Abstract
The authors examined the influence of positive aspects of caregiving (PAC) as a moderator of treatment outcome across 12 months in 1 of the original sites of the Resources for Enhancing Alzheimer’s Caregiver Health I project. They used multilevel random coefficients regression analysis to predict time-varying PAC, depression, behavioral bother, and daily care burden in Alzheimer’s caregivers (N = 243; mean age = 60.89, SD = 14.19). They found that time-varying PAC was predicted by time-varying daily care burden. They also found significant effects of time-varying PAC for depression, behavioral bother, and daily care burden. Notably, a PAC × Phase × Treatment effect was found for daily care burden, such that individuals who endorsed less PAC benefited most from the intervention across 12 months. The tendency to positively appraise the caregiving experience (i.e., PAC) in response to chronic stressors such as Alzheimer’s caregiving may affect individuals’ responsiveness to, and benefit from, interventions, whereas only daily care burden affected the tendency to find enjoyment in caregiving across 12 months. Future intervention research should assess individual PAC in order to better tailor interventions to caregiving needs.
Keywords: caregiving, race/ethnicity, intervention, coping, strength
As the longevity of the population increases, so does the prevalence of age-related disorders such as Alzheimer’s disease. Recent research conducted through the Centers for Disease Control and Prevention estimates prevalence rates of Alzheimer’s disease and related disorders (ADRD) in the United States at nearly 4.5 million, with expected escalation to 13.2 million by the year 2050 (Hebert, Scherr, Bienias, Bennett, & Evans, 2003). Care for individuals with ADRD is provided predominantly by family members and friends in the community or home; however, to date, the role of positive appraisals of the caregiving experience has been largely marginal in caregiving research (Roff et al., 2004; Tarlow et al., 2004). Moreover, no studies have examined the role of positive caregiving appraisal and responsiveness to caregiver interventions.
Psychological distress and increased risk for depression are consistently associated with caregiving (e.g., Haley, Levine, Brown, & Bartolucci, 1987; Hooker et al., 2002; Levine, 2003; Schulz, Visintainer, & Williamson, 1990). Although many of the hardships encountered by Alzheimer’s caregivers are shared with those caring for individuals with any chronic illness, caring for an individual with Alzheimer’s can be especially detrimental (Ory, Yee, Tennestedt, & Schulz, 1999). The functional impairments that are a fundamental part of dementia are accompanied by a number of behavior problems that make caring for a loved one particularly challenging. These unique functional and behavioral impairments can contribute substantially to the psychological and physical morbidity of the caregiver (e.g., Hooker et al., 2002).
Stress process models of coping have frequently been applied to the caregiving role to explain individual differences in the ability to manage the many stressors that caregivers face and to predict physical and emotional outcomes based on these factors (Haley et al., 1987, 1996; Pearlin, Mullan, Semple, & Skaff, 1990). For example, Lazarus and Folkman’s (1984) original stress process model explained stress within an individual as a process that is influenced by a number of contextual factors affecting how the individual appraises and copes with a given event. Similarly, Pearlin’s stress process framework (Pearlin et al., 1990) focused on contextual variables in terms of primary and secondary stressors, making a distinction between stressors that are directly related to the caregiving role (e.g., care recipient problem behaviors) and sources of stress that are indirectly related (e.g., financial strain). After additional research involving individuals caring for partners with AIDS, Folkman (1997) revised the original stress and coping model, incorporating positive psychological appraisals, specifically with respect to their beneficial effects on the maintenance of the coping process. When stressed caregivers do not engage in positive, or meaning-based, reappraisals, negative emotional outcomes may diminish the coping response. Therefore, intervention targeted at increasing positive reappraisal of caregiving outcomes may reinforce and facilitate continued emotional coping.
Positive aspects of caregiving (PAC) have been identified in a variety of ways, but they are typically defined as the rewards and satisfaction derived from the caregiving relationship (Kramer, 1997; Tarlow et al., 2004). Satisfaction with caregiving and rewarding appraisals of caregiving may reduce caregiving stress and improve emotional outcomes (Kinney & Stephens, 1989; Roff et al., 2004). C. A. Cohen, Colantonio, and Vernich (2002) asked 289 Canadian caregivers if they could identify and describe at least one positive aspect within their caregiving relationship. Results revealed that 73% of caregivers could identify at least one PAC, mentioning a range of positive experiences from feeling fulfilled, important, and responsible, to finding a sense of companionship and meaning within the relationship. Individuals with higher PAC reported less depression, burden, and better subjective health than those who did not endorse PAC. C. A. Cohen and colleagues concluded that the ability to identify positive aspects within the caregiving relationship might serve as a buffer against negative consequences. Notably, this research was cross-sectional, limiting the causal attributions that can be gleaned from the results.
Findings have recently emerged on cultural variations in the report of positive aspects of the caregiving experience (Burgio, Stevens, Guy, Roth, & Haley, 2003; Roff et al., 2004). Roff and colleagues explored racial differences in the report of PAC at baseline within White and African American Alzheimer’s caregivers from the original Resources for Enhancing Alzheimer’s Caregiver Health I (REACH I) project (National Institutes of Health [NIH], 2002). Like previous research in this area (Picot, Debanne, Namazi, & Wykle, 1997), Roff and colleagues observed higher levels of PAC in African American caregivers than in White caregivers. Moreover, religiosity partially mediated the relationship between PAC and race. Although symptoms of depression were not associated with baseline PAC, these researchers also found that lower anxiety levels, less behavioral bother, and lower socioeconomic status (SES) among African American caregivers appeared to contribute to their higher levels of PAC. However, this research was cross-sectional, leaving open the question of whether PAC is a time-varying individual difference variable that may be targeted, in itself, for intervention.
Notably, Tennen and Affleck (2002) cautioned that identifying positive aspects of stressful situations does not necessarily indicate that individuals use these appraisals to cope. They stated that the ability to recognize positive aspects or find benefits in adversity when prompted during an interview should be distinguished from active reliance on these coping mechanisms on a daily basis as a means of reducing stress. Tennen and Affleck framed this as a methodological weakness in the coping literature that has not yet been adequately addressed. Absent from the literature is evidence that PAC is, in itself, modifiable across time or, potentially, that PAC may moderate individuals’ effective use of interventions. Specifically, perhaps persons with fewer identified positive aspects of the caregiving role would benefit differentially than those already receiving some level of benefit from the caregiving context.
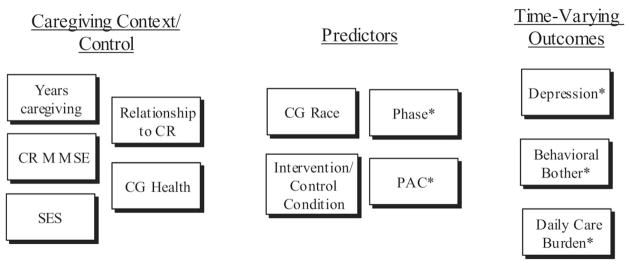
The current study was designed to address four limitations in the caregiving literature as it relates to PAC. Specifically, we (a) examined the impact of time-varying depression, behavioral bother, and daily care burden on time-varying PAC over 12 months; (b) examined the impact of time-varying PAC on negative emotional outcomes over 12 months; (c) explored the interaction between PAC and participation in intervention on time-varying negative emotional outcomes; and (d) examined whether the relations among these variables varied by race, limiting our sample to a comparison of White/Caucasian to Black/African American caregivers. By examining the potential of time-varying negative affect to serve as a predictor of time-varying PAC and the potential of PAC to serve as a moderator of the effects of intervention on time-varying depression, behavioral bother, and daily care burden associated with caregiving, we may provide evidence that positive appraisal of the caregiving experience does indeed reinforce the coping process, as predicted by Folkman’s (1997) revised stress and coping model. Our conceptual model of the interrelationship of PAC, depression, behavioral bother, and daily care burden as they unfold over time is depicted in Figure 1.
Figure 1.
