Abstract
Purpose
Growth factors such as platelet-derived growth factor (PDGF) exert potent effects on wound healing including the regeneration of periodontia. Pyridinoline cross-linked carboxyterminal telopeptide of type I collagen (ICTP) is a well-known biomarker of bone turnover, and as such is a potential indicator of osseous metabolic activity. The objective of this study was to evaluate the release of the ICTP into the periodontal wound fluid (WF) following periodontal reconstructive surgery using local delivery of highly purified recombinant human PDGF (rhPDGF)-BB.
Methods
Forty-seven human subjects at five treatment centres possessing chronic severe periodontal disease were monitored longitudinally for 24 weeks following PDGF regenerative surgical treatment. Severe periodontal osseous defects were divided into one of three groups and treated at the time of surgery with either: β-tricalcium phosphate (TCP) osteoconductive scaffold alone (active control), β-TCP+0.3 mg/ml of rhPDGF-BB, or β-TCP+1.0 mg/ml of rhPDGF-BB. WF was harvested and analysed for local ICTP levels by radioimmunoassay. Statistical analysis was performed using analysis of variance and an area under the curve analysis (AUC).
Results
The 0.3 and 1.0 mg/ml PDGF-BB treatment groups demonstrated increases in the amount of ICTP released locally for up to 6 weeks. There were statistically significant differences at the week 6 time point between β-TCP carrier alone group versus 0.3 mg/ml PDGF-BB group (p<0.05) and between β-TCP alone versus the 1.0 mg/ml PDGF-BB-treated lesions (p<0.03). The AUC analysis revealed no statistical differences amongst groups.
Conclusion
This study corroborates the release of ICTP as a measure of active bone turnover following local delivery of PDGF-BB to periodontal osseous defects. The amount of ICTP released from the WF revealed an early increase for all treatment groups. Data from this study suggests that when PDGF-BB is delivered to promote periodontal tissue engineering of tooth-supporting osseous defects, there is a direct effect on ICTP released from the wound.
Keywords: collagen telopeptides, growth factors, periodontal regeneration, periodontal wound repair, tissue engineering
Growth factors are required for the regeneration of periodontal tissues. They are intricately involved in periodontal development and healing through their effects on cell chemotaxis, proliferation and differentiation (Giannobile 1996). Growth factors such as platelet-derived growth factor (PDGF) can stimulate cells involved in tissue repair and enhance periodontal wound healing and regeneration (Lynch et al. 1991, Parkar et al. 2001).
Recently, an experimental periodontal grafting material consisting of a β-tricalcium phosphate (β-TCP) scaffold enriched with a highly purified recombinant human PDGF (rhPDGF-BB) has been evaluated (Nevins et al. 2005). PDGF-BB enhances β-TCP's physical and mechanical actions by promoting cellular ingrowth into the osseous defect and bone matrix. PDGF has been thoroughly studied in periodontics since first discovered to promote regeneration of bone, cementum and periodontal ligament (Lynch et al. 1989, 1991, Giannobile et al. 1994, Cho et al. 1995, Giannobile et al. 1996, Green et al. 1997, Nevins et al. 2003). These studies have demonstrated the mechanism of action of PDGF, showing the presence of cell-surface receptors for PDGF on periodontal and alveolar bone cells, and elucidated PDGF's stimulatory effect on the DNA replication and chemotaxis of these cells (Matsuda et al. 1992, McAllister et al. 1993, Mumford et al. 2001). An initial human clinical trial demonstrated that application of 0.15 mg/ml of rhPDGF-BB and 0.15 mg/ml recombinant human insulin-like growth factor I (rhIGF-I) resulted in a significant improvement in bone fill compared with conventional flap surgery (Howell et al. 1997). In a recent clinical trial, Nevins (2005), reported a mean percent bone fill of 57% with 0.3 mg/ml PDGF-BB plus β-TCP, 34% for the 1.0 mg/ml PDGF-BB plus β-TCP, and 18% for the β-TCP alone, respectively. Comparisons of both concentrations of rhPDGF-BB demonstrated statistically significant improvements compared with the β-TCP carrier alone for radiographic parameters of bone regeneration.
Pyridinoline cross-linked carboxyterminal telopeptide of Type I collagen (ICTP) has been demonstrated to be involved in bone turnover in a variety of clinical situations such as in osteoporosis and during periodontal bone remodeling (Eastell et al. 1993, Giannobile et al. 1995). The results from a single-centre trial evaluating ICTP suggest a relationship between wound repair and ICTP release (Cooke et al. in press). This study sought to evaluate an expanded patient population with regard to the release of ICTP into local periodontal wound fluid (WF) or gingival crevicular fluid (GCF) during periodontal repair.
