Abstract
Background
In chronic schizophrenia and chronic bipolar disorder, gamma band (30–100 Hz) auditory steady-state EEG responses (ASSRs) are reduced in power and phase locking, likely reflecting neural circuit dysfunction. Here we examined whether gamma ASSR deficits are also present at first hospitalization for psychosis.
Methods
Subjects were 16 first episode schizophrenia patients (SZ), 16 first episode affective disorder patients (AFF) (13 with bipolar disorder), and 33 healthy controls (HC). Stimuli were 20, 30, and 40 Hz binaural click trains. ASSR phase locking and evoked power were analyzed using the Morlet wavelet transform.
Results
At 40 Hz stimulation, SZ and AFF had significantly reduced phase locking compared to HC. This deficit was more pronounced over the left hemisphere in SZ. Evoked power at 40 Hz was also reduced in the patients compared to HC. At 30 Hz stimulation phase locking and evoked power were reduced in both patient groups. The 20 Hz ASSR did not differ between groups, but phase locking and evoked power of the 40 Hz harmonic of the 20 Hz ASSR were reduced in both SZ and AFF. Phase locking of this 40 Hz harmonic was correlated with total positive symptoms in SZ.
Conclusions
The gamma ASSR deficit is present at first hospitalization for both schizophrenia and affective disorder, but shows a left hemisphere bias in first hospitalized SZ. Some of the neural circuitry abnormalities underlying the gamma ASSR deficit may be common to psychoses in general, while others may be specific to particular disorders.
Keywords: schizophrenia, bipolar disorder, first episode psychosis, EEG, gamma oscillation, auditory steady-state response
The synchronous activity of neurons, mediated by oscillations in the gamma band (30–100 Hz) of the electroencephalogram (EEG), has been proposed to play an important role in the linking of neurons into cell assemblies that code information in the brain (1). Abnormalities of gamma oscillations in the scalp-recorded EEGs of schizophrenia patients have been hypothesized to reflect neural circuit abnormalities in this disorder (2–4). Much of this evidence has come from studies of auditory steady-state responses (ASSRs), in which simple auditory stimuli such as clicks are delivered at rapid rates and entrain the EEG at the stimulation frequency (5). The ASSR appears to have a “resonant” frequency at 40 Hz (6), at which frequency the power and phase locking of the ASSR are enhanced compared to other stimulation frequencies. While it is not thought that the ASSR itself reflects any process related to the formation of cell assemblies, its 40 Hz resonance suggests that the underlying neural circuits preferentially oscillate at this frequency and thus may rely on some of the same circuit and intrinsic neuron properties as non-driven (sensory evoked and cognitive-related) gamma oscillations. Chronic schizophrenia patients show a “gamma ASSR deficit” with reduced power and phase locking of ASSRs in the gamma band but not at lower frequencies (7–10) compared to healthy individuals. This deficit appears to be most pronounced for 40 Hz stimulation. The gamma ASSR deficit has also been reported in chronic bipolar disorder patients (11, 12) and early onset psychosis (13), suggesting that it may be a manifestation of a neural circuit disorder (or set of disorders) that is shared by psychoses in general.
However, it has remained unknown whether this deficit appears early in the course of the disorder, or only appears later, consistent with reports of progression of the pitch mismatch negativity and gray matter volume loss in Heschl’s gyrus (14) or possibly the result of chronic medication. Nor is it known whether this deficit is present in all psychoses or is specific to schizophrenia. This report describes our efforts to address these important questions in the first study (to our knowledge) of the ASSR in first-episode (first hospitalization) patients with schizophrenic and affective (mainly bipolar) psychosis and in healthy controls. We examined the ASSR at beta (13–29 Hz) and gamma stimulation frequencies. The two main aims of this study were, first to determine whether the gamma ASSR deficit was present at first hospitalization and second, to determine, if present, if the gamma ASSR deficit was specific to schizophrenic psychosis, thereby providing useful information on commonality or differences between these two psychoses.
Method
Subjects
This study was approved by the McLean Hospital Institutional Review Board. After complete description of the study to the subjects, written informed consent was obtained.
Subjects were 32 first-episode psychosis patients and 34 healthy controls (HC; 14 female) paid for their participation. The HC were recruited from the local community through newspaper advertisements and were free of Axis I or II disorders (SCID-NP, 15; SCID II, 16), as well as a history of Axis I disorders in first-degree relatives. One HC was classified as an outlier and his data were excluded because his 40 Hz evoked power was > 4 standard deviations (SD) above the group mean, and his 30 Hz evoked power and 40 Hz phase locking values were > 2 SD above the respective group means. This exclusion did not meaningfully change the pattern of results. The final HC sample consisted of 33 subjects (14 female).
Patients were diagnosed with schizophrenia (SZ, N=16; 4 female) or affective disorder (AFF, N=16; 5 female) according to DSM-IV criteria (SCID, 17). The diagnostic composition of the SZ group was: 12 paranoid, 3 schizoaffective, and 1 disorganized. The AFF group consisted of 13 patients (4 female) with bipolar disorder and 3 patients (1 female) with major depression, all with psychotic features. Diagnoses were confirmed at a follow-up interview at least 6 months after the initial hospitalization when possible (6 SZ, 4 AFF). Psychosis patients participated in the study at 13.6 +/− 10.8 days (mean +/− SD) days following admission. The time from admission to the EEG recording session did not differ between the patient groups (see Table 1).
Table 1.
