprising that the pinna also contribute to the ILD cue. From the difference of the functions in Figure 8a, the maximum contributions of the pinna to ILD were 2.2, 7.4, 7.8, 17.5, 10.9, 15.4, and 8.4 dB for frequencies of 5, 10, 15, 20, 25, 30, and 35 kHz, respectively. At locations nearer to the acoustic axis, for particular frequencies, the contribution of the pinna to ILD could be even larger. Contributions of the pinna to ILD by up to 9 dB have been reported in the ferret (Carlile and King, 1994; Schnupp et al., 1998; Parsons et al., 1999). In guinea pig (Carlile and Pettigrew, 1987) pinna removal reduced ILDs for frequencies >5 kHz; similar to that found here in the rat, the largest ILD reduction occurred for those locations and frequencies associated with the acoustic axis.
Finally, the monaural gains and ILDs measured in rats by Harrison and Downey (1970) for a few frequencies and locations along the horizontal plane were remarkably similar to the data we recorded here by using DTFs.
Both low-frequency ongoing fine-structure ITDs and high-frequency envelope-based ITDs were measured. These ITDs systematically and symmetrically varied about the midsagittal plane [Fig. 10a]. Maximum envelope-based ITDs averaged 127 μs (n=6 rats). This value is 13% larger than the predicted maximum ITD of 111 μs based on the Woodworth (1938) spherical head model by using the across-rat average head diameter of 29.6 mm (n=6). By using the same average head diameter, a maximum value of only 87 μs is predicted based on Kuhn’s (1977) model for high-frequency ITDs [ITD(θ)=2(r∕c)sin(θ)]. Our empirical measurements of low-frequency ongoing ITDs, which averaged 158 μs (n=6 rats), were 1.25 times larger than the empirical high frequency ITDs. A maximum value of 131 μs is predicted based on Kuhn’s (1977) model for low-frequency ITDs [ITD(θ)=3(r∕c)sin(θ)]. In all cases, the empirical maximum ITD values were substantially larger than the appropriate spherical head model predictions. None of the models takes into account the possible role of the pinna. Maki and Furukawa (2005) found maximum high-frequency ITDs in gerbils that were ∼30% larger than those predicted based on the Woodworth (1938) model and the measured head diameters.
The finding that the low-frequency ongoing ITDs were 1.25 times larger than high-frequency envelope-based ITDs is in agreement with the theoretical spherical head model of ITDs by Kuhn (1977). The measurement in humans and subsequent modeling by Kuhn suggests low-frequency ITDs should be ∼1.5 times larger than high-frequency ITDs due to the dispersion that occurs when sound waves encounter an object, such as the head. Similar differences between low- and high-frequency ITDs and the discrepancies between empirical ITDs and those predicted based on spherical head models have been observed (cat: Roth et al., 1980; monkey: Spezio et al., 2000; gerbil: Maki and Furukawa, 2005; guinea pig: Sterbing et al., 2003).
For sources between ±30° along the horizontal plane, the rates of change (i.e., ITD slope) of the ITD cue with azimuth were 1.72 and 2.2 μs∕deg for the high-frequency envelope ITD and low-frequency ITD, respectively. The ITD cues change nearly linearly for azimuths within ∼±30° of the midsagital plane.
We examined the role of the pinna in the formation of the ITD cue in three animals. The maximum high-frequency ITDs in those animals were reduced by 36%, from 120 to 77 μs, after removing the pinna. On average, across azimuths (excluding 0°), the ITDs were reduced by 27%. The maximum ITDs of 77 μs without the pinna (i.e., head-only ITDs) are comparable to the predictions of the maximum ITDs based on Kuhn’s high-frequency ITD model of 87 μs. These results together reveal that the pinna substantially contributed to the magnitude of high-frequency envelope ITDs, and do so more at larger lateral angles. Few studies have examined the role of the pinna in ITD cue generation. Roth et al. (1980) in the cat found that folding back the pinna in the cat had little effect on low-frequency ongoing ITDs, but did quite substantially reduce the high-frequency ITDs. We were surprised here to find that low-frequency ITDs in the rat were reduced 32% by pinna removal from an average of 158 to 108 μs. Our result might be due to different sound stimuli and∕or to the different methods of computing ITD relative to Roth et al. (1980). Alternatively, Roth et al. (1980) may have underestimated the role of the pinna because they did not completely remove the pinna, but rather only folded it back on itself. This procedure likely still leaves a considerable portion of the pinna and distal parts of the auditory meatus in the path of the sound. In future studies of the contribution of the pinna to ITDs, the pinna should be completely removed to avoid this potential confound.