The aim of this multi-centre trial was to better understand the contribution to wound healing of pyridinoline cross-linked ICTP when released into periodontal WF during tissue repair after local PDGF-BB reconstructive therapy.
Material and Methods
This investigation evaluated 47 patients from five different clinical centres, afflicted with chronic severe periodontal disease requiring periodontal regenerative surgery. The patients in this study were a subset of research subjects participating in a phase III trial designed to evaluate the safety and effectiveness of rhPDGF-BB to promote soft- and hard-tissue engineering of the periodontium (Nevins et al. 2005) and those recently reported from the single study cohort of 16 subjects (Cooke et al. in press). The subjects provided GCF at baseline or periodontal WF following the delivery of grafts containing β-TCP alone or β-TCP containing one of two dose levels of rhPDGF-BB (0.3 or 1.0 mg/ml) to severe periodontal osseous defects. Informed consent was obtained at the initial visit prior to administration of any research procedures. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in obtainment of approval by the University of Michigan's and the Western Regional human subjects research review committees.
Inclusion/exclusion criteria
Subjects were entered into the study if they were between 25 and 75 years of age and displayed no evidence of localized aggressive periodontitis. Patients qualified for the study if one tooth exhibited a soft tissue probing pocket depth measuring 7 mm or greater, and after surgical debridement, a 4 mm or greater vertical bone defect (BD) was present with at least one bony wall. Patients were excluded if they were unable to maintain the health of the site or were pregnant. The following were also exclusionary factors at baseline: diagnosis of oral cancer or HIV within 6 months, previous periodontal surgery within 1 year on the study tooth, tooth mobility greater than grade II, radiographic signs of untreated acute infection at the surgical site, or recent history of smoking more than 20 cigarettes/day.
Randomization and surgical treatment
Subjects were randomized into one of three treatment groups: β-TCP carrier alone (15 patients) (active control), β-TCP+0.3 mg/ml of rhPDGF-BB (14 patients) or β-TCP+1.0 mg/ml of rhPDGF-BB (18 patients). Teeth adjacent to the treatment site served as surgical controls when included as part of the flap design.
Before surgical treatment, each subject received non-surgical therapy consisting of scaling and root planing to control the disease process and prepare the defect site for surgical therapy. Surgical treatment consisted of full-thickness mucoperiosteal flaps to allow adequate visualization of the osseous lesion. Following debridement of the test site, the BD was measured and if found to be ≥4 mm vertically, final subject eligibility was confirmed and the root surfaces were decontaminated with a tetracycline paste. The test sites were then treated with β-TCP with buffer alone, or buffer containing one of two dose levels of rhPDGF-BB followed by the securing of flaps with interdental sutures to achieve primary closure. Subjects were instructed to utilize an oral rinse of chlorhexidine (0.12%) twice daily for 6 weeks. Amoxicillin 500 mg taken thrice daily for a minimum of 10 days (or another appropriate antibiotic regimen) was also prescribed.
Periodontal WF collection
Each patient had one surgical site that received the device: β-TCP containing buffer alone (active control), β-TCP, with buffer containing 0.3 mg/ml of rhPDGF-BB, or β-TCP, with buffer containing 1.0 mg/ml of rhPDGF-BB that provided a WF sample. WF samples were also collected from the teeth adjacent to the treatment site serving as surgical controls. The WF samples were taken directly from the defect site at the periodontal pocket. The area around each sample site was air-dried and the supragingival plaque biofilm removed. A sterile methylcellulose strip (Pro Flow Inc., Amityville, NY, USA) was gently inserted into the sulcus/pocket until slight resistance was felt. The fluid sample was then collected for 10 s and the strip was then immediately placed into an Eppendorf tube. The samples were subsequently kept on ice for transport to the laboratory where they were stored at − 20°C until needed for analysis of ICTP. The collection of WF occurred at six different time points (Fig. 1): baseline, weeks 3, 6, 12, 18 and 24 after re-constructive surgery.
Fig. 1.
