Abstract
X-linked hypophosphatemic rickets (XLH) is a dominantly inherited disorder characterized by renal phosphate wasting, aberrant vitamin D metabolism, and abnormal bone mineralization. XLH is caused by inactivating mutations in PHEX (phosphate-regulating gene with homologies to endopeptidases on the X chromosome). In this study, we sequenced the PHEX gene in subjects from 26 kindreds who were clinically diagnosed with XLH. Sequencing revealed 18 different mutations, of which thirteen have not been reported previously. In addition to deletions, splice site mutations, and missense and nonsense mutations, a rare point mutation in the 3’-untranslated region (3’-UTR) was identified as a novel cause of XLH. In summary, we identified a wide spectrum of mutations in the PHEX gene. Our data, in accord with those of others, indicate that there is no single predominant PHEX mutation responsible for XLH.
Keywords: Mutation, PHEX (phosphate-regulating gene with homologies to endopeptidases on the X chromosome), 3’-untranslated region, X-linked hypophosphatemic rickets
Introduction
X-linked hypophosphatemic rickets (XLH, OMIM #307800) is the most common genetic disorder of renal phosphate wasting with an approximate prevalence of 1 in 20,000. XLH is an X-linked dominant disease with high penetrance and little evidence of a gene dosage effect [1]. Clinical features of the disease include short stature, bone pain, enthesopathy (calcification of tendons and ligaments), and lower extremity deformities from rickets and osteomalacia. Biochemically, patients with XLH have hypophosphatemia secondary to renal phosphate wasting, as well as inappropriately normal 1,25-dihydroxyvitamin D concentrations.
XLH results from mutations in PHEX (phosphate-regulating gene with homologies to endopeptidases on the X-chromosome) [2]. Despite the fact that PHEX mutations cause renal phosphate wasting, PHEX is not expressed in the kidney. Instead, PHEX is predominantly expressed in bone (osteoblasts and osteocytes) and teeth (odontoblasts). Recent data indicates that plasma concentrations of a phosphaturic hormone, fibroblast growth factor 23 (FGF23), are elevated in most affected individuals [3, 4]. Furthermore, Fgf23 is overexpressed in the bone of the Hyp mouse, an animal model of XLH [5], suggesting that increased Fgf23 expression is the likely cause of the clinical phenotype of XLH.
Since the identification of the PHEX gene, over 180 mutations have been found in XLH patients (www.phexdb.mcgill.ca) [6]. Previously described PHEX mutations include deletions, insertions, and base-pair substitutions, all of which are predicted to be loss-of-function mutations. Even though the genetic cause of XLH is well established, the mechanism of the disorder has yet to be elucidated. However, identifying additional mutations, particularly missense mutations, may potentially reveal functionally important regions of the gene product. In this study, we report thirteen novel mutations.
Subjects and Methods
Study subjects
We evaluated subjects from 26 kindreds with a clinical history of hypophosphatemia and renal phosphate wasting, consistent with XLH. Multiple subjects from four kindreds (Kindreds G, S, U, and W) were included, resulting in 34 total subjects. We ascertained the presence of disease as previously reported [7]. Diagnosis was established based on physical examination, serum phosphorus concentration, and urinary phosphate excretion with or without skeletal radiographs. Some subjects had been previously treated for hypophosphatemia before recruitment. The study protocol was approved by the Indiana University-Purdue University Indianapolis (IUPUI)/Clarian and Duke University institutional review boards, and written informed consent was obtained from all subjects prior to participation.
Mutational analysis
Genomic DNA was extracted from whole blood samples using standard methods. All 22 exons, along with adjacent intronic sequences, were amplified using Multiplex PCR Kit (QIAGEN Inc., Valencia, CA). PCR primers are from Francis et al. [8] or redesigned to include larger non-coding sequences. Primer sequences are available upon request. PCR products were electrophoresed in 2% agarose gels and then purified for direct DNA sequencing. All exons were sequenced from forward or reverse PCR primers, using Big-Dye Terminator Cycle Sequencing Kit and ABI PRISM 3100 Genetic Analyzer (Applied Biosystems, Foster City, CA). In some instances, DNA sequencing was performed with Thermo Sequenase Radiolabeled Terminator Cycle Sequencing Kit according to the manufacturer’s specification (USB Corp., Cleveland, OH).