ACKNOWLEDGMENTS
Special thanks to Staci Stanford and Dr. Karl Pfenninger for providing the animals for these experiments. Thanks to Dr. John Middlebrooks, Dr. Henry Heffner, Dr. Tom Yin, and Dr. Philip Joris and also Heath Jones and Eric Lupo for comments on the manuscript. This work was supported by National Institutes of Deafness and Other Communicative Disorders Grant No. DC-006865 to DJT and the National Institutes of Child and Health Development Grant No. HD-2080 to HLR.
References
- Aytekin, M., Grassi, E., Sahota, M., and Moss, C. F. (2004). “The bat head-related transfer function reveals binaural cues for sound localization in azimuth and elevation,” J. Acoust. Soc. Am. 10.1121/1.1811412 116, 3594–3605. [DOI] [PubMed] [Google Scholar]
- Beecher, M. D., and Harrison, J. M. (1971).“Rapid acquisition of an auditory localization discrimination by rats,” J. Exp. Anal Behav. 16, 193–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyerl, B. D. (1978). “Afferent projections to the central nucleus of the inferior colliculus in the rat,” Brain Res. 145, 209–223. [DOI] [PubMed] [Google Scholar]
- Brand, A., Behrend, O., Marquardt, T., McAlpine, D., and Grothe, B. (2002). “Precise inhibition is essential for microsecond interaural time difference coding,” Nature (London) 10.1038/417543a 417, 543–547. [DOI] [PubMed] [Google Scholar]
- Burlile, C. J., Feldman, M. L., Craig, C., and Harrison, J. M. (1985). “Control of responding by the location of sound: Role of binaural cues,” J. Exp. Anal Behav. 43, 315–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calford, M. B., and Pettigrew, J. D. (1984). “Frequency dependence of directional amplification at the cat’s pinna,” Hear. Res. 10.1016/S0378-5955(00)00083-6 14, 13–19. [DOI] [PubMed] [Google Scholar]
- Carlile, S. (1990). “The auditory periphery of the ferret. I: Directional response properties and the pattern of interaural level differences,” J. Acoust. Soc. Am. 10.1121/1.400115 88, 2180–2195. [DOI] [PubMed] [Google Scholar]
- Carlile, S., and Pettigrew, A. G. (1987). “Directional properties of the auditory periphery in the guinea pig,” Hear. Res. 10.1016/0378-5955(87)90117-1 31, 111–122. [DOI] [PubMed] [Google Scholar]
- Carlile, S., and King, A. J. (1994). “Monaural and binaural spectrum level cues in the ferret: acoustics and the neural representation of auditory space,” J. Neurophysiol. 71, 785–801. [DOI] [PubMed] [Google Scholar]
- Chan, J. C. K., and Geisler, C. D. (1990). “Estimation of tympanic membrane acoustic pressure and of ear canal length from remote points in the canal,” J. Acoust. Soc. Am. 10.1121/1.398799 87, 1237–1247. [DOI] [PubMed] [Google Scholar]
- Chen, Q.-C., Cain, D., and Jen, P. H.-S. (1995). “Sound pressure transformation at the pinna of Mus Domesticus,” J. Exp. Biol. 198, 2007–2023. [DOI] [PubMed] [Google Scholar]
- Clopton, B. M., and Silverman, B. M. (1977). “Plasticity of binaural interaction. II. Critical period and changes in midline response,” J. Neurophysiol. 40, 1275–1280. [DOI] [PubMed] [Google Scholar]
- Coles, R. B., and Guppy, A. (1986). “Biophysical aspects of directional hearing in the tammar wallaby, Macropus eugenii,” J. Exp. Biol. 121, 371–394. [Google Scholar]
- Finlayson, P. G., and Caspary, D. M. (1991). “Low-frequency neurons in the lateral superior olive exhibit phase-sensitive binaural inhibition,” J. Neurophysiol. 65, 598–605. [DOI] [PubMed] [Google Scholar]