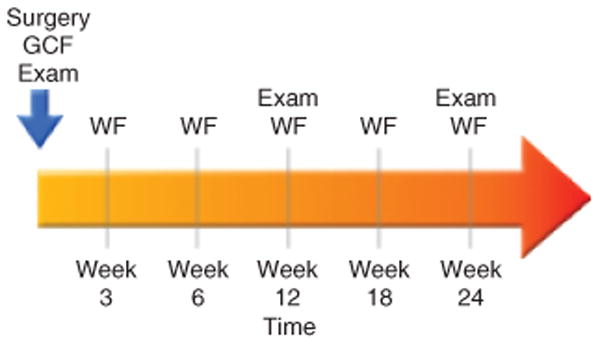
Clinical Trial Study Timeline. GCF, gingival crevicular fluid; WF, periodontal wound fluid; exam included collection of standard clinical parameters.
Biomarker analysis
ICTP evaluation
Frozen samples were thawed at room temperature and the proteins were then eluted through centrifugation × 5 in 12 × 75 ml polypropylene tubes at 3,000 rpm for 5 min. with 20 μl phosphate buffered saline (pH 7.4) containing 15 nM aprotinin, 1 mM PMSF and 0.1% of human serum albumin as described previously (Giannobile et al. 1995). GCF/WF ICTP levels were quantified using radioimmunoassay (DiaSorin Inc., Stillwater, MN, USA) as previously described (Risteli et al. 1993). ICTP was determined as total amount/time of collection (pg/site/patient).
Statistical analysis
The standard statistics and statistical modeling procedures using analysis of variance (anova) and a Fisher's PLSD post hoc test at the 5% level were performed to examine the differences in ICTP values among the treatment groups. A longitudinal analysis was used to take into account non-independence of the sample sites. Each model was run with the mediator level area under the curve (AUC) analysis as the dependent variable, and each of the surgery groups was run as the independent variables. AUC analysis was performed from Baseline-24 weeks. The Baseline-24 week AUC analysis accounts for the observation period that includes bone repair and maturation. AUC was calculated by determining the area under the line connecting each time point of the ICTP line chart when PWF was collected. The following formulae were used to determine the AUC for each of the three treatment groups:
Baseline-24 week AUC:
where y1 is the average ICTP at baseline and y2, y3, y4, y5, and y6 represents average ICTP at weeks 3, 6, 12, 18 and 24, respectively.
Statistical analysis using AUC was performed using anova Fisher's PSLD at the 5% level.
Results
Table 1 shows baseline patient demographics. The mean age for all patients was 51.4 years with a range of 26–73 years. Fifty-three percentage of the patients involved in the study were male. A total of 11 patients in the study were considered smokers and 36 were considered non-smokers. The β-TCP carrier alone group contained three current smokers, the 0.3 mg/ml of rhPDGF-BB group had four current smokers, and the 1.0 mg/ml of rhPDGF-BB group possessed four current smokers. For the test groups, baseline mean probing depth (PD) was 8.4 mm, baseline clinical attachment level was 9.4 mm, and the mean BD depth was 5.7 mm. No statistically significant differences were found between surgical treatment groups for these pretreatment measurements.
Table 1.
Baseline patient demographics
| Characteristic | Buffer alone | 0.3 mg/ml PDGF | 1.0 mg/ml PDGF | All patients Tx groups |
|---|---|---|---|---|
| Number of Patients | 15 | 14 | 18 | 47 |
| Age | ||||
| Mean | 54 | 48 | 52 | 51.4 |
| Range | 33–71 | 30–64 | 26–73 | 26–73 |
| %Males (#) | 57 (8) | 33 (5) | 70 (12) | 53 (25) |
| Smoking history | ||||
| Current | 3 | 4 | 4 | 11 |
| Non-smoker | 11 | 11 | 14 | 36 |
| Mean PD (mm) | 8.3 | 8.8 | 8.1 | 8.4 |
| Mean CAL (mm) | 9.2 | 10 | 9.1 | 9.4 |
PDGF, platelet-derived growth factor; PD, probing depth; CAL, clinical attachment level.
Figure 1 illustrates the timeline for WF collections. The collection of WF occurred at six different time points: baseline, week 3, 6, 12, 18 and 24 after surgery. Clinical and radiographic examinations occurred at the baseline time point pre-surgically, week 12 and 24.
Figure 2 shows an example of the experimental protocol including pre-treatment, surgical treatment, and post-treatment photographs as well as pre- and post-treatment radiographs. The pre-treatment radiograph shows bony defect on the mesial root of tooth #18. The pre-treatment PD was 7 mm. After flap reflection and degranulation an infrabony defect of 6 mm was present qualifying the patient for the study. The treatment group was then randomly assigned and the β-TCP carrier with or without PDGF-BB placed into infrabony defect. In this case the patient received the β-TCP carrier with 0.3 mg/ml PDGF-BB. Primary closure of the flap was obtained with Goretex® sutures (W.L. Gore Co., Flagstaff, AZ, USA). At week 24 in this case there is now a 4 mm PD and the week 24 radiograph shows the infrabony defect is absent and the crestal lamina dura is intact. The final result showed a 48% bone fill and 3 mm gain in clinical attachment.