Because the patient A1 had two nucleotide changes in exon 22, this exon was amplified with PfuUltra HF DNA polymerase (Stratagene, La Jolla, CA) and cloned into pCR4Blunt-TOPO vector (Zero Blunt® TOPO® PCR Cloning Kit for Sequencing, Invitrogen, Carlsbad, CA). Five clones were sequenced from T7 to verify whether the two changes occurred on the same chromosome or on separate chromosomes.
Sequencing results of the PCR products were aligned with the PHEX genomic sequence using the NCBI BLAST program (http://www.ncbi.nlm.nih.gov/blast/bl2seq/wblast2.cgi). The effect of nucleotide changes occurring in adjacent intronic regions were analyzed using a splice site prediction tool (http://www.fruitfly.org/seq_tools/splice.html). The mutations are reported according to mutation nomenclature guidelines given by the Human Genome Variation Society: Based on the cDNA sequence, +1 is the A of the ATG start codon [9].
Allele-specific PCR
To determine if the base pair change that was observed in the 3’-untranslated region (3’- UTR) of the patients was a mutation or a polymorphism, allele-specific PCR was performed in the patients and normal controls as previously described [10]. DNA from normal individuals was obtained from 341 whites (11 males, 330 females) and 99 blacks (2 males, 97 females). PCR primers were designed using Primer3 (Whitehead Institute; http://frodo.wi.mit.edu/cgibin/primer3/primer3_www.cgi). Forward primers were designed so that each of the two alleles could be differentiated by both the size and the color of the PCR product when used with a common reverse primer: Each 5’ end was labeled with either FAM or HEX, the 5’ end of the FAM-labeled primer had two additional bases, and the final 3’ nucleotide specifically matched the targeted allele (underlined in the sequences below). Using this technique, amplification occurs preferentially when the 3’ end of the forward primer exactly matches the DNA sequence. PCR was performed with the two forward primers and one reverse primer in a single reaction. Primer sequences were: 5’ CCTTCCCAGGTATTCATGCAC (reverse); 5’ FAM-GTTTCTTTAGAAAATCAAACCAACA (forward, wild-type); and 5’ HEX-TTCTTTAGAAAATCAAACCAACG (forward, mutant). PCR products were separated using a 3100 Genetic Analyzer (Applied Biosystems). The data were then analyzed using Genescan Analysis 3.7 software (Applied Biosystems).
Results
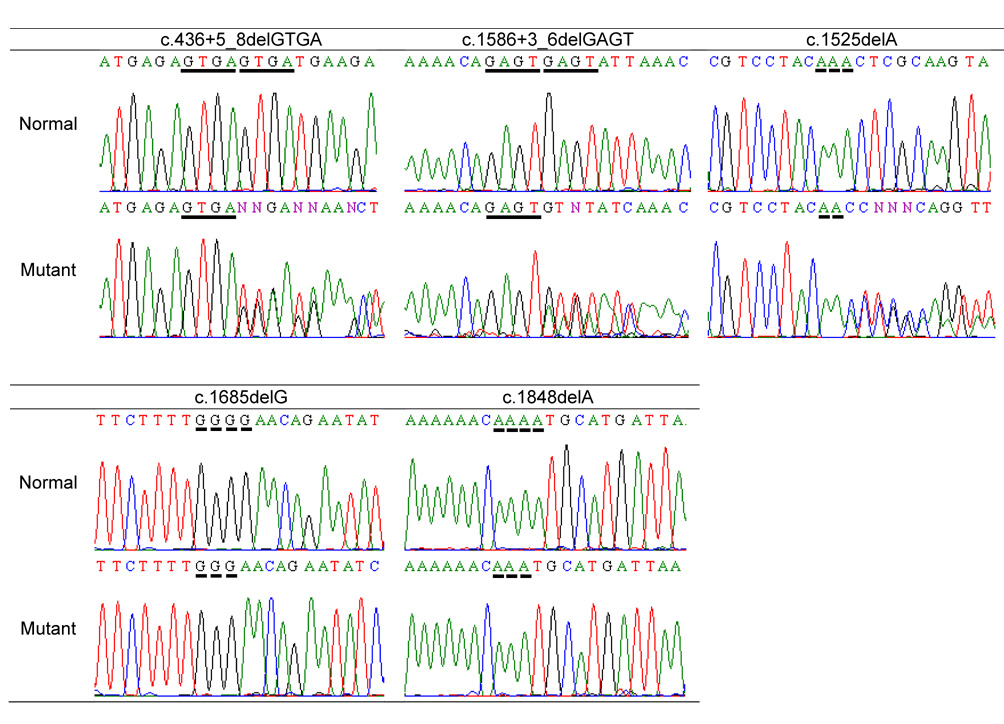
We identified 13 novel PHEX mutations and five mutations that have been previously described in the literature or entered in PHEXdb as personal submission [6] (Table 1). Of the 18 mutations, five were deletions, four were missense, seven disrupted normal splice sites, one was nonsense, and one was in the 3’-UTR. There was also a rare sequence variation in exon 3 (c.288A>G, Glu96Glu). We were unable to determine whether this is a rare synonymous polymorphism or a mutation affecting an exonic splicing enhancer. Sequence analysis revealed that several observed deletions occurred in short sequence repeats (Figure 1), which likely gave rise to misalignment during meiosis.