Subject demographic and clinical data. Mean +/− SD are listed for each group.
| HC | SZ | AFF | Statistic | p | |
|---|---|---|---|---|---|
| % female subjects | 42.4 | 25.0 | 31.3 | χ2[2] = 1.59 | 0.452 |
| Age (years) | 27.5 +/− 8.7 | 25.5 +/− 8.1 | 24.4 +/− 7.4 | F[2,62] = 0.850 | 0.432 |
| Parental socio-economic status | 1.6 +/− 0.8 | 1.8 +/− 1.1 | 1.4 +/− 0.6 | F[2,56] = 1.06 | 0.353 |
| Handedness | 0.76 +/− 0.14 | 0.78 +/− 0.21 | 0.76 +/− 0.12 | F[2,52] = 0.075 | 0.928 |
| Mini-Mental State Examination | 29.3 +/− 0.7 | 28.3 +/− 1.8 | 28.8 +/− 1.0 | F[2,52] = 4.35 | 0.018* |
| WAIS-R information subscale | 13.9 +/− 2.2 | 12.8 +/− 2.3 | 12.9 +/− 2.0 | F[2,52] = 1.77 | 0.180 |
| Days from admission | 14.9 +/− 11.9 range 3–42 |
12.3 +/− 9.8 range 4–37 |
F[1,29] = 0.404 | 0.530 | |
| Duration of continuous antipsychotic medication (days) | 14.7 +/− 12.1 range 2–42 |
12.3 +/− 9.8 range 4–37 |
F[1,29] = 0.354 | 0.557 | |
| Medication dosage (CPZ equivalents) | 338 +/− 196 range 50–800 |
220 +/− 125 range 75–556 |
F[1,25] = 3.54 | 0.072 | |
| Global Assessment Scale | 34.8 +/− 7.2 | 38.2 +/− 6.0 | F[1,25] = 1.82 | 0.189 | |
| PANSS total | 80.9 +/− 12.1 | 72.3 +/− 16.0 | F[1,29] = 2.84 | 0.103 | |
| PANSS: Positive symptom total | 20.9 +/− 4.5 | 20.9 +/− 7.2 | F[1,29] = 0.00 | 0.997 | |
| PANSS: Negative symptom total | 17.8 +/− 5.5 | 13.2 +/− 5.1 | F[1,29] = 5.83 | 0.022* | |
| PANSS: General symptom total | 37.7 +/− 7.0 | 32.9 +/− 6.5 | F[1,29] = 3.91 | 0.058 | |
| # trials: 20 Hz | 145 +/− 8 | 145 +/− 7 | 142 +/− 13 | F[2,62] = 0.590 | 0.557 |
| # trials: 30 Hz | 142 +/− 12 | 145 +/− 7 | 145 +/− 6 | F[2,62] = 0.733 | 0.485 |
| # trials: 40 Hz | 145 +/− 7 | 144 +/− 6 | 146 +/− 7 | F[2,62] = 0.252 | 0.778 |
Subjects were selected without regard for ethnicity and met our standard inclusion criteria: 1) right-handed as assessed by the Edinburgh Handedness Inventory (18); 2) no history of electroconvulsive treatment; 3) no history of neurological illness, including epilepsy; 4) no alcohol or drug dependence, or history of a “detox” admission within the last 5 years (DSM-IV criteria); 5) no present medication for medical disorders that would have deleterious EEG, neurological, or cognitive functioning consequences; and 6) estimated verbal IQ above 75.
Parental socioeconomic status was assessed with the Hollingshead two-factor index (19). The Mini-Mental State Examination (MMSE) was performed with all participants to rule out any dementia or delirium. The information subscale of the WAIS-R was used to estimate general fund of information. The Global Assessment Scale (GAS) (20) was administered to all patients to evaluate severity of illness and general level of functioning. Psychotic symptoms were rated with the Positive and Negative Syndrome Scale (PANSS) (21). Demographic and clinical data and inter-group comparisons are summarized in Table 1. The groups did not differ on age, gender proportion, handedness, parental socioeconomic status, or WAIS-R score. There was a significant effect of Group on MMSE score, with SZ scoring slightly lower than HC. SZ had significantly higher PANSS negative symptom ratings than AFF, and there was a trend for them to have higher general symptom ratings as well.
All patients received atypical antipsychotics, and 2 SZ and 2 AFF also received typical antipsychotics. Antipsychotic medication dosage was calculated in terms of chlorpromazine equivalents (22, 23). There was a trend for SZ to have a higher average medication dosage than AFF (Table 1). Within each group, the following numbers of subjects received additional medications: mood stabilizers – 4 SZ, 10 AFF; anti-depressants – 6 SZ, 9 AFF; anti-anxiolytics – 4 SZ, 2 AFF.
Stimuli and Experimental Design
Subjects were seated in a quiet room in a comfortable chair 1.1 m in front of a computer monitor. Stimuli were presented through headphones (55 dB sound pressure level) in three blocks of stimuli (150 per block): 20 Hz, 30 Hz, and 40 Hz stimulation rates. Stimuli consisted of trains of 1 ms white noise clicks (500 ms duration, 1100 ms stimulus onset asynchrony). Subjects were instructed to look at the fixation cross on the monitor and listen to the stimuli. The order of blocks was counterbalanced across subjects.
Electrophysiological Recording and Analysis
The EEG was recorded with Neuroscan Synamp 1 amplifiers (0.01–100 Hz, 500 Hz digitization) using sintered Ag/Ag-Cl electrodes in an electrode cap at 60 scalp sites, nosetip, and left mastoid, referenced to the right mastoid. The forehead (AFz) served as ground. Bipolar vertical and horizontal electro-oculograms were recorded from electrodes above and below the right eye and at the left and right outer canthi, respectively. Electrode impedances were <5 kΩ.