Fig. 2.
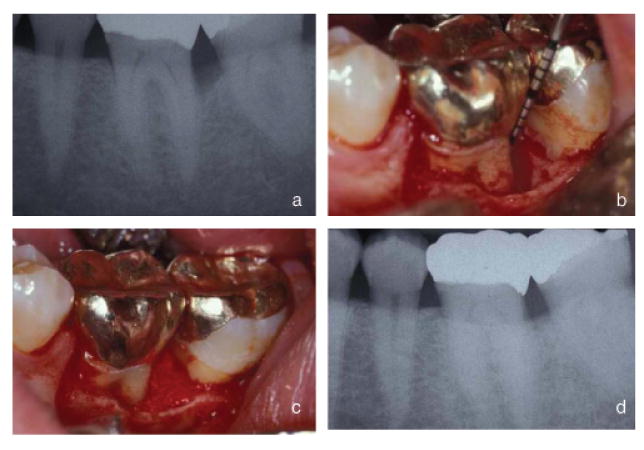
Pre-treatment, surgical procedure, and week 24 radiographs of a defect treated with the local delivery of 0.3 mg/ml of rhPDGF and β-tricalcium phosphate. (a) Pre-treatment radiograph shows bony defect on the mesial of tooth #18. (b) After flap reflection and degranulation an infrabony defect of 6 mm is present. (c) Bone graft placed into infrabony defect. (d) Week 24 post-operative radiograph shows the healing of the infrabony defect. The defect shows 48% bone fill and the crestal lamina dura intact.
Table 2 demonstrates ICTP levels (pg/site) over time for all groups. The β-TCP carrier alone group shows a decrease in the amount of ICTP released in the WF up to week 24. The 0.3 and 1.0 mg/ml PDGF-BB groups had an increase in the amount of ICTP released up to 6 weeks. Statistical differences were observed at week 6 between the β-TCP carrier alone group and the 0.3 mg/ml (p<0.05) and 1.0 mg/ml (p<0.03) PDGF-BB groups. At week 6 there is a greater than a twofold increase in the release of ICTP from the WF for the 0.3 and 1.0 mg/ml PDGF-BB groups compared with the β-TCP carrier alone. The amount of ICTP released from the β-TCP group was similar to the surgical control which remained < 100 pg/site for the entire observation period (data not shown). In addition, the AUC analysis of ICTP for all groups over 24 weeks is also presented in Table 2. No statistical differences were observed among treatment groups. The AUC was similar for the 0.3 mg/ml PDGF-BB group and the 1.0 mg/ml PDGF-BB group. The β-TCP carrier alone group displayed a trend of a lower AUC for ICTP, but this difference was not statistically significant. No centre effects were noted with respect to ICTP levels among the groups evaluated.
Table 2.
Effect of PDGF-BB on wound fluid ICTP levels
| Time (weeks) | ICTP (pg/10s) (mean+SEM) | ||
|---|---|---|---|
| Carrier | rhPDGF-BB
(0.3 mg/ml) |
rhPDGF-BB
(1.0 mg/ml) |
|
| Baseline | 64.9 ± 42.7 | 113.1 ± 43.7 | 109.7 ± 54.6 |
| 3 | 41.9 ± 33.6 | 72.6 ± 39.1 | 103.1 ± 33.7 |
| 6 | 45.1 ± 36.2 | 125.9 ± 63.1* | 139.7 ± 58.5* |
| 12 | 60.0 ± 35.5 | 65.6 ± 27.4 | 63.7 ± 25.4 |
| 18 | 50.7 ± 50.7 | 63.3 ± 27.6 | 60.2 ± 27.6 |
| 24 | 74.1 ± 50.7 | 63.3 ± 41.7 | 57.5 ± 21.7 |
| Overall (AUC) | 437.5 ± 287.1 | 641.7 ± 164.1 | 672.8 ± 183.0 |
p<0.05 versus carrier.
ICTP, carboxyterminal telopeptide of Type I collagen; AUC, area under the curve.