Table 1.
Mutational analysis of the XLH patients
| Patient a | Region | Mutation | Type | Previous Report |
|---|---|---|---|---|
| A1 | Exon 22 | c.*58C>T; c.*231A>G | 3'-UTR | |
| B1, C1, D1, E1, F1, G1–G2 | Exon 22 | c.*58C>T; c.*231A>G | 3'-UTR | |
| H1 | Intron 4 | c.436+1G>C | Splice Site | |
| I1 | Intron 4 | c.436+5_8delGTGA | Splice Site | |
| J1 | Intron 14 | c.1586+3_6delGAGT | Splice Site | [11, 14] |
| K1 | Intron 14 | c.1586+6T>C | Splice Site | |
| L1 | Intron 15 | c.1645+1G>A | Splice Site | [15, 16] |
| M1 | Intron 15 | c.1645+6T>C | Splice Site | |
| N1 | Intron 17 | c.1768+1G>C | Splice Site | |
| O1 | Exon 3 | c.328_330delAAT (Asn110del) | Deletion | |
| P1 | Exon 14 | c.1525delA | Deletion | [14] |
| Q1 | Exon 16 | c.1685delG | Deletion | |
| R1 | Exon 18 | c.1809_1816delGTCTACTG | Deletion | Personal submission in PHEXdb [6] |
| S1–S6 | Exon 18 | c.1848delA | Deletion | |
| T1 | Exon 10 | c.1109T>G (Met370Arg) | Missense | |
| U1–U2 | Exon 16 | c.1646G>C (Arg549Pro) | Missense | |
| V1 | Exon 17 | c.1735G>A (Gly579Arg) | Missense | [8, 11, 16–18] |
| W1–W2 | Exon 17 | c.1757T>C (Phe586Ser) | Missense | |
| X1, Y1 | Exon 16 | c.1699C>T (Arg567Stop) | Nonsense | |
| Z1 | Exon 3 | c.288A>G (Glu96Glu) | Polymorphism? |
Alphabet, kindred; number, individual.
Figure 1.
Sequence repeats near deletions. Horizontal bars indicate repeats in the genomic sequences, which may have promoted misalignment during meiosis. Two deletions (c.328_330delAAT and c.1809_1816delGTCTACTG) with no apparent repeats are not shown.
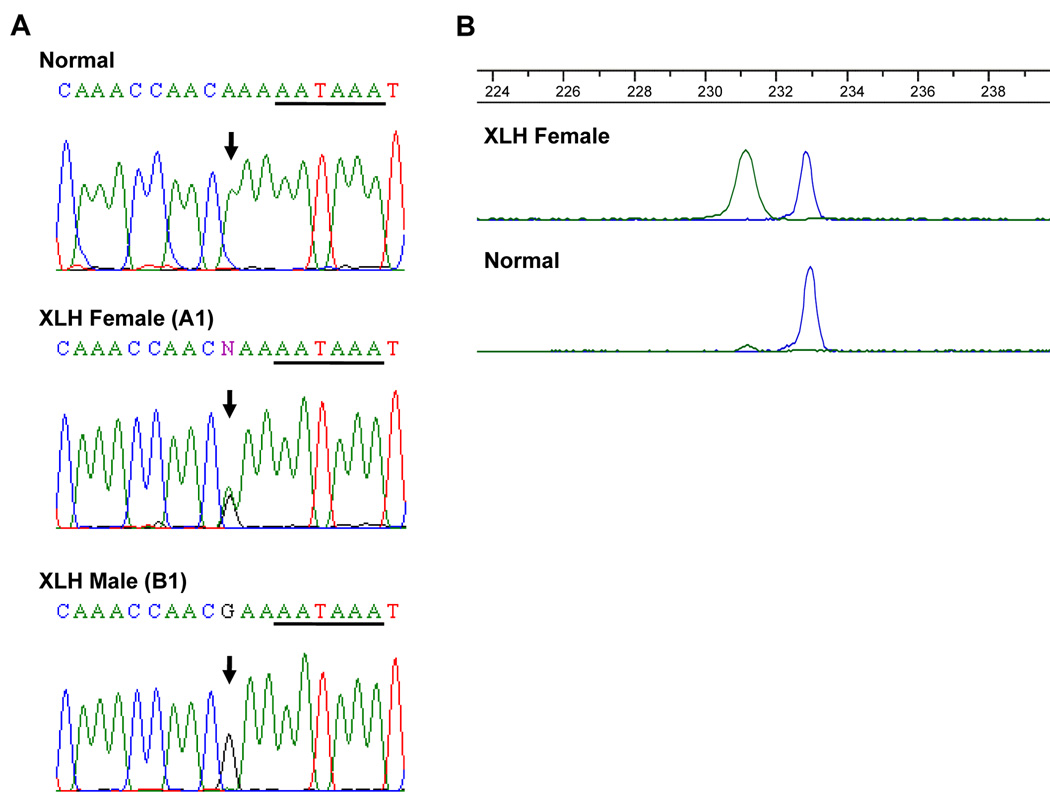
Due to its ambiguous mutation status, we further investigated the point mutation (c.*231A>G) that occurred in the 3’-UTR of the PHEX gene. This particular change was found in seven XLH patients (B1 to G2) from six different kindreds although these kindreds are from the same geographical region, consistent with a founder effect. The c.*231A>G transition is located three base pairs upstream of the putative polyadenylation signal (AAUAAA) (Figure 2A); however, it does not appear to affect any regulatory element in the region. Therefore, to determine if this change is a mutation or a polymorphism, we performed allele-specific PCR in 440 healthy individuals (427 females and 13 males; 867chromosomes) as well as our seven patients. Following