- Firzlaff, U., and Schuller. G., (2003). “Spectral directionality of the external ear of the lesser spear-nosed bat, Phyllostomus discolor,” Hear. Res. 10.1016/S0378-5955(03)00164-3 181, 27–39. [DOI] [PubMed] [Google Scholar]
- Flammino, F., and Clopton, B. M. (1975). “Neural responses in the inferior colliculus of albino rat to binaural stimuli,” J. Acoust. Soc. Am. 10.1121/1.380494 57, 692–695. [DOI] [PubMed] [Google Scholar]
- Fuzessery, Z. M. (1996), “Monaural and binaural spectral cues created by the external ears of the pallid bat,” Hear. Res. 10.1016/0378-5955(95)00223-5 95, 1–17. [DOI] [PubMed] [Google Scholar]
- Gratton, M. A., Bateman, K., Cannuscio, J. F., and Saunders, J. C. (2008). “Outer- and middle-ear contributions to presbycusis in the brown norway rat,” Audiol. Neuro-Otol. 10.1159/000107551 13, 37–52. [DOI] [PubMed] [Google Scholar]
- Harrison, J. M., and Downey, P. (1970). “Intensity changes at the ear as a function of the azimuth of a tone source: a comparative study,” J. Acoust. Soc. Am. 10.1121/1.1912082 47, 1509–1518. [DOI] [PubMed] [Google Scholar]
- Harrison, J. M. (1988). “Control of responding by sounds of different quality: An evolutionary analysis,” J. Exp. Anal Behav. 50, 521–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffner, H. E., and Heffner, R. S. (1985). “Sound localization in wild Norway rats (Rattus norvegicus),” Hear. Res. 10.1016/0378-5955(85)90119-4 19, 151–155. [DOI] [PubMed] [Google Scholar]
- Heffner, H. E., Heffner, R. S., Contos, C., and Ott, T. (1994). “Audiogram of the hooded Norway rat,” Hear. Res. 10.1016/0378-5955(94)90240-2 73, 244–247. [DOI] [PubMed] [Google Scholar]
- Heffner, R. S. (1997). “Comparative study of sound localization and its anatomical correlates in mammals,” Acta Oto-Laryngol., Suppl. 10.3109/00016489709126144 532, 46–53. [DOI] [PubMed] [Google Scholar]
- Hernández, O., Espinosa, N., Pérez-González, D., and Malmierca, M. S. (2005). “The inferior colliculus of the rat: A quantitative analysis of monaural frequency response areas,” Neuroscience (Oxford) 10.1016/j.neuroscience.2005.01.001 132, 203–217. [DOI] [PubMed] [Google Scholar]
- Inbody, S. B., and Feng, A. S. (1981). “Binaural response characteristics of single neurons in the medial superior olivary nucleus of the albino rat,” Brain Res. 210, 361–366. [DOI] [PubMed] [Google Scholar]
- Irvine, D. R. (1987). “Interaural intensity differences in the cat: Changes in sound pressure level at the two ears associated with azimuthal displacements in the frontal horizontal plane,” Hear. Res. 10.1016/0378-5955(87)90063-3 26, 267–286. [DOI] [PubMed] [Google Scholar]
- Irvine, D. R., Park, V. N., and Mattingley, J. B. (1995). “Responses of neurons in the inferior colliculus of the rat to interaural time and intensity differences in transient stimuli: Implications for the latency hypothesis,” Hear. Res. 10.1016/0378-5955(95)00040-B 85, 127–141. [DOI] [PubMed] [Google Scholar]
- Irvine, D. R., Park, V. N., and McCormick, L. (2001). “Mechanisms underlying the sensitivity of neurons in the lateral superior olive to interaural intensity differences,” J. Neurophysiol. 86, 2647–2666. [DOI] [PubMed] [Google Scholar]
- Irving, R., and Harrison, J. M. (1967). “The superior olivary complex and audition: a comparative study,” J. Comp. Neurol. 130, 77–86. [DOI] [PubMed] [Google Scholar]