Discussion
The results of this study show statistically significant differences at the week 6 time point between β-TCP carrier alone group versus 0.3 mg/ml PDGF-BB group (p<0.05) and between β-TCP carrier alone group versus 1.0 mg/ml PDGF-BB group (p<0.03) for WF ICTP levels. The 0.3 and 1.0 mg/ml PDGF-BB-treated groups demonstrated increases in the amount of ICTP released locally for up to 6 weeks following regenerative surgery. The results of this study expand up those reported in the single-centre investigation of a panel of biomarkers including ICTP found in 16 subjects reported by Cooke et al. in press.
For an evaluation of osseous remodeling following local PDGF-BB application, we studied ICTP, a member of a family of biomarkers which have emerged to be valuable for bone turnover in a multitude of osteolytic and osseous metabolic diseases including periodontal disease (Eyre 1987, Giannobile et al. 2003, Taba et al. 2005). Type I collagen comprises 90% of the organic matrix of bone and is the most abundant collagen of osseous tissue (Narayanan & Page 1983). Pyridinoline cross-links represent a class of mature collagen degradative molecules that include pyridinoline, deoxypyridinoline, N-telopeptides, and C-telopeptides (Eyre 1987, Calvo et al. 1996). Following procollagen synthesis and release into the maturing extracellular matrix, pyridinoline cross-links are formed in type I collagen by the enzyme lysyl oxidase on lysine and hydroxylysine residues in the carboxy- and amino-terminal telopeptide regions, increasing the mechanical stability of the structure (Last et al. 1990). Subsequent to osteoclastic bone re-sorption and collagen matrix degradation, cross-linked telopeptides of type I collagen are released into the circulation. As cross-linked telopeptides result from post-translational modification of collagen molecules, they cannot be reused during collagen synthesis, and are therefore precise indicators of bone re-sorption (Eriksen et al. 1993). In addition, contrary to other tissues, pyridinoline cross-links are specific to bone turnover (Charles et al. 1994).
Pyridinoline cross-links represent a potentially valuable diagnostic aid in periodontics, as biochemical markers specific for bone turnover may be useful in differentiating the presence of gingival inflammation from active periodontal and peri-implant bone turnover (Golub et al. 1997). Several investigations have recently explored the ability of pyridinoline cross-links to detect bone resorption in lesions of periodontitis (Talonpoika & Hämaläinen 1994, Giannobile et al. 1995, Golub et al. 1997, Shibutani et al. 1997, Palys et al. 1998) and peri-implantitis (Oringer et al. 1998). For instance, in a study of 25 periodontitis patients treated with scaling and root planing, significant correlations between GCF ICTP level and clinical periodontal disease parameters were found (Al-Shammari et al. 2001). In addition, elevated GCF ICTP levels at baseline, especially at shallow sites, were found to be predictive of subsequent attachment loss as early as one month after sampling (Oringer et al. 2002).
To monitor treatment, other studies have demonstrated that GCF ICTP levels are correlated to disease resolution. Golub et al. (1997) found that treatment of 18 chronic periodontitis patients with a matrix metalloproteinase inhibitor (subantimicrobial doxycycline hyclate, SDH) resulted in a 70% reduction in GCF ICTP levels after 1 month, concomitant with a 30% reduction in collagenase levels. Furthermore, Gapski et al. (2004) found that treatment of 24 chronic periodontitis patients with access flap surgery and SDH resulted in a potent decrease in ICTP levels soon after the surgical therapy at 3 months while the placebo controls demonstrated no change or increases in ICTP levels over a 12-month observation period.
In another related study PDGF-BB was found to have a direct effect on growth factors released from periodontal wounds. VEGF was induced during early wound repair (i.e. 3–5 days), while exogenous PDGF-BB possibly reduced the release of endogenous PDGF-AB from the wound site after several days of healing. There was also a marked increase in bone turnover during the first few days of wound healing when PDGF-BB was added to the osteoconductive scaffold, as the amount of ICTP release from the wound was increased for the early time points (Cooke et al. in press). In addition, non-surgical sites displayed very low (in general, non-detectable levels of ICTP) when compared with surgical wound sites (Cooke et al. 2006).