the allele-specific PCR, this particular change was found only in the DNA samples from the seven XLH patients, but not in the 440 healthy controls. The chromatogram of the PCR products shows the presence of both the mutated and normal alleles in a female XLH patient and the normal allele in a control female (Figure 2B). These results demonstrate that the c.*231A>G transition is a mutation and not a polymorphism. Subsequently, the same mutation was found in an unrelated XLH patient (A1) of a different race (i.e. African American compared to the seven American white patients) and geographic region than the other patients, increasing the number of patients with the 3’-UTR mutation to eight. Interestingly, this patient also had an additional 3’-UTR nucleotide change (c.*58C>T) on the other chromosome.
Figure 2.
A. Sequence of the 3’-UTR from XLH male and female patients alongside a normal control. Horizontal bars denote putative polyadenylation signal (AAUAAA). B. Allele-specific PCR chromatogram comparing a normal female and a XLH female. Chromatogram proves that the mutation is exclusive to individuals with XLH. Green, mutant allele; blue, normal allele.
Discussion
In this study, we identified 18 different PHEX mutations and one synonymous change in 26 XLH kindreds. To the best of our knowledge, thirteen of these mutations are novel and have not been reported. Although a relationship between PHEX and XLH is well established, identification of new mutations, particularly missense mutations, has merit as these mutations may shed a light on domains important in PHEX function. In this regard, it is worth noting that the majority of the mutations were found in the 3’ end of the gene (around exons 14 through 22). This trend appears to be consistent with a previous study [11] and mutations entered in PHEXdb [6], indicating that this region might encode for a critical region of the PHEX protein. Similarly, recurrent mutations found in unrelated kindreds have significant implications in genomic structure of PHEX. Mutations affecting the G nucleotide at 1735 have been found in multiple studies (Table 1), indicating that this may be a mutation hotspot. The presence of short sequence repeats around the deletions also suggests that those regions are susceptible to misalignment during meiosis.