- Jen, P. H., and Chen, D. M. (1988). “Directionality of sound pressure transformation at the pinna of echolocating bats,” Hear. Res. 10.1016/0378-5955(88)90098-6 34, 101–117. [DOI] [PubMed] [Google Scholar]
- Kandler, K., and Gillespie, D. C. (2005). “Developmental refinement of inhibitory sound-localization circuits,” Trends Neurosci. 10.1016/j.tins.2005.04.007 28, 290–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller, C. H., Hartung, K., and Takahashi, T. T. (1998). “Head-related transfer functions of the barn owl: Measurement and neural responses,” Hear. Res. 10.1016/S0378-5955(98)00014-8 118, 13–34. [DOI] [PubMed] [Google Scholar]
- Kelly, J. B. (1980). “Effects of auditory cortical lesions on sound localization by the rat,” J. Neurophysiol. 44, 1161–1174. [DOI] [PubMed] [Google Scholar]
- Kelly, J. B., and Glazier, S. J. (1978). “Auditory cortex lesions and discrimination of spatial location by the rat,” Brain Res. 145, 315–321. [DOI] [PubMed] [Google Scholar]
- Kelly, J. B., and Masterton, B. (1977). “Auditory sensitivity of the albino rat,” J. Comp. Physiol. Psychol. 10.1037/h0077356 91, 930–936. [DOI] [PubMed] [Google Scholar]
- Kelly, J. B., and Kavanagh, G. L. (1986). “Effects of auditory cortical lesions on pure-tone sound localization by the albino rat,” Behav. Neurosci. 10.1037/0735-7044.100.4.569 100, 569–575. [DOI] [PubMed] [Google Scholar]
- Kelly, J. B., and Judge, P. W. (1985). “Effects of medial geniculate lesions on sound localization by the rat,” J. Neurophysiol. 53, 361–372. [DOI] [PubMed] [Google Scholar]
- Kelly, J. B., and Sally, S. L. (1988). “Organization of auditory cortex in the albino rat: binaural response properties,” J. Neurophysiol. 59, 1756–1769. [DOI] [PubMed] [Google Scholar]
- Kelly, J. B., and Phillips, D. P. (1991). “Coding of interaural time differences of transients in auditory-cortex of rattus-norvegicus—implications for the evlolution of mammalian sound localization,” Hear. Res. 10.1016/0378-5955(91)90089-R 55, 39–44. [DOI] [PubMed] [Google Scholar]
- Kelly, J. B., Buckthought, A. D., and Kidd, S. A. (1998). “Monaural and binaural response properties of single neurons in the rat’s dorsal nucleus of the lateral lemniscus,” Hear. Res. 10.1016/S0378-5955(98)00082-3 122, 25–40. [DOI] [PubMed] [Google Scholar]
- Koay, G., Kearns, D., Heffner, H. E., and Heffner, R. S. (1998). “Passive sound-localization ability of the big brown bat (Eptesicus fuscus),” Hear. Res. 10.1016/S0378-5955(98)00037-9 119, 37–48. [DOI] [PubMed] [Google Scholar]
- Kuhn, G. F. (1977). “Model for the interaural time differences in the azimuthal plane,” J. Acoust. Soc. Am. 10.1121/1.381498 62, 157–167. [DOI] [Google Scholar]
- Leong, P., and Carlile, S. (1998). “Methods for spherical data analysis and visualization,” J. Neurosci. Methods 10.1016/S0165-0270(97)00201-X 80, 191–200. [DOI] [PubMed] [Google Scholar]
- Li, L., and Frost, B. J. (1996). “Azimuthal sensitivity of rat pinna reflex: EMG recordings from cervicoauricular muscles,” Hear. Res. 10.1016/0378-5955(96)00119-0 100, 192–200. [DOI] [PubMed] [Google Scholar]
- Li, L., and Kelly, J. B. (1992). “Binaural responses in rat inferior colliculus following kainic acid lesions of the superior olive: interaural intensity difference functions,” Hear. Res. 10.1016/0378-5955(92)90038-O 61, 73–85. [DOI] [PubMed] [Google Scholar]