Model depicting the relation between baseline control variables, predictor variables, and time-varying emotional outcomes. Asterisks indicate time-varying variables (i.e., values at baseline, 6 months, and 12 months are in the model); variables without an asterisk are baseline values only. CR = care recipient; MMSE = Mini-Mental State Examination; CG = caregiver; SES = socioeconomic status; PAC = positive aspects of caregiving.
For this project, data from the Philadelphia site of the original REACH I program (NIH, 2002), supported through the National Institute of Nursing Research and the National Institute on Aging, were chosen for the site’s successful intervention (Gitlin, Winter, et al., 2003), inclusion of a usual care control group, and roughly equal representation of White and African American participants. This usual care condition consisted of information and referral for services, which arguably resembled typical resources given to Alzheimer’s caregivers in the community. The intervention used at this site is described next.
Intervention
The environmental skill-building program implemented at the Philadelphia site (Gitlin, Winter, et al., 2003) was designed to increase mastery of caregivers through the introduction of strategies to modify the physical, social, and task dimensions of the home environment. This was done through education about the impact of the home environment on the care recipient’s behaviors and functional impairments. In the first session, a needs assessment was conducted by an occupational therapist who served as the interventionist and who assessed concern regarding 11 different caregiving domains (e.g., difficulty assisting with activities of daily living [ADLs], incontinence, wandering, home safety). From this assessment, treatment goals were developed. Following the initial needs assessment, occupational therapists worked in the home with caregivers to tailor a program addressing the specific needs of the dyad. Instruction and problem-solving techniques for manipulating the environment were then implemented. Once strategies were applied to the initial areas of complaint, caregivers were encouraged to generalize skills to new problems. During the active phase of treatment (i.e., the first 6 months), a trained occupational therapist conducted five 90-min home visits and one 30-min telephone call. Following the active phase of the intervention, 6 months of maintenance occurred that included one additional home visit and three telephone calls, which were used to reinforce previous strategies (Gitlin, Hauck, Dennis, & Winter, 2005). The Philadelphia site identified the 6-month assessment point as its primary posttreatment outcome; however, additional assessments were collected at 12 and 18 months in accordance with the REACH I protocol. Results revealed a significant intervention effect (p < .05) for behavioral bother as a result of memory-related behaviors, a decrease in need for assistance from others, and improvement in affect as determined by the Perceived Change Index, which was created by the authors (Machemer et al., 2000, as cited in Gitlin, Winter et al., 2003). For a full review of the protocol and results of the Philadelphia site, see Gitlin, Winter, et al., and Gitlin, Belle, et al. (2003). Philadelphia was chosen above other REACH I sites because the intervention directly targeted two of our primary dependent variables (i.e., behavioral bother and daily care burden).
Our model for PAC controlled for the effects of race and time-varying depression, behavioral bother, and daily care burden, as well as all two-way interactions between these variables. Our models for negative affect (e.g., time-varying depression, behavioral bother, and daily care burden) controlled for the effects of years of caregiving, caregiver’s relationship to the care recipient, SES, care recipient Mini-Mental State Examination (MMSE) score, and caregiver health (see Figure 1). Each of these variables has repeatedly been demonstrated in intervention research to impact the emotional outcomes of the caregiver (D. Cohen et al., 1990; Coon et al., 2004; Pinquart, & Sörensen, 2003; Sörensen & Pinquart, 2005). First, we hypothesized that PAC would vary across time and as a result of intervention, despite examining an intervention that was not designed to target increasing PAC as an outcome. Second, we predicted a main effect of PAC above and beyond control variables such that Alzheimer’s caregivers who reported more PAC across time also would demonstrate better treatment outcomes in the form of decreased negative affect across 12 months than caregivers who had lower levels of PAC across time. Third, we predicted that PAC would interact with intervention to produce changes in negative affect across time, but that negative affect would not influence time-varying PAC across 12 months. Finally, we expected to replicate previous cross-sectional analyses (e.g., Roff et al., 2004) demonstrating that African American Alzheimer’s caregivers report higher PAC across time in comparison with White caregivers as we explored the moderating effects of race and PAC across time for depression, behavioral bother, and daily care burden.
Method
Sample and Procedures
The original REACH I project (NIH, 2002), was a unique multisite project that implemented and evaluated nine different interventions across six sites for 18 months; however, due to attrition, only 12-month outcomes are examined in this analysis. Baseline data for 1,222 caregiver/care recipient dyads were collected through randomized clinical trials across the six sites: Birmingham, Boston, Memphis, Miami, Palo Alto, and Philadelphia. Detailed information about the overall REACH I study and recruitment procedures for each site are described elsewhere (Burgio et al., 2003; Burns, Nichols, Martindale-Adams, Graney, & Lummus, 2003; Eisdorfer et al., 2003; Gallagher-Thompson et al., 2003; Gitlin, Winter, et al., 2003; Mahoney, Tarlow, & Jones, 2003; Schulz et al., 2003). Baseline characteristics for the entire REACH I sample are described in Wisniewski et al. (2003).
Participants were recruited using a variety of methods from multiple community organizations including health and social settings, with special attention paid to the recruitment of minority individuals. Caregivers were included in the study if they were at least 21 years old, living with the care recipient, and providing 4 hr or more of care per day to a family member with at least two functional impairments of instrumental ADLs (IADLs) or one ADL (Katz, Ford, Moskowitz, Jackson, & Jaffe, 1963; Lawton & Brody, 1969). The care recipient had to have a diagnosis of ADRD or an MMSE score of 23 or less out of a possible score of 30 (Folstein, Folstein, & McHugh, 1975). In addition, caregivers had to have been in the caregiving role for at least 6 months, be reachable by phone, and plan on staying in the area for the duration of the study.
After obtaining informed consent from eligible participants, researchers collected baseline data before randomly assigning individuals to experimental groups. Subsequent interviews used the core battery of assessment (see Table 1), which was common to all sites, at 6 and 12 months after baseline (NIH, 2002). Modified batteries were given if caregiving status had changed as a result of care recipient death or institutionalization.
Table 1.
REACH I Core Battery of Assessment (Collected at Baseline, 6, 12, and 18 Months)
| Measure | Authors |
|---|---|
| Standard measures | |
| Activities of daily living | Katz, Ford, Moskowitz, Jackson, & Jaffe (1963) |
| Instrumental activities of daily living | Lawton & Brody (1969) |
| Revised Memory and Behavior Problems Checklist | Teri et al. (1992) |
| Center for Epidemiological Studies—Depression scale | Radloff (1977) |
| Mini-Mental State Examination | Folstein, Folstein, & McHugh (1975) |
| Modified measures | |
| Caregiver health and health behaviors | Posner, Jette, Smith, & Miller (1993) |
| Anxiety Inventory | Spielberger, Gorsuch, Lushene, Vagg, & Jacobs (1983) |
| Social support | Barrera, Sandler, & Ramsay (1981); Krause (1995); Krause & Markides (1990); Lubben (1988) |
| Measures designed by REACH researchers | |
| Vigilance | NIH (2002) |
| Formal care and services | NIH (2002) |
| Positive Aspects of Caregiving | Tarlow et al. (2004); NIH (2002) |
| Religiosity | Pargament & Rye, (1998); Schulz et al. (2001) |
| Social activities | NIH (2002) |
Note. REACH = Resources for Enhancing Alzheimer’s Caregiver Health.
Of the 253 Philadelphia caregivers providing care to a community-dwelling loved one with ADRD, 243 were included in the final analyses. The remaining 10 were excluded due to race; only African American and White caregivers were included in these analyses (African American caregivers, n = 121; White caregivers, n = 122). The mean age of the caregivers in this sample was 60.89 years (SD = 14.19). Caregivers were predominantly female (74.5%; see Table 2) and cared for care recipients with a mean age of 80.23 years (SD = 7.94). The mean MMSE score of the care recipients was 12.35 (SD = 7.1). Mean caregiver depression, PAC, and other characteristics of caregivers and care recipients are described in Table 3.