The epoching of continuous EEG files and artifact-related processing were performed using BrainVision Analyzer (Brain Products GmbH). Single-trial epochs were extracted from - 250 to 850 ms relative to stimulus onset and corrected for eye movements and blinks (24). Next, epochs containing artifacts were removed. The artifact criteria were: 1) > +/− 100 µV change in one time point; 2) amplitude range within an epoch exceeding 300 µV; and 3) <100 ms flat EEG (+/−0.5 µV range). These criteria were visually tested and verified. Finally, artifact-free single epochs were re-referenced to averaged mastoids. There were no differences between the subject groups in the number of trials per condition after artifact rejection (Table 1).
Averaging and time-frequency analyses were performed using custom software written in the IDL programming environment (ITT Visual Information Solutions). ERPs were averaged for each driving stimulus frequency. For ASSR analyses, the Morlet wavelet transform was applied to the single epochs in the 20–100 Hz frequency range of the EEG from −250 to 772 ms. Event-related spectral measures were computed on the wavelet-transformed epochs for each stimulus condition at each time point and wavelet frequency to yield time-frequency maps (25). Phase locking measures the variance of phase across single trials, and is mathematically independent of power. Phase locking is computed as one minus the circular variance of phases and ranges from 0 (random distribution of phases) to 1 (perfect phase locking). Evoked power measures the power of the average ERP, in which the contribution of non-stimulus-locked activity is minimized.
Average baseline values were subtracted from each time-frequency map (−150 to −50 ms). (There were no significant effects on baseline values for either spectral measure.) Average spectral measures were computed at the time points (30–530 ms), electrodes (Fp1/2, F1/2, F3/4, FC1/2, FC3/4, C1/2, C3/4), and wavelet frequencies (20 Hz: 20.3–22.1 Hz; 30 Hz: 31.3–34.1 Hz; 40 Hz: 40.5–44.1 Hz) where both ASSR phase locking and evoked power were maximal. Latency effects are not reported here, as inspection of the data and preliminary analyses indicated that the observed group differences did not vary strongly with latency, consistent with the findings of Light et al. (10).
Statistical Analysis
Dependent variables (phase locking and evoked power) were analyzed with ANOVA in the design Group (SZ/AFF/HC) X Hemisphere (Left/Right) X Site (7 per hemisphere). Separate ANOVAs were performed for each stimulation frequency (20, 30, and 40 Hz, as well as the 40 Hz harmonic of the 20 Hz ASSR). The Greenhouse-Geisser correction for inhomogeneity of variance (26) is reflected in the reported p values. Dunnett’s t was used for post-hoc comparisons, testing that SZ and AFF values were less than HC values and correcting for multiple comparisons. Spearman’s ρ was used for correlation analyses (2-tailed). For all statistical analyses, α = 0.05 (unless otherwise noted).
Results
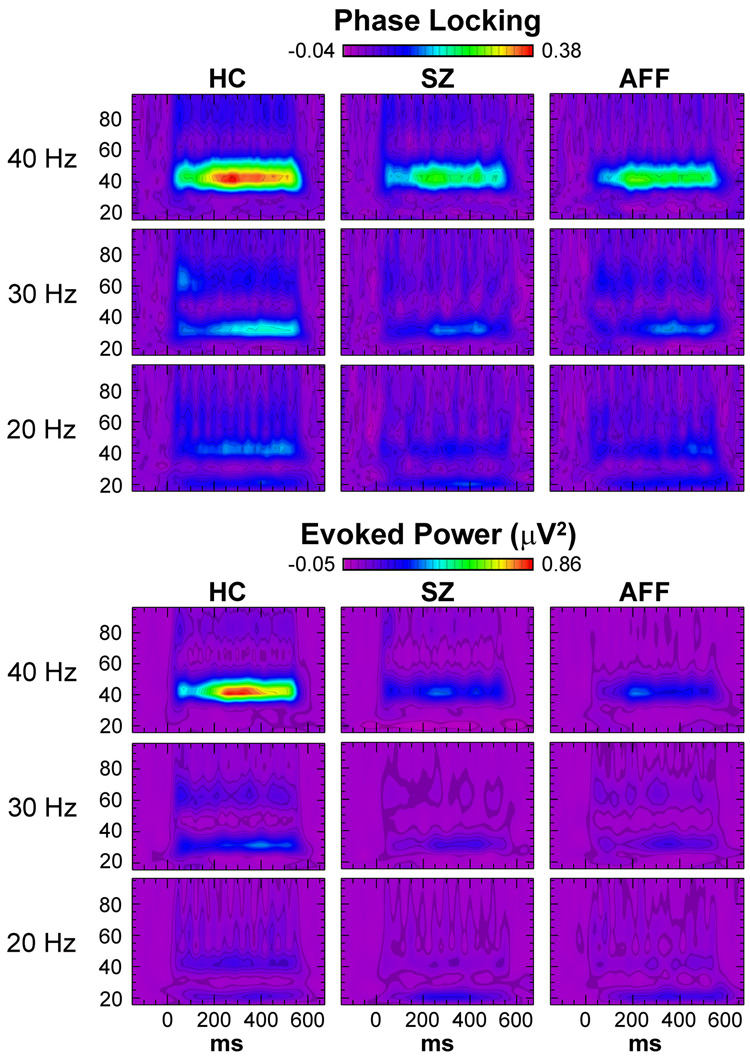
As is typical, the ASSR was maximal for 40 Hz stimulation in both the phase locking and evoked power data (Fig. 1). Also, during 20 Hz stimulation a robust harmonic was apparent at 40 Hz.
Fig. 1.
Time-frequency maps of ASSR phase locking (top) and evoked power (bottom) at electrode Cz for each subject group for 20, 30, and 40 Hz stimulation. Subject groups are healthy controls (HC), schizophrenia patients (SZ), and affective disorder patients (AFF).
