Abstract
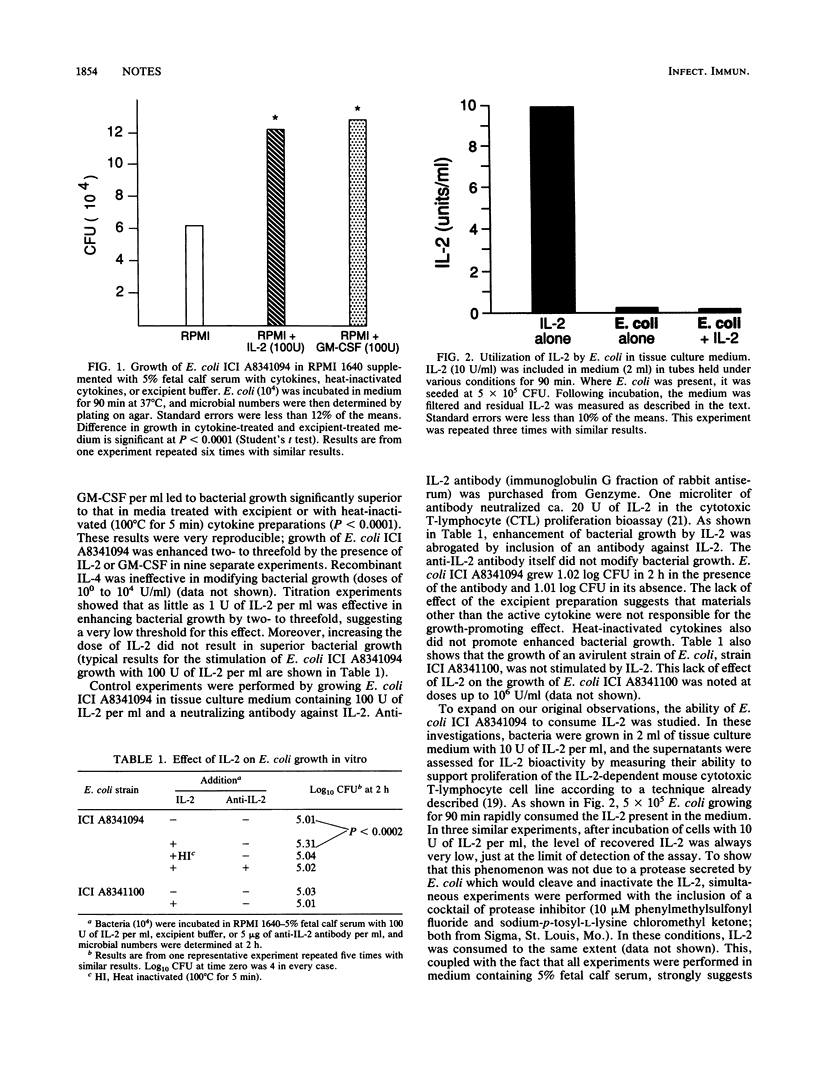
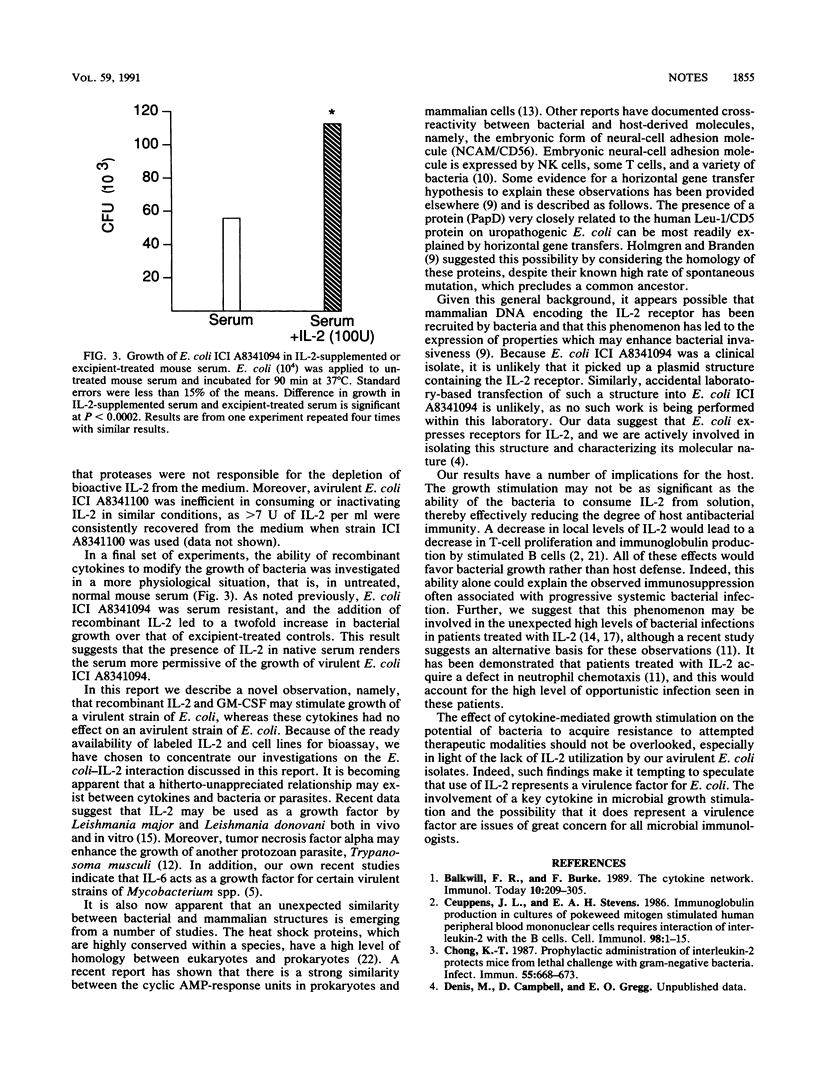
The effect of human recombinant interleukin-2 (IL-2) and human recombinant granulocyte-macrophage colony-stimulating factor on the growth of a virulent strain of Escherichia coli in tissue culture medium and in untreated, normal mouse serum was investigated. Both of these cytokines enhanced the growth of the microorganism two- to threefold in tissue culture medium with or without additional fetal calf serum and in untreated mouse serum. IL-4 did not have any effect on the growth of this microbe under the conditions tested. That the enhancement of growth seen with recombinant IL-2 was due to the active cytokine was shown by the following data: (i) addition of an antibody to IL-2 abrogated the growth-promoting effect; (ii) the excipient buffer, which contained everything except the active cytokine, was inactive in modifying bacterial growth; and (iii) heat-inactivated recombinant IL-2 did not promote enhanced microbial growth. The enhancement of growth with IL-2 was significant with concentrations as low as 1 U/ml. Growth of an avirulent strain of E. coli was not stimulated by IL-2. Moreover, addition of IL-2 to growth virulent E. coli in tissue culture medium led to rapid removal of the cytokine from the medium. Collectively, these data suggest that cytokines may act as growth factors for some virulent bacteria.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ceuppens J. L., Stevens E. A. Immunoglobulin production in cultures of pokeweed mitogen stimulated human peripheral blood mononuclear cells requires interaction of interleukin 2 with the B cells. Cell Immunol. 1986 Mar;98(1):1–7. doi: 10.1016/0008-8749(86)90261-3. [DOI] [PubMed] [Google Scholar]