Table 2.
Caregiver Characteristics by Experimental Condition
| Experimental condition
|
||
|---|---|---|
| Intervention (n = 124)
|
Control (n = 119)
|
|
| Caregiver characteristic | n (%) | n (%) |
| Race | ||
| White | 62 (50.0) | 60 (50.4) |
| African American | 62 (50.0) | 59 (49.6) |
| Gender | ||
| Male | 32 (25.8) | 30 (25.2) |
| Female | 92 (74.2) | 89 (74.8) |
| Years of education | ||
| Less than high school | 31 (25.0) | 29 (24.4) |
| High school diploma | 42 (33.9) | 39 (32.8) |
| Some college | 35 (28.2) | 36 (30.3) |
| College or more | 16 (12.9) | 15 (12.6) |
| Relation to care recipient | ||
| Spouse | 49 (39.5) | 46 (38.7) |
| Nonspouse | 75 (60.5) | 73 (61.3) |
Table 3.
Means for Variables of Interest by Experimental Condition
| Experimental condition
|
||
|---|---|---|
| Intervention (n = 124)
|
Control (n = 119)
|
|
| Sample characteristic | M (SD) | M (SD) |
| Caregiver | ||
| Years caregiving | 4.25 (3.72) | 4.25 (3.89) |
| Nam-Power’s Index | 54.54 (22.38) | 58.04 (21.21) |
| Subjective health | 2.74 (1.09) | 2.72 (1.00) |
| Total positive aspects of caregiving | 34.02 (8.69) | 33.05 (9.01) |
| Center for Epidemiological Studies—Depression | 14.63 (12.11) | 15.27 (11.40) |
| Revised Memory and Behavior Problems Checklist bother | 1.47 (.88) | 1.44 (.93) |
| Daily care burden | 0.85 (0.83) | 0.96 (1.01) |
| Care recipient | ||
| Mini-Mental State Examination | 12.14 (7.09) | 12.67 (6.95) |
| Activity of daily living impairmenta | 10.26 (3.42) | 10.27 (3.31) |
Note. No significant differences were observed between groups at baseline.
Sum of the amount of assistance needed by the care recipient to complete activities of daily living.
Measures
Background and stress variables
Sociodemographic information for caregivers and care recipients was collected through caregiver report for items including gender, age, primary racial/ethnic group, marital status, education level, household income, and caregiver’s relationship to the care recipient. Due to the importance of the caregiver’s relationship to the care recipient (e.g., D. Cohen et al., 1990; Mittelman, 2003; Pinquart & Sörensen, 2003), particularly its potential impact on responsiveness to interventions (Mahoney et al., 2003), we coded caregivers as either spouse or nonspouse for the final analyses. Information regarding primary occupation was later coded into SES scores according to the Nam-Powers criteria for estimating SES (Nam & Terrie, 1988). These criteria rank U.S. occupations according to the median education and income of individuals employed in each profession; scores range from 0 to 100, with service sector jobs scoring on the low end and professional or technical occupations scoring on the higher end. The higher individual Nam-Powers score within each dyad was used as an indicator of SES.
Caregiver health consisted of 19 items assessing four domains: perceived health, comorbidity, health behaviors and diet, and stress-related symptoms. Items from each domain were based on health questionnaires used elsewhere (Ware et al., 1995). A single item for subjective health, used in final analyses, measured the individual’s perception of his or her health status; response options ranged from poor to excellent along a 5-point scale. This type of subjective assessment of overall health status is widely used and is generally accepted as a valid measure of health status (Knauper & Turner, 2003). However, because this was a single-item measure, Cronbach’s alpha was not computed.
Care-recipient-related variables
The cognitive status of the care recipient was assessed using the MMSE (Folstein et al., 1975). The MMSE includes several tasks that cover orientation; memory; attention; and ability to name objects, follow verbal and written commands, write a sentence, and copy a complex design. Scores range from 0 to 30, with scores equal to or less than 24 indicating cognitive impairment. This measure of cognitive functioning has been well validated, with test–retest correlations of .80 to .95 (Tombaugh & McIntyre, 1992).
The seven-item Activities of Daily Living Scale (Katz et al., 1963) was used to assess the care recipient’s ability to perform tasks of daily functioning independently (e.g., bathing, dressing, toileting, eating, grooming, and transferring). If the care recipient required assistance in a given area as indicated by either a yes or no response, the caregiver was asked whether he or she provided the assistance (Cronbach’s α for our sample = .83). Total level of assistance needed for ADLs was summed, with higher scores indicating more impairment. We did not assess the need for assistance with IADLs or associated IADL care burden because previous research has shown that most moderately to severely demented community-dwelling care recipients require assistance with these activities (potentially restricting variability in our measure) and that IADL care burden is relatively independent of the care recipient’s ability to perform IADL and ADL tasks (Gitlin, Roth, et al., 2005).
PAC
The nine-item Positive Aspects of Caregiving scale presents statements about a caregiver’s mental or affective state in the context of the caregiving experience (Tarlow et al., 2004). Responses are provided on a 5-point agree/disagree scale and are designed to assess the perception of benefits within the caregiving context, such as feeling useful, feeling appreciated, and finding meaning. Higher scores represent more positive appraisals. In these analyses, PAC was allowed to vary across time. In other words, our analysis of PAC was not limited to REACH I baseline data.
Psychometric analyses of this measure using the entire REACH I sample (N = 1,229) revealed a Cronbach’s alpha of .89 (Tarlow et al., 2004). Scores ranged from 9 to 45, with higher scores indicating higher levels of PAC within the care-giving context. Psychometric analyses of the REACH I baseline sample found factor invariance for two factors (e.g., self-affirmation and outlook on life) across numerous subgroups (e.g., age quartiles, gender, and race). Additionally, Tarlow and colleagues established convergent validity by demonstrating positive bivariate associations of PAC with (a) the four-item Well-Being subscale of the Center for Epidemiological Studies—Depression scale (CES–D; Radloff, 1977), (b) self-reported health, and (c) satisfaction with social support from the Inventory of Socially Supportive Behaviours (as modified by Krause, 1995). Similarly, convergent validity was also supported by negative bivariate correlations with (a) Behavioral Bother subscales of the Revised Memory and Behavior Problems Checklist (RMBPC; Teri et al., 1992) and (b) negative social interactions (Inventory of Socially Supportive Behaviours; Krause, 1995). Furthermore, support for discriminant validity included the negative bivariate association with the Somatic Signs subscale of the CES–D. It is interesting to note that the bivariate associations demonstrating convergent validity were smaller than predicted (Tarlow et al., 2004). For example, the overall association between total PAC and the CES–D Well-Being subscale was r = .24. This suggests that PAC represents a unique construct distinguishable from well-being, negative affect, and social support.
Depression
Caregiver depression was assessed using the CES–D (Radloff, 1977). The CES–D is a 20-item scale that asks about the frequency with which respondents have experienced depressive symptoms within the past week. It comprises four subscales: Depressive Affect, Positive Affect or Well-Being, Interpersonal Distress, and Somatic Signs. Response options range from 0 to 3 for each item (0 = rarely or none of the time, 1 = some or a little of the time, 2 = moderately or much of the time, 3 = most or almost all of the time), with total scores ranging from 0 to 60. Higher scores indicate elevated levels of depressive symptoms, and a score of 16 or greater indicates that the individual may have clinically significant depressive symptoms (Radloff, 1977). A high degree of internal consistency has been documented, with Cronbach’s alpha reliabilities for older populations reported between .86 and .89 (Schein & Koenig, 1997).