- Maki, K., and Furukawa, S. (2005). “Acoustical cues for sound localization by the Mongolian gerbil, Meriones unguiculatus,” J. Acoust. Soc. Am. 10.1121/1.1944647 118, 872–886. [DOI] [PubMed] [Google Scholar]
- Martin, R. L., and Webster, W. R. (1989). “Interaural sound pressure level differences associated with sound-source locations in the frontal hemifield of the domestic cat,” Hear. Res. 10.1016/0378-5955(89)90072-5 38, 289–302. [DOI] [PubMed] [Google Scholar]
- McAlpine, D., and Grothe, B. (2003). “Sound localization and delay lines—do mammals fit the model?,” Trends Neurosci. 10.1016/S0166-2236(03)00140-1 26, 347–350. [DOI] [PubMed] [Google Scholar]
- Middlebrooks, J. C., and Pettigrew, J. D. (1981). “Functional classes of neurons in primary auditory cortex of the cat distinguished by sensitivity to sound location,” J. Neurosci. 1, 107–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middelbrooks, J. C., and Knudsen, E. I. (1987). “Changes in external ear position modify the spatial tuning of auditory units in the cat’s superior colliculus,” J. Neurophysiol. 57, 672–687. [DOI] [PubMed] [Google Scholar]
- Middlebrooks, J. C., Makous, J. C., and Green, D. M. (1989). “Directional sensitivity of sound-pressure levels in the human ear canal,” J. Acoust. Soc. Am. 10.1121/1.398224 86, 89–108. [DOI] [PubMed] [Google Scholar]
- Middlebrooks, J. C., and Green, D. M. (1990). “Directional dependence of interaural envelope delays,” J. Acoust. Soc. Am. 10.1121/1.399183 87, 2149–2162. [DOI] [PubMed] [Google Scholar]
- Middlebrooks, J. C. (1999). “Individual differences in external-ear transfer functions reduced by scaling in frequency,” J. Acoust. Soc. Am. 10.1121/1.427176 106, 1480–1492. [DOI] [PubMed] [Google Scholar]
- Moiseff, A. (1989). “Binaural disparity cues available to the barn owl for sound localization,” J. Acoust. Soc. Am. 10.1121/1.380961 59, 1222–1226. [DOI] [PubMed] [Google Scholar]
- Moore, D. R., and Irvine, D. R. F. (1979). “A developmental study of the sound pressure transformation by the head of the cat,” Acta Oto-Laryngol. 10.3109/00016487909126447 87, 434–440. [DOI] [PubMed] [Google Scholar]
- Musicant, A. D., and Butler, R. A. (1984). “The psychophysical basis of monaural localization,” Hear. Res. 10.1016/S0378-5955(00)00113-1 14, 185–190. [DOI] [PubMed] [Google Scholar]
- Musicant, A. D., Chan, J. C., and Hind, J. E. (1990). “Direction-dependent spectral properties of cat external ear: New data and cross-species comparisons,” J. Acoust. Soc. Am. 10.1121/1.399545 87, 757–781. [DOI] [PubMed] [Google Scholar]
- Obrist, M. K., Fenton, M. B., Eger, J. L., and Schlegel, P. A. (1993). “What ears do for bats: A comparative study of pinna sound pressure transformation in chiroptera,” J. Exp. Biol. 180, 119–152. [DOI] [PubMed] [Google Scholar]
- Parsons, C. H., Lanyon, R. G., Schnupp, J. W. H., and King, A. J. (1999). “Effects of altering spectral cues in infancy on horizontal and vertical sound localization by adult ferrets,” J. Neurophysiol. 82, 2294–2309. [DOI] [PubMed] [Google Scholar]
- Palmer, A. R., and King, A. J. (1985). “A monaural space map in the guinea-pig superior colliculus,” Hear. Res. 10.1016/0378-5955(85)90071-1 17, 267–280. [DOI] [PubMed] [Google Scholar]
- Phillips, D. P., Calford, M. B., Pettigrew, J. D., Aitkin, L. M., and Semple, M. N. (1982). “Directionality of sound pressure transformation at the cat’s pinna,” Hear. Res. 10.1016/0378-5955(95)00130-4 8, 13–28. [DOI] [PubMed] [Google Scholar]