This study evaluated release of ICTP following PDGF-BB application as a measure of active bone turnover in a multi-centre investigation. The amount of ICTP released from the WF showed early increases for the 0.3 mg/ml PDGF-BB and the 1.0 mg/ml PDGF-BB groups compared with scaffold alone. In particular, at 6 weeks, sites treated with PDGF-BB showed a greater amount of ICTP release from the WF. However, the total amount of released ICTP over time, as shown by AUC analysis, was unchanged with active treatment. Final clinical outcomes from the multi-centre parent study show an average increase in linear bone growth of 2.6 mm for the 0.3 mg/ml PDGF-BB group, 1.5 mm for the 1.0 mg/ml PDGF-BB group, and 0.9 mm for the β-TCP carrier alone group (Nevins et al. 2005). Therefore, in contrast to previous studies where ICTP levels were indicative of further disease, the present study demonstrates that elevated ICTP as a result of a regenerative attempt is suggestive as a sign of bone turnover given the interrelationship between bone formation and bone resorption. In addition, as could be anticipated with periodontal regeneration, early events are likely decisive to treatment outcome.
In conclusion, data from this study shows that when rhPDGF-BB is delivered to promote periodontal tissue engineering of tooth-supporting osseous defects, there is a direct effect on ICTP released from the wound. Future studies with expanded patient populations and earlier time intervals of WF collection will be needed to better understand the effects of rhPDGF-BB on periodontal wound healing.
Clinical Relevance
PDGF has shown potential to regenerate periodontal defects. However, little information is available regarding the mechanisms on how PDGF regulates bone turnover. This study examined 47 patients following reconstructive periodontal surgery combining PDGF treatment on the expression of a key marker of bone turnover, the pyridinoline cross-link of type I collagen (ICTP). It was noted that following therapy, ICTP was strongly induced during the early stages of tissue repair. These results provide better information on the role of PDGF in affecting periodontal wound healing and bone turnover.
Acknowledgments
This study was supported by NIH/NIDCR Grants T-35DE07101, DE13397 and by BioMimetic Pharmaceuticals, Inc. The authors thank Mary Gilson Layher, Louis Whitesman and Sarah Webb for their technical assistance and contributions to the project.
References
- Al-Shammari KF, Giannobile WV, Aldredge WA, Iacono VJ, Eber RM, Wang HL, Oringer RJ. Effect of non-surgical periodontal therapy on C-telopeptide pyridinoline cross-links (ICTP) and interleukin-1 levels. Journal of Periodontology. 2001;72:1045–1051. doi: 10.1902/jop.2001.72.8.1045. [DOI] [PubMed] [Google Scholar]
- Calvo MS, Eyre DR, Gundberg CM. Molecular basis and clinical application of biological markers of bone turnover. Endocrine Review. 1996;17:333–368. doi: 10.1210/edrv-17-4-333. [DOI] [PubMed] [Google Scholar]
- Charles P, Mosekilde L, Risteli L, Risteli J, Eriksen EF. Assessment of bone remodeling using biochemical indicators of type I collagen synthesis and degradation: relation to calcium kinetics. Bone and Mineral. 1994;24:81–94. doi: 10.1016/s0169-6009(08)80147-x. [DOI] [PubMed] [Google Scholar]
- Cho MI, Lin WL, Genco RJ. Platelet-derived growth factor-modulated guided tissue regenerative therapy. Journal of Periodontology. 1995;66:522–530. doi: 10.1902/jop.1995.66.6.522. [DOI] [PubMed] [Google Scholar]
- Cooke JW, Sarment DP, Whitesman LA, Miller SE, Jin Q, Lynch SE, Giannobile WV. Effect of platelet derived growth factor on mediators of periodontal wound repair. Tissue Engineering. doi: 10.1089/ten.2006.12.1441. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastell R, Robins SP, Colwell T, Assiri AM, Riggs BL, Russell RG. Evaluation of bone turnover in type I osteoporosis using biochemical markers specific for both bone formation and bone resorption. Osteoporosis International. 1993;3:255–260. doi: 10.1007/BF01623829. [DOI] [PubMed] [Google Scholar]