Phase locking
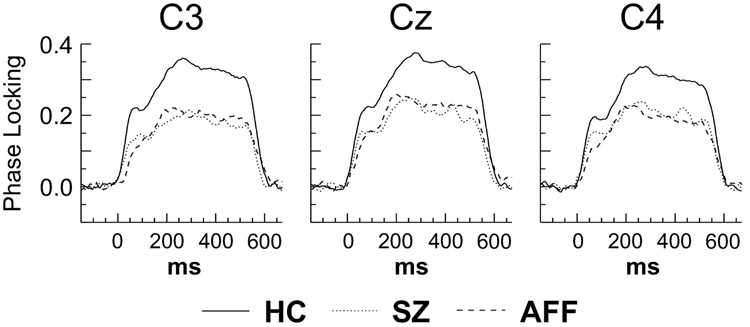
During 40 Hz stimulation the phase locking aspect of the ASSR was reduced in SZ and AFF compared to HC. The main effect of Group was significant (F[2,62] = 4.09, p < 0.05), as were post-hoc comparisons between SZ and HC (p < 0.05) and AFF and HC (p < 0.05). In addition, the Group X Hemisphere interaction was significant (F[2,62] = 3.49, p < 0.05). When the analysis was repeated for each patient group compared to the HC separately, the Group X Hemisphere interaction was significant for SZ (F[1,47] = 6.41, p < 0.05) but not AFF (F[1,47] = 1.63, p = 0.208). This pattern of effects reflected a larger reduction of phase locking in SZ over the left hemisphere (HC – SZ = 0.114, p < 0.05) compared to the right hemisphere (HC – SZ = 0.091, p < 0.05) (see Fig. 2).
Fig. 2.
Phase locking time series from the 40 Hz ASSR at left hemisphere (C3), midline (Cz), and right hemisphere (C4) electrodes. The time series were extracted from the frequency bands of the maximal response in Fig. 1 (40.5–44.1 Hz wavelet center frequencies).
For 30 Hz stimulation there was a significant reduction of phase locking in patients (F[2,62] = 3.25, p < 0.05). Post-hoc tests found that this reduction was significant for SZ (p < 0.05) and marginally significant for AFF (p = 0.053). The Group X Hemisphere interaction was not significant (F[2,62] = 0.152, p = 0.859).
During 20 Hz stimulation the ASSR at 20 Hz did not differ between groups (F[2,62] = 0.029, p = 0.972). The Group X Hemisphere interaction also was not significant (F[2,62] = 0.095, p = 0.909). In contrast, there was a significant effect of Group on the 40 Hz harmonic of the 20 Hz ASSR (F[2,62] = 3.26, p < 0.05). Post-hoc comparisons found that the 40 Hz harmonic was significantly reduced in SZ compared to HC (p < 0.05). In AFF the reduction compared to HC was marginally significant (p = 0.051). The Group X Hemisphere interaction for the 40 Hz harmonic was not significant (F[2,62] = 1.48, p = 0.235).
Evoked power
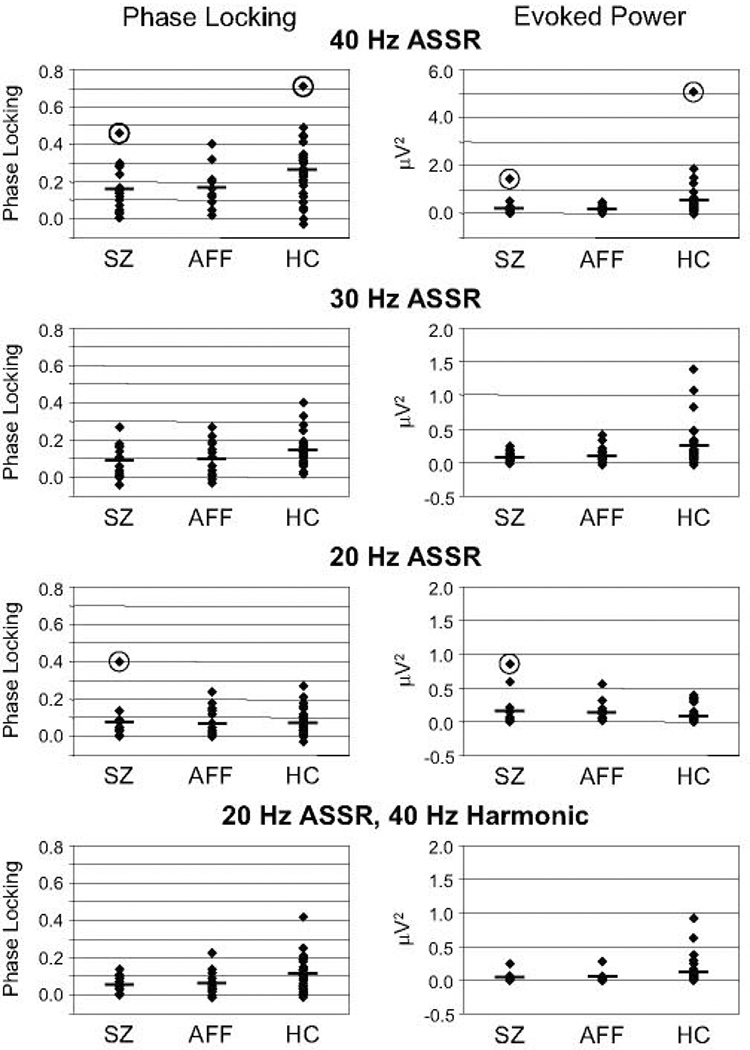
While ASSR evoked power during 40 Hz stimulation appeared to be reduced in SZ and AFF compared to HC, the main effect of Group only approached significance (F[2,62] = 2.63, p = 0.080), and the Group X Hemisphere interaction was not significant (F[2,62] = 0.473, p = 0.626). Since the reduction of 40 Hz evoked power has been a consistent finding of ASSR studies of psychosis, we examined this measure further. In post-hoc tests the reduction approached significance in SZ (p = 0.075) and was marginally significant in AFF (p = 0.052). Inspection of the distributions of individual values (Fig. 3, top plots) revealed outliers in the SZ and HC groups. In the other measures these subjects’ values were not outliers, so we decided not to exclude them from analyses (unlike the HC subject described in the Subjects section). Therefore, to reduce the influence of these outliers, we analyzed the Group effect with the non-parametric Kruskal-Wallis χ2 test, and found a significant effect (χ2[2] = 10.3, p < 0.01). In paired comparisons with the Mann-Whitney U test, 40 Hz evoked power was significantly reduced in SZ (U = 136, p < 0.05) and AFF (U = 149, p < 0.05) compared to HC (correcting for multiple comparisons).