Behavioral bother
Appraisal of caregiver burden was operationalized in terms of two components, the first of which was upset with memory-related and disruptive behaviors. The RMBPC (Teri et al., 1992) was used to assess the presence of 24 problem behaviors (7 memory related, 8 depressive, and 9 disruptive) that the care recipient may have exhibited in the past week. Presence or absence of the behaviors was adapted to a yes/no format rather than the 5-point scale used in the original. If caregivers responded yes, they were asked how bothered or upset they were for each reported behavior using a 5-point scale ranging from 0 (not at all) to 4 (extremely). A total caregiver upset score was found for behavioral bother by totaling responses to each of these subscales, with higher scores indicating more subjective burden. The average score was then calculated, with scores ranging from 0 to 4. This conditional bother score was used for this analysis because it indicated how reactive or upset the caregiver was to the average problem experienced, regardless of the total number of behaviors displayed. In addition, this made comparisons across caregivers more meaningful. This method for computing average upset scores is described in more detail in Gitlin, Roth, et al. (2005); the condition is referred to as behavioral bother for these analyses. Psychometric properties for the REACH I revision of the RMBPC were reported by Roth et al. (2003) based on data from REACH I baseline.
Daily care burden
The second, novel component of caregiver burden was operationalized as degree of bother associated with the tasks of providing daily care, or assistance with ADLs (Katz et al., 1963). Although it is standard practice to consider the level of impairment of individuals with ADRD based on the amount of assistance required, until recently the amount of burden or caregiver reaction to providing that care has been omitted (Gitlin, Roth, et al., 2005). Although this type of burden has also been referred to as caregiver upset (Gitlin, Roth, et al.), we refer to this construct here as daily care burden to distinguish it from burden resulting from behavioral disturbances.
Psychometric properties for this scale were reported by Gitlin, Roth, et al. (2005) based on data from REACH I baseline. For each item, caregivers rated their level of upset on a 5-point scale from 0 (no upset) to 4 (extremely upset). First, a total caregiver upset score was found for daily care burden by summing responses to each of these subscales, with higher scores indicating more subjective burden. Next, average burden experienced by the caregiver for each daily care routine (i.e., ADLs) was determined. This burden is the focus of these analyses, similar to the analyses for behavioral bother to ensure comparability of upset scores across caregivers. Scores of average daily care burden could range from 0 to 4; higher scores indicate more burden.
Data Analysis
In order to test the proposed hypotheses, we first examined whether time-varying PAC remained stable or varied by time, intervention, and time-varying negative affect (e.g., depression, behavioral bother, and daily care burden). Our model used multilevel random coefficients regression analysis (PROC MIXED, SAS Institute, Cary, NC) with time-varying PAC as the primary outcome variable. All two-way interactions were included in the model. A first-order autoregressive error structure was specified to allow for autocorrelation among the three time points for each individual caregiver. Next, we examined the main effect of time-varying PAC above and beyond control variables on the time-varying treatment outcomes of depression, behavioral bother, and daily care burden using multilevel random coefficients regression analysis (PROC MIXED). Time-lagged independent variables were not considered. In other words, we did not include any predictors that used baseline assessments to predict later performance (i.e., 6-month or 12-month outcomes); our predictors were always measured at the same time point. Depression, behavioral bother, and daily care burden were examined in three different models. The following control variables served as covariates: SES, years of caregiving, caregiver’s relationship to the care recipient, subjective health of the caregiver, and MMSE of the care recipient. Our primary predictor variables of interest included treatment group, phase, PAC, and race (see Figure 1). The model predicting daily care burden also included total ADL/IADL functional impairment. All interactions, including the PAC × Treatment interaction, were included in the models.
Our hypothesis that PAC might interact with treatment across time among ADRD caregivers would be supported by a significant PAC × Treatment interaction for each outcome variable (i.e., caregiver depression, behavioral bother, and daily care burden). We represented interactions with time-varying PAC, measured continuously, by graphing the 90th (e.g., high PAC), 50th (mean PAC), and 10th (low PAC) percentiles. All of our outcome variables were centered, and standard scores were used to represent the y-axis in all of our figures.
Results
Predicting PAC
The model predicting time-varying PAC included treatment condition, phase, race, and time-varying depression, behavioral bother, and daily care burden, as well as all two-way interactions. We found a significant effect for race, F(1, 104) = 4.12, p = .0448, with African American caregivers reporting higher levels of PAC across 12 months. Additionally, there was a main effect of time-varying daily care burden, F(1, 162) = 15.38, p < .0001, indicating that decreases in daily care burden across time were associated with increases in PAC. The main effect of phase was not a significant predictor of PAC in this model, which suggests that time alone does not impact PAC. It is interesting to note that although there were significant bivariate relations between time-varying PAC and time-varying depression, F(1, 490) = 23.38, p < .0001, time-varying behavioral bother, F(1, 495) = 19.19, p < .0001, and time-varying daily care bother, F(1, 176) = 26.12, p < .0001, the model indicated that only daily care bother had a significant independent relation with PAC.
Predicting Depression
As expected, time-varying PAC uniquely predicted variance in time-varying depression beyond other effects in the model (e.g., relationship to the care recipient, care recipient MMSE), F(1, 447) = 17.12, p < .0001, such that persons with higher values of PAC had lower levels of depression across time. In addition, caregiver health, F(1, 460) = 33.98, p < .0001; race, F(1, 224) = 14.38, p < .0001; and SES, F(1, 209) = 4.11, p = .0438, were also identified as unique predictors of depression. Caregivers who reported better subjective health, were African American, and had higher SES reported lower levels of depression.
Predicting Behavioral Bother
Significant predictors of the caregiver’s level of time-varying bother for behavioral problems or disturbances were PAC, F(1, 463) = 4.35, p = .0375; caregiver health, F(1, 435) = 13.65, p = .0002; race, F(1, 248) = 16.52, p < .0001; and phase, F(2, 276) = 4.75, p = .0094. Similar to the model predicting depression, caregivers reporting better subjective health and higher PAC reported lower levels of behavioral bother across time. Furthermore, African Americans also reported lower levels of behavioral bother than their White counterparts. Finally, the significant main effect for phase indicated that behavioral bother varied over time, with the highest levels reported at baseline and the lowest at 12 months.
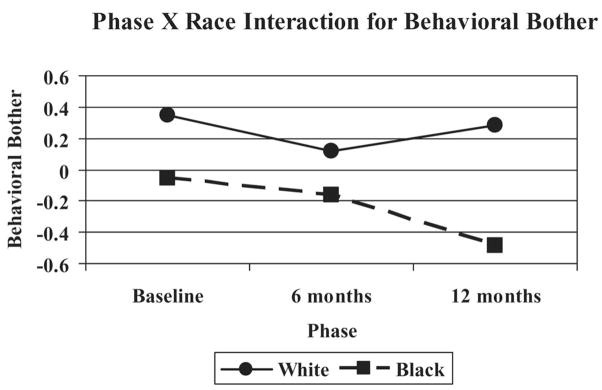
In addition, a significant interaction for Phase × Race, F(2, 276) = 4.1, p = .0177, was found to uniquely predict variance in behavioral bother, such that African American caregivers reported decreasing behavioral bother from baseline to 12 months. However, White caregivers reported relatively stable levels of behavioral bother across time (see Figure 2), with a slight reduction at 6 months that returned to baseline levels by 12 months.
Figure 2.
White and African American caregivers’ ratings of behavioral bother over 12 months.