Although various mutations in PHEX are known to cause XLH, a rare point mutation in the 3’-UTR (c.*231A>G) is noteworthy. This mutation upstream of the polyadenylation signal of PHEX was found in eight patients from seven different kindreds. To our knowledge, no mutations in the 3’-UTR have been associated with the occurrence of XLH. Since the identified change is not in the PHEX coding region, it is challenging to prove that this is indeed a disease-causing mutation. However, several observations support our conclusion: (1) This rare point mutation was found in all affected subjects from seven apparently unrelated kindreds (patients A1 to G2). However, it was not found in over 800 normal chromosomes. (2) Six of the seven kindreds came from the same geographical region, while the seventh with a different racial background was identified in a distant state. Although their relationship was not determined, it is possible that the mutation is a result of a founder effect. (3) Several of these patients were members of kindreds where linkage was established between XLH and Xp22.1 [12, 13], making other genetic hypophosphatemic disorders (autosomal dominant hypophosphatemic rickets, autosomal recessive hypophosphatemic rickets and hereditary hypophosphatemic rickets with hypercalciuria) unlikely. (4) These XLH patients did not have any other nucleotide changes in PHEX. Since patient A1 had another rare variation in the 3’-UTR, we cannot rule out the possibility that the additional variation was pathogenic in this patient. However, these observations overwhelmingly support the notion that the 3’-UTR nucleotide variation (c.*231A>G) is in fact a mutation responsible for XLH.
The location of this mutation was three base pairs upstream of the potential polyadenylation signal (AAUAAA), and therefore, it might prevent generation of polyadenylated 3’ end (poly(A) tail), resulting in less stable mRNA. Alternatively, since microRNA usually binds to the 3’-UTR of mRNA, this mutation may increase microRNA binding, leading to inhibition of protein translation or degradation of mRNA. In any event, if a protein were made from the mutant allele, we would predict that it would result in a normal protein. Thus, although a functional study is necessary, this mutation likely decreases mRNA levels or inhibits translation of the PHEX mRNA.
In conclusion, we identified a variety of PHEX mutations in patients with XLH, including thirteen novel mutations. Although there were a few recurrent mutations, no single mutation is predominant in XLH. This mutational survey also indicates that variation in the 3’-UTR could potentially cause XLH. Therefore, molecular diagnosis of XLH may require screening of untranslated regions of the PHEX gene.
Acknowledgements
This work was supported by NIH grants R01 AR42228 and M01 RR00750. SAE was supported by a grant to the IU Cancer Center, CA82706-04S1.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brame LA, White KE, Econs MJ. Renal phosphate wasting disorders: clinical features and pathogenesis. Semin Nephrol. l2004;24:39–47. doi: 10.1053/j.semnephrol.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 2.The HYP Consortium. A gene (PEX) with homologies to endopeptidases is mutated in patients with X-linked hypophosphatemic rickets. The HYP Consortium. Nat Genet. l1995;11:130–136. doi: 10.1038/ng1095-130. [DOI] [PubMed] [Google Scholar]
- 3.Jonsson KB, Zahradnik R, Larsson T, White KE, Sugimoto T, Imanishi Y, Yamamoto T, Hampson G, Koshiyama H, Ljunggren O, Oba K, Yang IM, Miyauchi A, Econs MJ, Lavigne J, Juppner H. Fibroblast growth factor 23 in oncogenic osteomalacia and X-linked hypophosphatemia. N Engl J Med. l2003;348:1656–1663. doi: 10.1056/NEJMoa020881. [DOI] [PubMed] [Google Scholar]
- 4.Yamazaki Y, Okazaki R, Shibata M, Hasegawa Y, Satoh K, Tajima T, Takeuchi Y, Fujita T, Nakahara K, Yamashita T, Fukumoto S. Increased circulatory level of biologically active full-length FGF-23 in patients with hypophosphatemic rickets/osteomalacia. J Clin Endocrinol Metab. l2002;87:4957–4960. doi: 10.1210/jc.2002-021105. [DOI] [PubMed] [Google Scholar]