Fig. 3.
Scatterplots illustrating intersubject variability in phase locking and evoked power, measured at fronto-central electrodes. Horizontal bars indicate the mean values for each group for comparison with the individual values. Outliers are circled for both measures. Note that the y-axis on the 40 Hz ASSR evoked power plot has a different scale than the other plots to accommodate the outlying values.
During 30 Hz stimulation evoked power was reduced in SZ and AFF compared to HC (Group: F[2,62] = 3.74, p < 0.05; post-hoc tests: p’s < 0.05). The Group X Hemisphere interaction was not significant (F[2,62] = 0.672, p = 0.514).
For 20 Hz stimulation, evoked power at 20 Hz appeared to be larger in SZ than AFF and HC, but the main effect of Group was not significant (F[2,62] = 0.192, p = 0.826). The increase in power for SZ and AFF was due to the presence of outliers who also had extremely large phase locking scores (Fig. 3). The Group X Hemisphere interaction was not significant (F[2,62] = 0.064, p = 0.938). The 40 Hz harmonic of the 20 Hz ASSR was significantly reduced in both patient groups (Group: F[2,62] = 3.18, p < 0.05; post-hoc tests: p’s < 0.05). The Group X Hemisphere interaction for the 40 Hz harmonic was not significant (F[2,62] = 0.051, p = 0.950).
Correlations with demographic and clinical variables
To rule out confounding effects, correlations were calculated between ASSR measures of interest and demographic variables. The ASSR measures of interest (those that differed between groups) were phase locking and evoked power at 40 Hz, 30 Hz, and the 40 Hz harmonic of the 20 Hz ASSR, averaged across the fronto-central electrodes (see Methods). In no group were there correlations with age, parental socio-economic status, handedness, medication dosage, or time from admission.
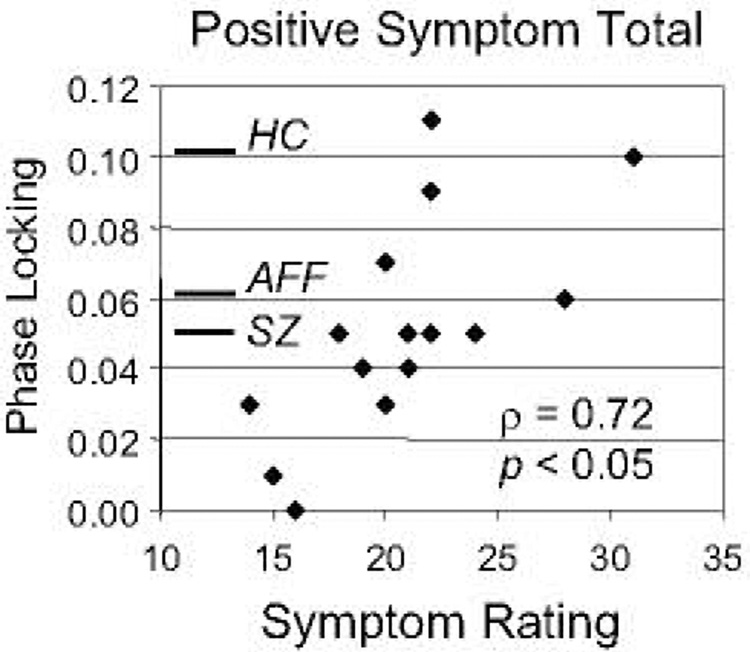
Next we conducted exploratory correlations between the ASSR measures of interest and clinical symptom ratings on the PANSS. We examined correlations with the PANSS Positive, Negative, and General Symptom subtotals, applying a Bonferroni correction with α= 0.05 (6 ASSR measures X 3 symptom totals = 18 correlations per group). No significant correlations were found in the AFF group. In the SZ group, phase locking of the 40 Hz harmonic of the 20 Hz ASSR was correlated with the Positive Symptom Total (N=15, ρ = 0.72, p < 0.05 [p = 0.0026 uncorrected]) (Fig. 4). The correlation between 40 Hz harmonic phase locking and the Hallucinations symptom score appeared to make the largest contribution to the overall positive symptom correlation, but was not significant at the corrected p level (N=15, ρ = 0.67, p = 0.123 [p = 0.0068 uncorrected]).
Fig. 4.
Scatterplot illustrating the correlation between the PANSS Positive Symptom Total score and phase locking of the 40 Hz harmonic of the 20 Hz ASSR in SZ. The mean phase locking values for each group are indicated with horizontal bars. Note that only the SZ with the most positive phase locking values were near the mean of the HC group.