Predicting Daily Care Burden
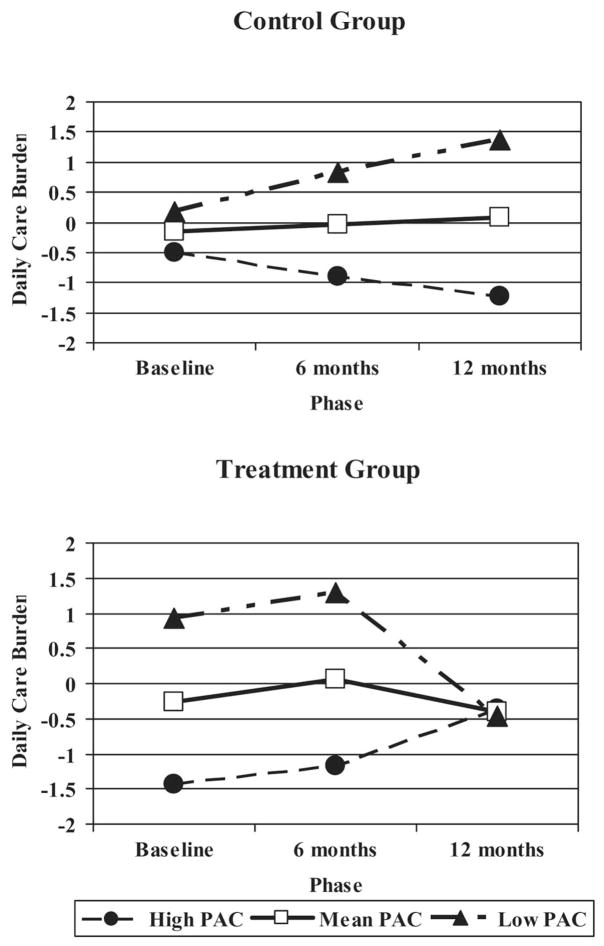
As predicted, time-varying PAC accounted for a significant amount of the variance in the prediction of time-varying daily care burden beyond other variables in the model, F(1, 148) = 23.59, p < .0001, such that caregivers who were able to identify more PAC were less upset by providing assistance with daily care. Moreover, the Treatment Condition × PAC × Phase interaction was a significant predictor of daily care burden across time, F(2, 89.2) = 5.94, p = .0038, such that individuals low in PAC showed the greatest benefit from intervention across 12 months (see Figure 3).
Figure 3.
Three-way interaction of positive aspects of caregiving (PAC), treatment condition, and phase on daily care burden across 12 months.
Discussion
Our conceptual framework (see Figure 1), based on Folkman’s (1997) revised stress and coping model, proposed that Alzheimer’s caregivers’ positive subjective appraisals of the caregiving experience (e.g., PAC) over time would predict positive and negative emotional outcomes. In Folkman’s model, unfavorable resolution of stressful events or negative emotional outcomes may lead to meaning-based coping, consisting of positive reappraisals, revised goals, spiritual beliefs, and engagement in positive events. Such positive reappraisal reinforces and sustains the coping process, functionally enabling the caregiver to approach the ongoing stress anew with a greater experience of meaning in the process. In support of this conceptualization, we found that time-varying PAC did indeed predict time-varying depression, behavioral bother, and daily care burden, with caregivers who appraised the caregiving experience more positively across time showing better emotional outcomes across time (see also Kinney & Stephens, 1989; Kramer, 1997). Moreover, time-varying PAC interacted with treatment condition and phase to predict daily care burden, such that caregivers who appraised the caregiving experience less positively across time showed the greatest benefit of intervention. However, other aspects of our conceptual framework regarding the role of PAC in the stress and coping process across time were not supported.
Looking at our findings in relation to our expectations more closely, we see that our first hypothesis was that PAC, consisting of two factors labeled self-affirmation and outlook on life (Tarlow et al., 2004), would vary with time and intervention. We considered PAC as a measure of positive subjective appraisal that might vary across time. Instead, we found that time-varying PAC was predicted by race (e.g., the fourth hypothesis) and time-varying daily care burden (one aspect of the third hypothesis). Our results did not support the notion that PAC is variable with time and intervention. It is possible that self-affirmation and outlook on life are relatively stable aspects of an individual’s appraisal of the Alzheimer’s caregiving experience. Future research should examine, both in cross-sectional and longitudinal studies, the relation of PAC and individual difference variables including resilience (Wagnild & Young, 1993), emotional stability (Costa & McCrae, 1988), and well-being (Lawton, Moss, Kleban, Glicksman, & Rovine, 1991; Ryff, 1989). Future research with Alzheimer’s caregivers should also examine Folkman’s (1997) revised stress and coping model longitudinally, particularly the role of meaning-based coping after unfavorable resolution of stressful events or negative emotional outcomes. Perhaps PAC as currently measured in REACH I does not adequately represent Folkman’s latent construct of meaning-based coping. Kramer (1997) highlighted the need for greater methodological precision in the measurement of PAC and the need to distinguish PAC from positive affect.
Our second and third hypotheses involved the longitudinal interrelationship of PAC, depression, behavioral bother, and daily care burden. We first examine our findings regarding time-varying negative affect as a predictor of time-varying PAC. In a meta-analysis of 228 studies including aspects of caregiving such as PAC, depression, and behavioral bother, Pinquart and Sörensen (2003) found that lower levels of caregiver depression and behavioral bother were associated with positive appraisals of the care-giving experience. However, these relations were relatively modest for dementia caregivers (rs = −.17 and −.26, respectively). Moreover, previous cross-sectional analyses of the REACH I data failed to show meaningful associations among these variables (Roff et al., 2004; Tarlow et al., 2004). For example, Roff and colleagues did not find a relation between PAC and depression across three REACH I sites at baseline, and we did not find that time-varying depression predicted time-varying PAC. Moreover, the factor analysis of the PAC measure across all six REACH I sites at baseline revealed only small associations between PAC and the Well-Being subscale (Spearman’s r = .24) and the Somatic Signs subscale (Spearman’s r = −.17) of the CES–D and between PAC and behavioral bother (Spearman’s r = −.23). We also did not find that time-varying behavioral bother predicted time-varying PAC, even though Roff and colleagues found that behavioral bother predicted PAC at baseline. It seems, then, that available longitudinal evidence suggests that time-varying depression and behavioral bother are not predictive of time-varying PAC. However, because these variables are highly related, multicollinearity between the constructs may have contributed to this null finding.
Time-varying daily care burden, however, did significantly predict time-varying PAC. Alzheimer’s caregivers who reported lower burden associated with the provision of ADL care to their care recipient across time also reported higher PAC across time. This study is the first to examine the longitudinal relation of these two constructs. Gitlin, Roth, and colleagues (2005) did not examine the relation between PAC and daily care burden (measured across ADLs and IADLs), and, as attempts to measure daily care burden are relatively new, prior meta-analytic studies have not examined this association (Pinquart & Sörensen, 2003). This finding is interesting in light of prior research indicating that more positive appraisals of the caregiving experience are associated with greater functional impairments among care recipients (Lawton, Kleban, Moss, Rovine, & Glicksman, 1989; Lawton et al., 1991). Perhaps those dementia caregivers who are most likely to report daily care burden are the least likely to report positive aspects of the caregiving relationship. It may be, then, that these caregivers could benefit most from active, multicomponent skills-building interventions, particularly if such interventions contained a component specifically designed to enhance positive reappraisal.
Notably, time-varying PAC had a significant main effect for each time-varying negative affect variable (i.e., depression, behavioral bother, and daily care burden), such that caregivers reporting greater PAC across time reported less depression, behavioral bother, and daily care burden across time. For the most part, this effect was observed over and above the effect of control variables including phase, SES, years of caregiving, caregiver’s relationship to the care recipient (spouse, nonspouse), and MMSE of the care recipient, with specific exceptions. Time-varying depression was also predicted by caregiver health, SES, and race, such that those who reported better subjective health, higher SES, and African American race reported lower levels of depression across time. Like depression, time-varying behavioral bother was also predicted by caregiver health and race, as well as phase. Caregivers who reported better subjective health and those who were African American reported lower levels of behavioral bother across time. There was also a significant main effect of phase for time-varying behavioral bother, such that the highest levels of bother were reported at baseline and the lowest at 12 months. Thus, in support of our conceptual model, it appears that positive reappraisal of the dementia caregiving experience across time reinforces the coping process and ameliorates negative emotional outcomes regardless of many caregiver and care recipient characteristics (Folkman, 1997; Kinney & Stephens, 1989; Kramer, 1997).