- 5.Liu S, Guo R, Simpson LG, Xiao ZS, Burnham CE, Quarles LD. Regulation of fibroblastic growth factor 23 expression but not degradation by PHEX. J Biol Chem. l2003;278:37419–37426. doi: 10.1074/jbc.M304544200. [DOI] [PubMed] [Google Scholar]
- 6.Sabbagh Y, Jones AO, Tenenhouse HS. PHEXdb, a locus-specific database for mutations causing X-linked hypophosphatemia. Hum Mutat. l2000;16:1–6. doi: 10.1002/1098-1004(200007)16:1<1::AID-HUMU1>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 7.Econs MJ, Pericak-Vance MA, Betz H, Bartlett RJ, Speer MC, Drezner MK. The human glycine receptor: a new probe that is linked to the X-linked hypophosphatemic rickets gene. Genomics. l1990;7:439–441. doi: 10.1016/0888-7543(90)90180-3. [DOI] [PubMed] [Google Scholar]
- 8.Francis F, Strom TM, Hennig S, Boddrich A, Lorenz B, Brandau O, Mohnike KL, Cagnoli M, Steffens C, Klages S, Borzym K, Pohl T, Oudet C, Econs MJ, Rowe PS, Reinhardt R, Meitinger T, Lehrach H. Genomic organization of the human PEX gene mutated in X-linked dominant hypophosphatemic rickets. Genome Res. l1997;7:573–585. doi: 10.1101/gr.7.6.573. [DOI] [PubMed] [Google Scholar]
- 9.den Dunnen JT, Antonarakis SE. Mutation nomenclature extensions and suggestions to describe complex mutations: a discussion. Hum Mutat. l2000;15:7–12. doi: 10.1002/(SICI)1098-1004(200001)15:1<7::AID-HUMU4>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 10.Carn G, Koller DL, Peacock M, Hui SL, Evans WE, Conneally PM, Johnston CC, Jr, Foroud T, Econs MJ. Sibling pair linkage and association studies between peak bone mineral density and the gene locus for the osteoclast-specific subunit (OC116) of the vacuolar proton pump on chromosome 11p12-13. J Clin Endocrinol Metab. l2002;87:3819–3824. doi: 10.1210/jcem.87.8.8740. [DOI] [PubMed] [Google Scholar]
- 11.Filisetti D, Ostermann G, von Bredow M, Strom T, Filler G, Ehrich J, Pannetier S, Garnier JM, Rowe P, Francis F, Julienne A, Hanauer A, Econs MJ, Oudet C. Non-random distribution of mutations in the PHEX gene, and under-detected missense mutations at non-conserved residues. Eur J Hum Genet. l1999;7:615–619. doi: 10.1038/sj.ejhg.5200341. [DOI] [PubMed] [Google Scholar]
- 12.Econs MJ, Fain PR, Norman M, Speer MC, Pericak-Vance MA, Becker PA, Barker DF, Taylor A, Drezner MK. Flanking markers define the X-linked hypophosphatemic rickets gene locus. J Bone Miner Res. l1993;8:1149–1152. doi: 10.1002/jbmr.5650080916. [DOI] [PubMed] [Google Scholar]
- 13.Econs MJ, Rowe PS, Francis F, Barker DF, Speer MC, Norman M, Fain PR, Weissenbach J, Read A, Davis KE, et al. Fine structure mapping of the human X-linked hypophosphatemic rickets gene locus. J Clin Endocrinol Metab. l1994;79:1351–1354. doi: 10.1210/jcem.79.5.7962329. [DOI] [PubMed] [Google Scholar]
- 14.Holm IA, Huang X, Kunkel LM. Mutational analysis of the PEX gene in patients with Xlinked hypophosphatemic rickets. Am J Hum Genet. l1997;60:790–797. [PMC free article] [PubMed] [Google Scholar]
- 15.Holm IA, Nelson AE, Robinson BG, Mason RS, Marsh DJ, Cowell CT, Carpenter TO. Mutational analysis and genotype-phenotype correlation of the PHEX gene in X-linked hypophosphatemic rickets. J Clin Endocrinol Metab. l2001;86:3889–3899. doi: 10.1210/jcem.86.8.7761. [DOI] [PubMed] [Google Scholar]
- 16.Popowska E, Pronicka E, Sulek A, Jurkiewicz D, Rowe P, Rowinska E, Krajewska-Walasek M. X-linked hypophosphatemia in Polish patients. 1. Mutations in the PHEX gene. J Appl Genet. l2000;41:293–302. [PubMed] [Google Scholar]
- 17.Dixon PH, Christie PT, Wooding C, Trump D, Grieff M, Holm I, Gertner JM, Schmidtke J, Shah B, Shaw N, Smith C, Tau C, Schlessinger D, Whyte MP, Thakker RV. Mutational analysis of PHEX gene in X-linked hypophosphatemia. J Clin Endocrinol Metab. l1998;83:3615–3623. doi: 10.1210/jcem.83.10.5180. [DOI] [PubMed] [Google Scholar]
- 18.Rowe PS, Oudet CL, Francis F, Sinding C, Pannetier S, Econs MJ, Strom TM, Meitinger T, Garabedian M, David A, Macher MA, Questiaux E, Popowska E, Pronicka E, Read AP, Mokrzycki A, Glorieux FH, Drezner MK, Hanauer A, Lehrach H, Goulding JN, O'Riordan JL. Distribution of mutations in the PEX gene in families with X-linked hypophosphataemic rickets (HYP) Hum Mol Genet. l1997;6:539–549. doi: 10.1093/hmg/6.4.539. [DOI] [PubMed] [Google Scholar]