- Chong K. T. Prophylactic administration of interleukin-2 protects mice from lethal challenge with gram-negative bacteria. Infect Immun. 1987 Mar;55(3):668–673. doi: 10.1128/iai.55.3.668-673.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denis M., Gregg E. O. Recombinant tumour necrosis factor-alpha decreases whereas recombinant interleukin-6 increases growth of a virulent strain of Mycobacterium avium in human macrophages. Immunology. 1990 Sep;71(1):139–141. [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A., Mier J. W. Lymphokines. N Engl J Med. 1987 Oct 8;317(15):940–945. doi: 10.1056/NEJM198710083171506. [DOI] [PubMed] [Google Scholar]
- Edwards J. R., Williams S., Nairn K. Therapeutic activity of meropenem in experimental infections. J Antimicrob Chemother. 1989 Sep;24 (Suppl A):279–285. doi: 10.1093/jac/24.suppl_a.279. [DOI] [PubMed] [Google Scholar]
- Fauci A. S., Rosenberg S. A., Sherwin S. A., Dinarello C. A., Longo D. L., Lane H. C. NIH conference. Immunomodulators in clinical medicine. Ann Intern Med. 1987 Mar;106(3):421–433. doi: 10.7326/0003-4819-106-3-421. [DOI] [PubMed] [Google Scholar]
- Holmgren A., Bränden C. I. Crystal structure of chaperone protein PapD reveals an immunoglobulin fold. Nature. 1989 Nov 16;342(6247):248–251. doi: 10.1038/342248a0. [DOI] [PubMed] [Google Scholar]
- Klempner M. S., Noring R., Mier J. W., Atkins M. B. An acquired chemotactic defect in neutrophils from patients receiving interleukin-2 immunotherapy. N Engl J Med. 1990 Apr 5;322(14):959–965. doi: 10.1056/NEJM199004053221404. [DOI] [PubMed] [Google Scholar]
- Kongshavn P. A., Ghadirian E. Enhancing and suppressive effects of tumour necrosis factor/cachectin on growth of Trypanosoma musculi. Parasite Immunol. 1988 Sep;10(5):581–588. doi: 10.1111/j.1365-3024.1988.tb00245.x. [DOI] [PubMed] [Google Scholar]