The only evidence in support of our third hypothesis that time-varying PAC would interact with the intervention to produce further changes in time-varying negative affect was the significant Treatment Condition × PAC × Phase interaction found for time-varying daily care burden (see Figure 3). Although daily care burden did not differ significantly among intervention and control group caregivers at baseline, caregivers low in PAC benefited more from the intervention across time. On the one hand, it is not surprising that the caregivers who experience less PAC (e.g., fulfillment, meaning) within the caregiving role have the most to benefit from an intervention designed to build skills to help them manage the stress of their situation. On the other hand, as can be seen among control group caregivers, perhaps individuals with high PAC are relying more heavily on internally generated coping mechanisms to maintain the coping process across time, as suggested by Folkman’s (1997) revised stress and coping model. Among intervention group caregivers, those reporting the lowest PAC (i.e., low self-affirmation and more negative outlook on life) might have continued to benefit across time with additional practice of their newly learned skills (see Figure 3). Notably, the REACH I intervention at the Philadelphia site was specifically designed to increase mastery of caregivers through the introduction of strategies to modify the physical, social, and task dimensions of the home environment through education about the impact of the environment on the care recipient’s functional impairments and behaviors. The intervention was not designed, however, to specifically target daily care burden or positive reappraisals.
It is interesting that time-varying PAC interacted with treatment to improve daily care burden but not behavioral bother. Perhaps providing care for daily needs is perceived differently by caregivers with high and low PAC when compared with behavioral disturbances, which may be less directly related to feeling appreciated or finding meaning in the caregiving role across time. Furthermore, although depression, behavioral bother, and daily care bother all had significant bivariate relations with PAC, only daily care bother was able to predict unique variability in PAC. Helping with basic daily care activities may be more intimate and therefore more rewarding in comparison with responding to behavioral disturbances across time.
Our fourth hypothesis involved racial/ethnic differences in PAC and interactions between PAC and race. Similar to cross-sectional findings from the REACH I data regarding the relation of PAC with race (Roff et al., 2004), we found that African Americans reported higher PAC across time. Additionally, similar to prior research (Haley et al., 1995; Knight, Silverstein, McCallum, & Fox, 2000; Pinquart & Sörensen, 2003), we found a significant Race × Phase interaction for behavioral bother. African American caregivers experienced less behavioral bother than White caregivers across time, such that there was a continuous decrease in behavioral bother among African American caregivers from baseline to 12 months (see Figure 2). In contrast, White caregivers appeared to return to baseline levels of behavioral bother at 12 months. Prior research with REACH I baseline data has shown that the relation between race and PAC is partially but not fully mediated by African Americans’ lower SES, lower negative affect, and higher religiosity in comparison with White dementia caregivers (Roff et al., 2004). Clearly, additional variables need to be examined with regard to the longitudinal trajectories of PAC and negative affect among dementia caregivers of various racial/ethnic backgrounds. Future longitudinal studies should incorporate measures of potential cultural differences in the justification of caregiving (Dilworth-Anderson, Goodwin, & Williams, 2004; Dilworth-Anderson, Williams, & Gibson, 2002) and use of emotion-focused coping (Knight et al., 2000) in the examination of the role of PAC in the stress and coping process.
We acknowledge the limitations of this study. Our study only compared longitudinal outcomes between White and African American caregivers of moderately impaired dementia care recipients at a single site across 12 months. Thus, the potential relation of PAC to treatment outcome and the stress process among caregivers of other racial/ethnic backgrounds or of care recipients with mild or end-stage levels of the disease, of care recipient and caregiver dyads living in different geographical locations, or across longer periods of time is unknown. This limits the generalizability of our findings. Moreover, although our findings were based on multilevel random coefficients regression analysis (PROC MIXED), the analytic design remains correlational. More longitudinal research is needed with intervention designs specifically targeting the role of positive appraisal of the caregiving experience in the stress process. Finally, limitations of most caregiving intervention research include the relative lack of attention to translation (or how effective interventions can be implemented and maintained in the real world) and the sparse attention afforded care recipient emotional outcomes. Greater research attention needs to be devoted to policy and real-world costs of implementation surrounding these issues.
Cutting-edge research such as the recently published REACH II intervention (Belle et al., 2006), the first multisite psychosocial clinical trial for dementia caregivers funded by the National Institutes of Health, has demonstrated significant improvements in quality-of-life outcomes for diverse dementia caregivers. Notably, the multicomponent intervention was not found to be effective among African American nonspousal caregivers, and the REACH II intervention did not specifically target skills training in positive reappraisal of the caregiving experience. Given the large body of evidence documenting racial/ethnic differences in the report of PAC, if PAC consistently predicts better emotional outcomes (e.g., time-varying depression, behavioral bother, daily care burden), then this seems a promising target for future interventions (Kinney & Stephens, 1989; Kramer, 1997; Pinquart & Sörensen, 2003). The impact of negative emotional states such as depression and stress in older adults and caregivers has been linked to increased mortality, strokes, high blood pressure, and other negative health outcomes (Adamson, Price, Breeze, Bulpitt, & Fletcher, 2005; Haley et al., 1995; Krishnan et al., 2005; Schulz et al., 1997). Effective interventions, perhaps targeting individuals with low levels of PAC, could reduce health care costs and be cost effective because individuals who have difficulty positively reappraising the caregiving relationship may have the most to gain from interventions targeting such skills.
Two interventions stemming from research in palliative and end-of-life care show promise with regard to the potential role of the positive reappraisal of stress. First, dignity therapy (Chochinov, 2006; Chochinov et al., 2005) is delivered individually to patients receiving home-based or acute palliative care. Treatment consists of an audiotaped semistructured interview involving life review. Dignity therapy is effective in reducing physical suffering and depressive symptoms and increasing the will to live among individuals nearing the end of life (Chochinov, 2006). Second, our research group has piloted the Legacy intervention (Allen, Hilgeman, Ege, Shuster, & Burgio, 2007) with community-dwelling caregivers of older adults living with chronic, life-limiting illnesses. Preliminary analyses have revealed positive emotional outcomes for both care recipients and caregivers in the Legacy intervention when compared with individuals receiving supportive telephone calls. It is possible that these interventions can be modified to target the identification of PAC among dementia caregivers (Kinney & Stephens, 1989; Kramer, 1997; Pinquart & Sörensen, 2003) and, perhaps, care recipients. Moreover, these interventions easily could be implemented by community volunteers. Translational trials of these interventions within the context of dementia care are needed.