Discussion
This study demonstrates that the gamma ASSR deficit is present at first hospitalization for both schizophrenia and affective disorder, particularly bipolar disorder. (We note that the effects reported for the AFF group did not change appreciably when the non-bipolar patients were removed from the analyses.) Thus, the gamma ASSR deficit that has been observed in chronic psychosis patients does not appear to be due to brain abnormalities that only arise due to the chronic state, although progressive changes in severity might still occur. Nor does the gamma ASSR deficit appear to be due to long-term consequences of antipsychotic medication.
While both the SZ and AFF groups showed reduced gamma-frequency ASSRs compared to HC, there were differences between the groups in the expression of the gamma ASSR deficit. First, for 40 Hz phase locking the gamma ASSR deficit was more pronounced over the left hemisphere in SZ. The lateralization of the gamma ASSR deficit to the left hemisphere in the schizophrenia patients is consistent with a large body of evidence for structural and functional abnormalities of the left hemisphere in schizophrenia (e.g., 27–31). The current data suggest that schizophrenia is characterized by left hemisphere pathology, particularly during the early phase of the disease. In contrast, the deficit in affective psychosis patients is bilateral.
Second, the SZ demonstrated a correlation between symptom ratings and the ASSR, while no correlations were found for the AFF. Counterintuitively, this correlation was in the positive direction, indicating that the ASSR was more normal in SZ who were more symptomatic. In the scatterplot of the Positive Symptom Total vs. 40 Hz harmonic phase locking values in Fig. 4, the mean phase locking values for each group are indicated to demonstrate the how intersubject variability relates to the overall between-group differences. The SZ with the highest symptom ratings have phase locking values near the HC mean, while the majority of SZ have much lower phase locking values.
A similar pattern was also found in our second study of Gestalt perception, in which the phase locking of a beta oscillation associated with visual object perception was positively correlated in SZ with positive symptoms such as visual hallucinations and thought disorder (3). Here positive symptoms were again involved. We have hypothesized that such positive correlations, particularly involving positive symptoms, may reflect a propensity for a dysfunctional cortical network to synchronize inappropriately in psychosis (3, 32). However, given the unusual nature of this correlation, replication of this finding is crucial. Another important question is why this correlation was found for the 40 Hz harmonic of the 20 Hz ASSR, but not for the 40 Hz ASSR itself. Longitudinal testing of this cohort should determine whether this positive correlation remains, or whether patients that remain psychotic begin to show less synchronous activity.
In the present study the 30 Hz ASSR was reduced in both patient groups compared to controls. In reviewing the ASSR studies in schizophrenia and bipolar disorder to date that tested the ASSR at ~30 Hz, it appears that the occurrence of deficits at this frequency is variable. Brenner et al. (7) and Light et al. (10) reported 30 Hz deficits in schizophrenics, while Kwon et al. (9) and Hong et al. (8) did not. In bipolar disorder, O’Donnell et al. (12) found a 30 Hz ASSR deficit, while O’Donnell et al. (11) did not. Furthermore, Brenner et al. (7) and O’Donnell et al. (12) found ASSR deficits across a wide range of frequencies (although the deficits appeared greatest in the gamma band). As more ASSR studies are conducted (hopefully with a wider range of frequencies), it will be important to determine whether the frequency range of ASSR deficits reflects factors such as symptom patterns or medication type.
Finally, in the present study we observed an outlier in the SZ group with extremely large (> 2 SD) 20 Hz phase locking and evoked power values (Fig. 3). This subject caused the SZ group to have a larger 20 Hz evoked power ASSR than the HC group, although the difference was not significant. Similarly, Kwon et al. (9) reported that 20 Hz ASSR evoked power in chronic schizophrenia patients was non-significantly larger than in healthy controls (as well as a 20 Hz subharmonic during 40 Hz stimulation that we did not observe), as did Light et al. (10). In examining the outlier’s demographic and clinical data we could not find any particular measures that distinguished this patient from the rest of the SZ group. Individual patients with extremely large 20 Hz ASSRs have not been reported in the three other published ASSR studies of schizophrenia patients (7, 8, 10). Further research will be necessary to determine whether these patients may in fact represent a particular subtype of schizophrenia.
These data demonstrate that the gamma driving deficit is already present at first hospitalization for psychosis in both schizophrenia and affective disorder, but they also raise the issue of diagnostic specificity. Both patient groups evinced similar patterns of responses across frequencies, suggesting that the same neural circuit abnormalities to which the ASSR is sensitive are present in schizophrenia and affective psychosis. This would not be a surprising result, since there is a considerable degree of overlap in inhibitory interneuron-related neural circuit abnormalities in schizophrenia and bipolar disorder (33–37). However, we are not aware of studies that have examined neural circuitry in the auditory cortex of affective disorders, and to date little work has been done in schizophrenia, so it remains to be determined whether auditory cortical abnormalities at the microcircuit level are the basis for similarities in the gamma driving deficit. On the other hand, this study demonstrates that the gamma driving deficits are not identical, as the difference in the laterality of the deficit clearly differentiates schizophrenia (left-sided) and affective psychosis (bilateral) and therefore provides diagnostic specificity. In addition, since the gamma driving deficit in bipolar disorder appears to be state-dependent (11), it is possible that the deficits in schizophrenia and bipolar disorder may be differentiated by their state vs. trait dependence. The disorders may also be distinguished by the presence of individuals with extremely large 20 Hz responses in schizophrenia, but not in affective psychosis.
To date, two other studies have examined gamma oscillations in first-episode schizophrenia patients. In these studies, subjects performed auditory oddball tasks. Gallinat et al. (38) observed a reduction in the power of a late evoked gamma oscillation but did not find any differences in the power of the early stimulus-evoked oscillation. In contrast, Symond et al. (39) found that a measure of inter-electrode phase synchrony was reduced in first episode patients compared to controls, as in chronic patients (40). Taken together, the results of the present study and those of Gallinat et al. and Symond et al. imply that stages of auditory processing are differentially affected in early-stage psychosis.