- Lin Y. S., Green M. R. Similarities between prokaryotic and eukaryotic cyclic AMP-responsive promoter elements. Nature. 1989 Aug 24;340(6235):656–659. doi: 10.1038/340656a0. [DOI] [PubMed] [Google Scholar]
- Maoleekoonpairoj S., Mittelman A., Savona S., Ahmed T., Puccio C., Gafney E., Skelos A., Arnold P., Coombe N., Baskind P. Lack of protection against bacterial infections in patients with advanced cancer treated by biologic response modifiers. J Clin Microbiol. 1989 Oct;27(10):2305–2308. doi: 10.1128/jcm.27.10.2305-2308.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazingue C., Cottrez-Detoeuf F., Louis J., Kweider M., Auriault C., Capron A. In vitro and in vivo effects of interleukin 2 on the protozoan parasite leishmania. Eur J Immunol. 1989 Mar;19(3):487–491. doi: 10.1002/eji.1830190312. [DOI] [PubMed] [Google Scholar]
- Mizel S. B. The interleukins. FASEB J. 1989 Oct;3(12):2379–2388. doi: 10.1096/fasebj.3.12.2676681. [DOI] [PubMed] [Google Scholar]
- Pahwa R., Chatila T., Pahwa S., Paradise C., Day N. K., Geha R., Schwartz S. A., Slade H., Oyaizu N., Good R. A. Recombinant interleukin 2 therapy in severe combined immunodeficiency disease. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5069–5073. doi: 10.1073/pnas.86.13.5069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg S. A., Lotze M. T. Cancer immunotherapy using interleukin-2 and interleukin-2-activated lymphocytes. Annu Rev Immunol. 1986;4:681–709. doi: 10.1146/annurev.iy.04.040186.003341. [DOI] [PubMed] [Google Scholar]
- Smith K. A. Interleukin-2: inception, impact, and implications. Science. 1988 May 27;240(4856):1169–1176. doi: 10.1126/science.3131876. [DOI] [PubMed] [Google Scholar]
- Young R. A., Elliott T. J. Stress proteins, infection, and immune surveillance. Cell. 1989 Oct 6;59(1):5–8. doi: 10.1016/0092-8674(89)90861-1. [DOI] [PubMed] [Google Scholar]