References
- Adamson JA, Price GM, Breeze E, Bulpitt CJ, Fletcher AE. Are older people dying of depression? Findings from the Medical Research Council trial of the assessment and management of older people in the community. Journal of the American Geriatrics Society. 2005;53:1128–1132. doi: 10.1111/j.1532-5415.2005.53355.x. [DOI] [PubMed] [Google Scholar]
- Allen RS, Hilgeman MM, Ege MA, Shuster J, Burgio LD. Legacy activities as interventions near the end of life. 2007 doi: 10.1089/jpm.2007.0294. Manuscript submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrera M, Jr, Sandler IN, Ramsay TB. Preliminary development of a scale of social support: Studies on college students. American Journal of Community Psychology. 1981;9:435–447. [Google Scholar]
- Belle SH, Burgio L, Burns R, Coon D, Czaja SJ, Gallagher-Thompson D, et al. Enhancing the quality of life of dementia caregivers from different ethnic or racial groups. Annals of Internal Medicine. 2006;145:727–738. doi: 10.7326/0003-4819-145-10-200611210-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgio L, Stevens A, Guy D, Roth D, Haley W. Impact of two psychological interventions on White and African American family caregivers of individuals with dementia. The Gerontologist. 2003;43:568–579. doi: 10.1093/geront/43.4.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns R, Nichols L, Martindale-Adams J, Graney M, Lummus A. Primary care interventions for dementia caregivers: 2-year outcomes from the REACH study. The Gerontologist. 2003;43:547–555. doi: 10.1093/geront/43.4.547. [DOI] [PubMed] [Google Scholar]
- Chochinov HM. Dying, dignity, and new horizons in palliative end-of-life care. CA: A Cancer Journal for Clinicians. 2006;56:84–103. doi: 10.3322/canjclin.56.2.84. [DOI] [PubMed] [Google Scholar]
- Chochinov HM, Hack T, Hassard T, Kristjanson LJ, McClement S, Harlos M. Dignity therapy: A novel psychotherapeutic intervention for patients near the end of life. Journal of Clinical Oncology. 2005;23:5520–5525. doi: 10.1200/JCO.2005.08.391. [DOI] [PubMed] [Google Scholar]
- Cohen CA, Colantonio A, Vernich L. Positive aspects of caregiving: Rounding out the caregiver experience. International Journal of Geriatric Psychiatry. 2002;17:184–188. doi: 10.1002/gps.561. [DOI] [PubMed] [Google Scholar]
- Cohen D, Luchins D, Eisdorfer C, Paveza G, Ashford JW, Gorelick P, et al. Caring for relatives with Alzheimer’s disease: The mental health risks to spouses, adult children, and other family caregivers. Behavior, Health, and Aging. 1990;1:171–182. [Google Scholar]
- Coon DW, Rubert M, Solano N, Mausbach B, Kraemer H, Arguelles T, et al. Well-being, appraisal, and coping in Latina and Caucasian female dementia caregivers: Findings from the REACH study. Aging & Mental Health. 2004;8:330–345. doi: 10.1080/13607860410001709683. [DOI] [PubMed] [Google Scholar]
- Costa PT, McCrae RR. Personality in adulthood: A six year longitudinal study of self-reports and spouse ratings of the NEO Personality Inventory. Journal of Personality and Social Psychology. 1988;54:853–863. doi: 10.1037//0022-3514.54.5.853. [DOI] [PubMed] [Google Scholar]
- Dilworth-Anderson P, Goodwin PY, Williams SW. Can culture help explain the physical health effects of caregiving over time among African American caregivers? Journals of Gerontology, Series B: Psychological Sciences and Social Sciences. 2004;59:S138–S145. doi: 10.1093/geronb/59.3.s138. [DOI] [PubMed] [Google Scholar]
- Dilworth-Anderson P, Williams IC, Gibson BE. Issues of race, ethnicity, and culture in caregiving research: A 20-year review (1980–2000) The Gerontologist. 2002;42:237–272. doi: 10.1093/geront/42.2.237. [DOI] [PubMed] [Google Scholar]
- Eisdorfer C, Czaja SJ, Loewenstein DA, Rubert MP, Arguelles S, Mitrani VB, et al. The effect of a family therapy and technology-based intervention on caregiver depression. The Gerontologist. 2003;43:521–531. doi: 10.1093/geront/43.4.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkman S. Positive psychological states and coping with severe stress. Social Science Medicine. 1997;45:1207–1221. doi: 10.1016/s0277-9536(97)00040-3. [DOI] [PubMed] [Google Scholar]
- Folstein M, Folstein S, McHugh P. Mini-mental state:” A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gallagher-Thompson D, Coon DW, Solano N, Ambler C, Rabinowitz Y, Thompson LW. Change of indices of distress among Latino and Anglo female caregivers of elderly relatives with dementia: Site-specific results from the REACH national collaborative study. The Gerontologist. 2003;43:580–591. doi: 10.1093/geront/43.4.580. [DOI] [PubMed] [Google Scholar]
- Gitlin LN, Belle SH, Burgio LD, Czaja SJ, Mahoney D, Gallagher-Thompson D, et al. Effect of multicomponent interventions on caregiver burden and depression: The REACH multisite initiative at 6-month follow-up. Psychology and Aging. 2003;18:361–374. doi: 10.1037/0882-7974.18.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitlin LN, Hauck WW, Dennis MP, Winter L. Maintenance of effects of the home environmental skill-building program for family caregivers and individuals with Alzheimer’s disease and related disorders. Journals of Gerontology, Series A: Biological Sciences and Medical Sciences. 2005;60:368–374. doi: 10.1093/gerona/60.3.368. [DOI] [PubMed] [Google Scholar]
- Gitlin LN, Roth DL, Burgio LD, Loewenstein DA, Winter L, Nichols L, et al. Caregiver appraisals of functional dependence in individuals with dementia and associated caregiver upset: Psychometric properties of a new scale and response patterns by caregiver and care recipient characteristics. Journal of Aging and Health. 2005;17:148–171. doi: 10.1177/0898264304274184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitlin LN, Winter L, Corcoran M, Dennis MP, Schinfeld S, Hauck WW. Effects of the home environmental skill-building program on the caregiver–care recipient dyad: 6-month outcomes from the Philadelphia REACH initiative. The Gerontologist. 2003;43:532–546. doi: 10.1093/geront/43.4.532. [DOI] [PubMed] [Google Scholar]
- Haley WE, Levine EG, Brown SL, Bartolucci AA. Stress, appraisal, coping, and social support as predictors of adaptational outcome among dementia caregivers. Psychology and Aging. 1987;2:323–330. doi: 10.1037//0882-7974.2.4.323. [DOI] [PubMed] [Google Scholar]
- Haley WE, Roth DL, Coleton MI, Ford GR, West CAC, Collins RP, et al. Appraisal, coping, and social support as mediators of well-being in Black and White family caregivers of patients with Alzheimer’s disease. Journal of Consulting and Clinical Psychology. 1996;64:121–129. doi: 10.1037//0022-006x.64.1.121. [DOI] [PubMed] [Google Scholar]
- Haley WE, West CAC, Wadley VG, Ford GR, White FA, Barrett JJ, et al. Psychological, social, and health impact of caregiving: A comparison of Black and White dementia family caregivers and noncaregivers. Psychology and Aging. 1995;10:540–552. [PubMed] [Google Scholar]
- Hebert LE, Scherr PA, Bienias JL, Bennett DA, Evans DA. Alzheimer disease in the US population: Prevention estimates using the 2000 census. Archives of Neurology. 2003;60:1119–1122. doi: 10.1001/archneur.60.8.1119. [DOI] [PubMed] [Google Scholar]
- Hooker K, Bowman S, Coehlo DP, Lim SR, Kaye J, Guariglia R, et al. Behavioral change in persons with dementia: Relationships with mental and physical health of caregivers. Journals of Gerontology, Series B: Psychological Sciences and Social Sciences. 2002;57:P453–P461. doi: 10.1093/geronb/57.5.p453. [DOI] [PubMed] [Google Scholar]
- Katz S, Ford A, Moskowitz R, Jackson B, Jaffe M. Studies of illness and the aged: The index of ADL, a standardized measure of biological and psychological function. Journal of the American Medical Association. 1963;185:914–919. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- Kinney JM, Stephens MAP. Hassles and uplifts of giving care to a family member with dementia. Psychology and Aging. 1989;4:402–408. doi: 10.1037//0882-7974.4.4.402. [DOI] [PubMed] [Google Scholar]
- Knauper B, Turner PA. Measuring health: Improving the validity of health assessments. Quality of Life Research. 2003;12(Suppl 1):81–89. doi: 10.1023/a:1023589907955. [DOI] [PubMed] [Google Scholar]