In conclusion, the present findings demonstrate that the gamma ASSR deficit is present at first hospitalization for psychosis in patients with schizophrenia and affective psychoses. The phase locking aspect of the deficit is more pronounced at left hemisphere electrode sites in SZ. These results suggest that schizophrenia and affective psychosis may share some common neural circuitry abnormalities that are expressed in the gamma ASSR deficit, but the differing patterns of expression of this deficit (such as lateralization and correlations with clinical variables) imply that it is related to different aspects of psychosis. However, we note that to the extent to which medication effects might be responsible for the present results, similarities in the gamma ASSR deficit between schizophrenic and affective psychosis patients might be due to the use of the same medications.
Acknowledgments
Supported by a Department of Veterans Affairs Research Enhancement Award Program and Schizophrenia Center (RWM); National Institute of Mental Health grants R01 40799 (RWM), R01 58704 (DFS), and R03 076760 (KMS); and a Young Investigator Award from the National Alliance for Research in Schizophrenia and Depression (KMS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure
Drs. Spencer, Salisbury, Shenton, and McCarley reported no biomedical financial interests or potential conflicts of interest.
References
- 1.Singer W. Neuronal synchrony: a versatile code for the definition of relations? Neuron. 1999;24:49–65. doi: 10.1016/s0896-6273(00)80821-1. [DOI] [PubMed] [Google Scholar]
- 2.Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- 3.Spencer KM, Nestor PG, Perlmutter R, Niznikiewicz MA, Klump MC, Frumin M, Shenton ME, McCarley RW. Neural synchrony indexes disordered perception and cognition in schizophrenia. Proc Natl Acad Sci USA. 2004;101:17288–17293. doi: 10.1073/pnas.0406074101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Uhlhaas PJ, Singer W. Neural synchrony in brain disorders: relevance for cognitive dysfunctions and pathophysiology. Neuron. 2006;52:155–168. doi: 10.1016/j.neuron.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 5.Galambos R, Makeig S, Talmachoff J. A 40 Hz auditory potential recorded from the human scalp. Proc Natl Acad Sci USA. 1981;78:2643–2647. doi: 10.1073/pnas.78.4.2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pastor MA, Artieda J, Arbizu J, Marti-Climent JM, Peñuelas I, Masdeu JC. Activation of human cerebral and cerebellar cortex by auditory stimulation at 40 Hz. J Neurosci. 2002;22:10501–10506. doi: 10.1523/JNEUROSCI.22-23-10501.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brenner CA, Sporns O, Lysaker PH, O'Donnell BF. EEG synchronization to modulated auditory tones in schizophrenia, schizoaffective disorder, and schizotypal personality disorder. Am J Psychiatry. 2003;160:2238–2240. doi: 10.1176/appi.ajp.160.12.2238. [DOI] [PubMed] [Google Scholar]
- 8.Hong LE, Summerfelt A, McMahon R, Adami H, Francis G, Elliott A, Buchanan RW, Thaker GK. Evoked gamma band synchronization and the liability for schizophrenia. Schizophr Res. 2004;70:293–302. doi: 10.1016/j.schres.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 9.Kwon JS, O’Donnell BF, Wallenstein GV, Greene RW, Hirayasu Y, Nestor PG, Hasselmo ME, Potts GF, Shenton ME, McCarley RW. Gamma frequency-range abnormalities to auditory stimulation in schizophrenia. Arch Gen Psychiatry. 1999;56:1001–1005. doi: 10.1001/archpsyc.56.11.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Light GA, Hsu JL, Hsieh MH, Meyer-Gomes K, Sprock J, Swerdlow NR, Braff DL. Gamma band EEG oscillations reveal neural network cortical coherence dysfunction in schizophrenia patients. Biol Psychiatry. 2006;60:1231–1240. doi: 10.1016/j.biopsych.2006.03.055. [DOI] [PubMed] [Google Scholar]
- 11.O’Donnell BF, Hetrick WP, Bodkins M, Vohs JL, Bismark A, Skosnik PD, Johannesen JK, Carroll CA, Shekhar A. New Developments in Mania Research. Hauppauge, NY: Nova Science Press; 2006. Event-related potential abnormalities in bipolar disorder: relationship to symptoms, medication, and substance disorders; pp. 115–133. [Google Scholar]
- 12.O’Donnell BF, Hetrick WP, Vohs JL, Krishnan GP, Carroll CA, Shekhar A. Neural synchronization deficits to auditory stimulation in bipolar disorder. NeuroReport. 2004;15:1369–1372. doi: 10.1097/01.wnr.0000127348.64681.b2. [DOI] [PubMed] [Google Scholar]
- 13.Wilson TW, Hernandez O, Asherin RM, Teale PD, Reite ML, Rojas DC. Cortical gamma generators suggest abnormal auditory circuitry in early-onset psychosis. Cereb Cortex. 2008;18:371–378. doi: 10.1093/cercor/bhm062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salisbury DF, Kuroki N, Kasai K, Shenton ME, McCarley RW. Progressive and interrelated functional and structural evidence of post-onset brain reduction in schizophrenia. Arch Gen Psychiatry. 2007;64:521–529. doi: 10.1001/archpsyc.64.5.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spitzer RL, Williams JBW, Gibbon M, First MB. Structured Clinical Interview for DSM-III-R Non-Patient Edition (SCID-NP, Version 1.0) Washington, D.C: American Psychiatric Press; 1990. [Google Scholar]