- Eriksen EF, Charles P, Melsen F, Mosekilde L, Risteli L, Risteli J. Serum markers of type I collagen formation and degradation in metabolic bone disease: correlation with bone histomorphometry. Journal of Bone and Mineral Research. 1993;8:127–132. doi: 10.1002/jbmr.5650080202. [DOI] [PubMed] [Google Scholar]
- Eyre D. Collagen cross-linking amino acids. Methods in Enzymology. 1987;144:115–139. doi: 10.1016/0076-6879(87)44176-1. [DOI] [PubMed] [Google Scholar]
- Gapski R, Barr JL, Sarment DP, Layher MG, Socransky SS, Giannobile WV. Effect of systemic matrix metalloproteinase inhibition on periodontal wound repair: a proof of concept trial. Journal of Periodontology. 2004;75:441–452. doi: 10.1902/jop.2004.75.3.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannobile WV. Periodontal tissue engineering by growth factors. Bone. 1996;19:23S–37S. doi: 10.1016/s8756-3282(96)00127-5. [DOI] [PubMed] [Google Scholar]
- Giannobile WV, Al-Shammari KF, Sarment DP. Matrix molecules and growth factors as indicators of periodontal disease activity. Periodontology 2000. 2003;31:125–134. doi: 10.1034/j.1600-0757.2003.03108.x. [DOI] [PubMed] [Google Scholar]
- Giannobile WV, Finkelman RD, Lynch SE. Comparison of canine and non-human primate animal models for periodontal regenerative therapy: results following a single administration of PDGF/IGF-I. Journal of Periodontology. 1994;65:1158–1168. doi: 10.1902/jop.1994.65.12.1158. [DOI] [PubMed] [Google Scholar]
- Giannobile WV, Hernandez RA, Finkelman RD, Ryan S, Kiritsy CP, D'Andrea M, Lynch SE. Comparative effects of platelet-derived growth factor-BB and insulin-like growth factor-I, individually and in combination, on periodontal regeneration in Macaca fascicularis. Journal of Periodontal Research. 1996;31:301–312. doi: 10.1111/j.1600-0765.1996.tb00497.x. [DOI] [PubMed] [Google Scholar]
- Giannobile WV, Lynch SE, Denmark RG, Paquette DW, Fiorellini JP, Williams RC. Crevicular fluid osteocalcin and pyridinoline cross-linked carboxy-terminal telopeptide of type I collagen (ICTP) as markers of rapid bone turnover in periodontitis. A pilot study in beagle dogs. Journal of Clinical Periodontology. 1995;22:903–910. doi: 10.1111/j.1600-051x.1995.tb01793.x. [DOI] [PubMed] [Google Scholar]
- Golub LM, Lee HM, Greenwald RA, Ryan ME, Sorsa T, Salo T, Giannobile WV. A matrix metalloproteinase inhibitor reduces bone-type collagen degradation fragments and specific collagenases in gingival crevicular fluid during adult periodontitis. Inflammation Research. 1997;46:310–319. doi: 10.1007/s000110050193. [DOI] [PubMed] [Google Scholar]
- Green RJ, Usui ML, Hart CE, Ammons WF, Narayanan AS. Immunolocalization of platelet-derived growth factor A and B chains and PDGF-alpha and beta receptors in human gingival wounds. Journal of Periodontal Research. 1997;32:209–214. doi: 10.1111/j.1600-0765.1997.tb00526.x. [DOI] [PubMed] [Google Scholar]
- Howell TH, Fiorellini JP, Paquette DW, Offenbacher S, Giannobile WV, Lynch SE. A phase I/II clinical trial to evaluate a combination of recombinant human platelet-derived growth factor-BB and recombinant human insulin-like growth factor-I in patients with periodontal disease. Journal of Periodontology. 1997;68:1186–1193. doi: 10.1902/jop.1997.68.12.1186. [DOI] [PubMed] [Google Scholar]
- Last JA, Armstrong LG, Reiser KM. Biosynthesis of collagen crosslinks. International Journal of Biochemistry. 1990;22:559–564. doi: 10.1016/0020-711x(90)90031-w. [DOI] [PubMed] [Google Scholar]
- Lynch SE, de Castilla GR, Williams RC, Kiritsy CP, Howell TH, Reddy MS, Antoniades HN. The effects of short-term application of a combination of platelet-derived and insulin-like growth factors on periodontal wound healing. Journal of Periodontology. 1991;62:458–467. doi: 10.1902/jop.1991.62.7.458. [DOI] [PubMed] [Google Scholar]
- Lynch SE, Williams RC, Polson AM, Howell TH, Reddy MS, Zappa UE, Antoniades HN. A combination of platelet-derived and insulin-like growth factors enhances periodontal regeneration. Journal of Clinical Periodontology. 1989;16:545–548. doi: 10.1111/j.1600-051x.1989.tb02334.x. [DOI] [PubMed] [Google Scholar]