- Rice, J. J., May, B. J., Spirou, G. A., and Young, E. D. (1992). “Pinna-based spectral cues for sound localization in cat,” Hear. Res. 10.1016/0378-5955(92)90123-5 58, 132–152. [DOI] [PubMed] [Google Scholar]
- Rife, D. D., and Vanderkooy, J. (1989). “Transfer-function measurement with maximum-length sequences,” J. Audio Eng. Soc. 37, 419–444. [Google Scholar]
- Roth, G. L., Kochhar, R. K., and Hind, J. E. (1980). “Interaural time differences: Implications regarding the neurophysiology of sound localization,” J. Acoust. Soc. Am. 10.1121/1.385196 68, 1643–1651. [DOI] [PubMed] [Google Scholar]
- Schnupp, J. W. H., King, A. J., and Carlile, S. (1998). “Altered spectral localization cues disrupt the development of the auditory space map in the superior colliculus of the ferret,” J. Neurophysiol. 79, 1053–1069. [DOI] [PubMed] [Google Scholar]
- Schnupp, J. W. H., Booth, J., and King., A. J. (2003). “Modeling individual differences in ferret external ear transfer functions,” J. Acoust. Soc. Am. 10.1121/1.1547460 113, 2021–2030. [DOI] [PubMed] [Google Scholar]
- Silverman, M. S., and Clopton, B. M. (1977). “Plasticity of binaural interaction. I. Effect of early auditory deprivation,” J. Neurophysiol. 40, 1266–1274. [DOI] [PubMed] [Google Scholar]
- Spezio, M. L., Keller, C. H., Marrocco, R. T., and Takahashi, T. T. (2000). “Head-related transfer functions of the rhesus monkey,” Hear. Res. 10.1016/S0378-5955(00)00050-2 144, 73–88. [DOI] [PubMed] [Google Scholar]
- Sterbing, S. J., Hartung, K., and Hoffmann, K.-P. (2003). “Spatial tuning to virtual sounds in the inferior colliculus of the guinea pig,” J. Neurophysiol. 10.1152/jn.00348.2003 90, 2648–2659. [DOI] [PubMed] [Google Scholar]
- Tollin, D. J. (2004). “The development of the acoustical cues to sound location in cats,” Assoc. Res. Otolaryngol. Abstr. 27, 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesolek, C. M., Koay, G., and Heffner, H. E. (2007). “Laboratory rats do not use binaural time cues to localize sound,” J. Acoust. Soc. Am. 10.1121/1.2431330 121, 3093 (Abstract). [DOI] [Google Scholar]
- Wiener, F. M., Pfeiffer, R. R., and Backus, A. S. N. (1966). “On the sound pressure transformation by the head and auditory meatus of the cat,” Acta Oto-Laryngol. 10.3109/00016486609127062 61, 255–269. [DOI] [PubMed] [Google Scholar]
- Wightman, F. L., and Kistler, D. J. (1989). “Headphone simulation of free-field listening. I: Stimulus synthesis,” J. Acoust. Soc. Am. 10.1121/1.397557 85, 858–867. [DOI] [PubMed] [Google Scholar]
- Woodworth, R. S. (1938). Experimental Psychology (Henry Holt and Co., New York: ). [Google Scholar]
- Wotton, J. M., Haresign, T., and Simmons, J. A. (1995). “Spatially dependent acoustic cues generated by the external ear of the big brown bat, Eptesicus fuscus,” J. Acoust. Soc. Am. 10.1121/1.413410 98, 1423–1445. [DOI] [PubMed] [Google Scholar]
- Xu, L., and Middlebrooks, J. C. (2000). “Individual differences in external-ear transfer functions of cats,” J. Acoust. Soc. Am. 10.1121/1.428432 107, 1451–1459. [DOI] [PubMed] [Google Scholar]
- Young, E. D., Rice, J. J., and Tong, S. C. (1996). “Effects of pinna position on head-related transfer functions in the cat,” J. Acoust. Soc. Am. 10.1121/1.414883 99, 3064–3076. [DOI] [PubMed] [Google Scholar]