- 16.First MB, Gibbon M, Spitzer RL, Williams JBW. Structured Clinical Interview for DSM-IV Axis II Personality Disorders (SCID-II) Washington, D.C.: American Psychiatric Press; 1997. [Google Scholar]
- 17.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders – Patient Edition. New York, NY: New York State Psychiatric Institute; 1995. [Google Scholar]
- 18.Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 19.Hollingshead AB. Two Factor Index of Social Position. New Haven, CT: Yale University Press; 1965. [Google Scholar]
- 20.Endicott J, Spitzer RL, Fleiss JL, Cohen J. The Global Assessment Scale: a procedure for measuring overall severity of psychiatric disturbance. Arch Gen Psychiatry. 1976;33:766–771. doi: 10.1001/archpsyc.1976.01770060086012. [DOI] [PubMed] [Google Scholar]
- 21.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 22.Stoll AL. The Psychopharmacology Reference Card. Belmont, MA: McLean Hospital; 2001. [Google Scholar]
- 23.Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003;64:663–667. doi: 10.4088/jcp.v64n0607. [DOI] [PubMed] [Google Scholar]
- 24.Gratton G, Coles MGH, Donchin E. A new method for off-line removal of ocular artifact. Electroencephalogr clin Neurophysiol. 1983;75:468–484. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- 25.Tallon-Baudry C, Bertrand O. Oscillatory gamma activity in humans and its role in object representation. Trends Cogn Sci. 1999;3:151–162. doi: 10.1016/s1364-6613(99)01299-1. [DOI] [PubMed] [Google Scholar]
- 26.Keselman HJ, Rogan JC. Repeated measures F tests and psychophysiological research: controlling the number of false positives. Psychophysiology. 1980;17:499–503. doi: 10.1111/j.1469-8986.1980.tb00190.x. [DOI] [PubMed] [Google Scholar]
- 27.Salisbury DF, Shenton ME, Sherwood AR, Fischer IA, Yurgelun-Todd DA, Tohen M, McCarley RW. First episode schizophrenic psychosis differs from first episode affective psychosis and controls in P300 amplitude over left temporal lobe. Arch Gen Psychiatry. 1998;55:173–180. doi: 10.1001/archpsyc.55.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salisbury DF, Shenton ME, McCarley RW. P300 topography differs in schizophrenia and manic psychosis. Biol Psychiatry. 1999;45:99–106. doi: 10.1016/s0006-3223(98)00208-x. [DOI] [PubMed] [Google Scholar]
- 29.Bruder G, Kayser J, Tenke C, Amador X, Friedman M, Sharif Z, Gorman J. Left temporal lobe dysfunction in schizophrenia: event-related potential and behavioral evidence from phonetic and tonal dichotic listening tasks. Arch Gen Psychiatry. 1999;56:267–276. doi: 10.1001/archpsyc.56.3.267. [DOI] [PubMed] [Google Scholar]
- 30.Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophr Res. 2001;49:1–52. doi: 10.1016/s0920-9964(01)00163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sommer I, Aleman A, Ramsey N, Bouma A, Kahn R. Handedness, language lateralisation and anatomical asymmetry in schizophrenia: meta-analysis. Brit J Psychiatry. 2001;178:344–351. doi: 10.1192/bjp.178.4.344. [DOI] [PubMed] [Google Scholar]
- 32.Spencer KM, McCarley RW. Visual hallucinations, attention, and neural circuitry: perspectives from schizophrenia research. Behav Brain Sci. 2005;28:774. [Google Scholar]
- 33.Akbarian S, Huang H-S. Molecular and cellular mechanisms of altered GAD1/GAD67 expression in schizophrenia and related disorders. Brain Res Rev. 2006;52:293–304. doi: 10.1016/j.brainresrev.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 34.Benes FM, Berretta S. GABAergic interneurons: implications for understanding schizophrenia and bipolar disorder. Neuropsychopharmacol. 2001;25:1–27. doi: 10.1016/S0893-133X(01)00225-1. [DOI] [PubMed] [Google Scholar]
- 35.Benes FM, Lim B, Matzilevich D, Walsh JP, Subburaju S, Minns M. Regulation of the GABA cell phenotype in hippocampus of schizophrenics and bipolars. Proc Natl Acad Sci USA. 2007;104:10164–10169. doi: 10.1073/pnas.0703806104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Torrey EF, Barci BM, Webster MJ, Bartko JJ, Meador-Woodruff JH, Knable MB. Neurochemical markers for schizophrenia, bipolar disorder, and major depression in postmortem brains. Biol Psychiatry. 2005;57:252–260. doi: 10.1016/j.biopsych.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 37.Woo T-U, Walsh JP, Benes FM. Density of glutamic acid decarboxylase 67 messenger RNA-containing neurons that express the N-methyl-D-aspartate receptor subunit NR2A in the anterior cingulate cortex in schizophrenia and bipolar disorder. Arch Gen Psychiatry. 2004;61:649–657. doi: 10.1001/archpsyc.61.7.649. [DOI] [PubMed] [Google Scholar]
- 38.Gallinat J, Winterer G, Herrmann CS, Senkowski D. Reduced oscillatory gammaband responses in unmedicated schizophrenic patients indicate impaired frontal network processing. Clin Neurophysiol. 2004;115:1863–1874. doi: 10.1016/j.clinph.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 39.Symond MB, Harris AWF, Gordon E, Williams LM. “Gamma synchrony” in firstepisode schizophrenia: a disorder of temporal connectivity? Am J Psychiatry. 2005;162:459–465. doi: 10.1176/appi.ajp.162.3.459. [DOI] [PubMed] [Google Scholar]
- 40.Lee K-H, Williams LM, Haig A, Gordon E. Gamma (40 Hz) phase synchronicity and symptom dimensions in schizophrenia. Cognitive Neuropsychiatry. 2003;8:57–71. doi: 10.1080/713752240. [DOI] [PubMed] [Google Scholar]