- Matsuda N, Lin WL, Kumar NM, Cho MI, Genco RJ. Mitogenic, chemotactic, and synthetic responses of rat periodontal ligament fibroblastic cells to polypeptide growth factors in vitro. Journal of Periodontology. 1992;63:515–525. doi: 10.1902/jop.1992.63.6.515. [DOI] [PubMed] [Google Scholar]
- McAllister B, Leeb-Lundberg F, Olsen MS. Bradykinin inhibition of epidermal growth factor and platelet-derived growth factor-induced DNA synthesis in human fibroblasts. American Journal of Physiology. 1993;265:C477–C484. doi: 10.1152/ajpcell.1993.265.2.C477. [DOI] [PubMed] [Google Scholar]
- Mumford JH, Carnes DL, Cochran DL, Oates TW. The effects of platelet-derived growth factor-BB on periodontal cells in an in vitro wound model. Journal of Periodontology. 2001;72:331–340. doi: 10.1902/jop.2001.72.3.331. [DOI] [PubMed] [Google Scholar]
- Narayanan AS, Page RC. Connective tissues of the periodontium: a summary of current work. Collagen and Related Research. 1983;3:33–64. [PubMed] [Google Scholar]
- Nevins M, Camelo M, Nevins ML, Schenk RK, Lynch SE. Periodontal regeneration in humans using recombinant human platelet-derived growth factor-BB (rhPDGF-BB) and allogenic bone. Journal of Periodontology. 2003;74:1282–1292. doi: 10.1902/jop.2003.74.9.1282. [DOI] [PubMed] [Google Scholar]
- Nevins M, Giannobile WV, McGuire MK, Kao RT, Mellonig JT, Hinrichs JE, McAllister BS, Murphy KS, McClain PK, Nevins ML, Paquette DW, Han TH, Reddy MS, Lavin PT, Genco RJ, Lynch SE. Platelet-derived growth factor (rhPDGF-BB) Stimulates bone fill and rate of attachment level gain: results of a large multi-center randomized controlled trial. Journal of Periodontology. 2005;76:2205–2215. doi: 10.1902/jop.2005.76.12.2205. [DOI] [PubMed] [Google Scholar]
- Oringer RJ, Al-Shammari KF, Aldrege WA, Iacono VJ, Eber RM, Wang HL, Berwald B, Nejat R, Giannobile WV. Effect of locally delivered minocycline microspheres on markers of bone resorption. Journal of Periodontology. 2002;73:835–842. doi: 10.1902/jop.2002.73.8.835. [DOI] [PubMed] [Google Scholar]
- Oringer RJ, Palys MD, Iranmanesh A, Fiorellini JP, Haffajee AD, Socransky SS, Giannobile WV. C-telopeptide pyridinoline cross-links (ICTP) and periodontal pathogens associated with endosseous oral implants. Clinical Oral Implants Research. 1998;9:365–373. doi: 10.1034/j.1600-0501.1996.090602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palys MD, Haffajee AD, Socransky SS, Giannobile WV. Relationship between C-telopeptide pyridinoline cross-links (ICTP) and putative periodontal pathogens in periodontitis. Journal of Clinical Periodontology. 1998;25:865–871. doi: 10.1111/j.1600-051x.1998.tb02383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkar MH, Kuru L, Giouzeli M, Olsen I. Expression of growth-factor receptors in normal and regenerating human periodontal cells. Archives of Oral Biology. 2001;46:275–284. doi: 10.1016/s0003-9969(00)00099-6. [DOI] [PubMed] [Google Scholar]
- Risteli J, Elomaa I, Niemi S, Novamo A, Risteli L. Radioimmunoassay for the pyridinoline cross-linked carboxy-terminal telopeptide of type I collagen: a new serum marker of bone collagen degradation. Clinical Chemistry. 1993;39:635–640. [PubMed] [Google Scholar]
- Shibutani T, Murahashi Y, Tsukada E, Iwayama Y, Heersche JN. Experimentally induced periodontitis in beagle dogs causes rapid increases in osteoclastic resorption of alveolar bone. Journal of Periodontology. 1997;68:385–391. doi: 10.1902/jop.1997.68.4.385. [DOI] [PubMed] [Google Scholar]
- Taba M, Jr, Kinney J, Kim AS, Giannobile WV. Diagnostic biomarkers for oral and periodontal diseases. Dental Clinics of North America. 2005;49:551–571. doi: 10.1016/j.cden.2005.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talonpoika JT, Hämaläinen MM. Type I collagen carboxyterminal telopeptide in human gingival crevicular fluid in different clinical conditions and after periodontal treatment. Journal of Clinical Periodontology. 1994;21:320–326. doi: 10.1111/j.1600-051x.1994.tb00720.x. [DOI] [PubMed] [Google Scholar]