- Knight BG, Silverstein M, McCallum TJ, Fox LS. A sociocultural stress and coping model for mental health outcomes among African American caregivers in southern California. Journals of Gerontology, Series B: Psychological Sciences and Social Sciences. 2000;55:P142–P150. doi: 10.1093/geronb/55.3.p142. [DOI] [PubMed] [Google Scholar]
- Kramer BJ. Gain in the caregiving experience: Where are we? What next? The Gerontologist. 1997;37:218–232. doi: 10.1093/geront/37.2.218. [DOI] [PubMed] [Google Scholar]
- Krause N. Negative interaction and satisfaction with social support among older adults. Journals of Gerontology, Series B: Psychological Sciences and Social Sciences. 1995;50:P59–P73. doi: 10.1093/geronb/50b.2.p59. [DOI] [PubMed] [Google Scholar]
- Krause N, Markides K. Measuring social support among older adults. International Journal of Aging and Human Development. 1990;30:37–53. doi: 10.2190/CY26-XCKW-WY1V-VGK3. [DOI] [PubMed] [Google Scholar]
- Krishnan M, Mast BT, Ficker LJ, Lawhorne L, Lichtenberg PA, Morley JE. The effects of preexisting depression on cerebrovascular health outcomes in geriatric continuing care. Journals of Gerontology, Series A: Biological Sciences and Medical Sciences. 2005;60:915–919. doi: 10.1093/gerona/60.7.915. [DOI] [PubMed] [Google Scholar]
- Lawton M, Brody E. Assessment of older people: Self-maintaining and instrumental activities of daily living. The Gerontologist. 1969;9:179–186. [PubMed] [Google Scholar]
- Lawton MP, Kleban MH, Moss M, Rovine M, Glicksman A. Measuring caregiving appraisal. Journals of Gerontology, Series B: Psychological Sciences and Social Sciences. 1989;44:P61–P71. doi: 10.1093/geronj/44.3.p61. [DOI] [PubMed] [Google Scholar]
- Lawton MP, Moss M, Kleban MH, Glicksman A, Rovine M. A two-factor model of caregiving appraisal and psychological well-being. Journals of Gerontology, Series B: Psychological Sciences and Social Sciences. 1991;46:P181–P189. doi: 10.1093/geronj/46.4.p181. [DOI] [PubMed] [Google Scholar]
- Lazarus RS, Folkman S. Stress, appraisal, and coping. New York: Springer; 1984. [Google Scholar]
- Levine C. Depression in caregivers of patients with dementia: A greater role for physicians. Journal of General Internal Medicine. 2003;18:1058–1059. doi: 10.1111/j.1525-1497.2003.31003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubben JE. Assessing social networks among elderly populations. Family Community Health. 1988;11:42–52. [Google Scholar]
- Mahoney DF, Tarlow BJ, Jones RN. Effects of an automated telephone support system on caregiver burden and anxiety: Findings from the REACH for TLC intervention study. The Gerontologist. 2003;43:556–567. doi: 10.1093/geront/43.4.556. [DOI] [PubMed] [Google Scholar]
- Mittelman MS. Community caregiving. Alzheimer’s Care Quarterly. 2003;4:273–285. [Google Scholar]
- Nam CB, Terrie EW. 1980-based Nam-Powers Occupational Status scores, (Working Paper Series 88–148) Tallahassee: Florida State University, Center for the Study of Population; 1988. [Google Scholar]
- National Institutes of Health. Resources for Enhancing Alzheimer’s Caregiver Health (1996–2001) 2002 Available from http://clinicaltrials.gov/show/NCT00178165.
- Ory MG, Yee JL, Tennestedt SL, Schulz R. The extent and impact of dementia care: Unique challenges experienced by family caregivers. In: Schulz R, editor. Handbook on dementia caregiving: Evidence based interventions for family caregivers. New York: Springer; 1999. pp. 1–32. [Google Scholar]
- Pargament KI, Rye MS. Forgiveness as a method of religious coping. In: Worthington EL Jr, editor. Dimensions of forgiveness: Psychological research and theological perspectives. Philadelphia: Templeton Foundation Press; 1998. pp. 59–78. [Google Scholar]
- Pearlin LI, Mullan JT, Semple SJ, Skaff MM. Caregiving and the stress process: An overview of concepts and their measures. The Gerontologist. 1990;30:583–594. doi: 10.1093/geront/30.5.583. [DOI] [PubMed] [Google Scholar]
- Picot SJ, Debanne SM, Namazi KH, Wykle ML. Religiosity and perceived rewards of Black and White caregivers. The Gerontologist. 1997;37:89–101. doi: 10.1093/geront/37.1.89. [DOI] [PubMed] [Google Scholar]
- Pinquart M, Sörensen S. Predictors of burden and depressive mood: A meta-analysis. Journals of Gerontology, Series B: Psychological Sciences and Social Sciences. 2003;58:P112–P128. doi: 10.1093/geronb/58.2.p112. [DOI] [PubMed] [Google Scholar]
- Posner BM, Jette AM, Smith KW, Miller DR. Nutrition and health risks in the elderly: The Nutrition Screening Initiative. American Journal of Public Health. 1993;83:972–978. doi: 10.2105/ajph.83.7.972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS. The CES–D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Roff LL, Burgio LD, Gitlin L, Nichols L, Chaplin W, Hardin JM. Positive aspects of Alzheimer’s caregiving: The role of race. Journals of Gerontology, Series B: Psychological Sciences and Social Sciences. 2004;59:P185–P190. doi: 10.1093/geronb/59.4.p185. [DOI] [PubMed] [Google Scholar]
- Roth DL, Burgio LD, Gitlin LN, Gallagher-Thompson D, Coon DW, Belle SH, et al. Psychometric analysis of the Revised Memory and Behavior Problems Checklist: Factor structure of occurrence and reaction ratings. Psychology and Aging. 2003;18:906–915. doi: 10.1037/0882-7974.18.4.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryff CD. In the eye of the beholder: Views of psychological well-being among middle-aged and older adults. Psychology and Aging. 1989;4:195–201. doi: 10.1037//0882-7974.4.2.195. [DOI] [PubMed] [Google Scholar]
- Schein RS, Koenig HG. The Center for Epidemiological Studies—Depression (CES–D) scale: Assessment of depression in the medically ill elderly. International Journal of Geriatric Psychiatry. 1997;12:436–446. [PubMed] [Google Scholar]
- Schulz R, Burgio L, Burns R, Eisdorfer C, Gallagher-Thompson D, Gitlin LN, et al. Resources for Enhancing Alzheimer’s Caregiver Health (REACH): Overview, site-specific outcomes, and future directions. The Gerontologist. 2003;43:514–520. doi: 10.1093/geront/43.4.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz R, Newsom JT, Mittlemark M, Burton L, Hirsh C, Jackson S. Health effects of caregiving: The Caregiver Health Effects Study. Annals of Behavioral Medicine. 1997;12:110–116. doi: 10.1007/BF02883327. [DOI] [PubMed] [Google Scholar]
- Schulz R, Visintainer P, Williamson GM. Psychiatric and physical morbidity effects of caregiving. Journals of Gerontology, Series B: Psychological Sciences and Social Sciences. 1990;45:P181–P191. doi: 10.1093/geronj/45.5.p181. [DOI] [PubMed] [Google Scholar]
- Sörensen S, Pinquart M. Racial and ethnic differences in the relationship of caregiving stressors, resources, and sociodemographic variables to caregiver depression and perceived physical health. Aging & Mental Health. 2005;9(5):482–495. doi: 10.1080/13607860500142796. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the State–Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- Tarlow BJ, Wisniewski SR, Belle SH, Rubert M, Ory MG, Gallagher-Thompson D. Positive aspects of caregiving, contributions of the REACH project to the development of a new measure for Alzheimer’s caregiving. Research on Aging. 2004;26:429–453. [Google Scholar]
- Tennen H, Affleck G. Benefit-finding and benefit-reminding. In: Snyder CR, Lopez SJ, editors. Handbook of positive psychology. New York: Oxford; 2002. pp. 584–597. [Google Scholar]
- Teri L, Truax P, Logsdon R, Uomoto J, Zarit S, Vitaliano PP. Assessment of behavioral problems in dementia: The Revised Memory and Behavior Problems Checklist (RMBPC) Psychology and Aging. 1992;7:622–631. doi: 10.1037//0882-7974.7.4.622. [DOI] [PubMed] [Google Scholar]
- Tombaugh TN, McIntyre NJ. The Mini-Mental State Examination: A comprehensive review. Journal of the American Geriatrics Society. 1992;40:922–935. doi: 10.1111/j.1532-5415.1992.tb01992.x. [DOI] [PubMed] [Google Scholar]
- Wagnild GM, Young HM. Development and psychometric evaluation of the Resilience Scale. Journal of Nursing Measurement. 1993;1:165–178. [PubMed] [Google Scholar]
- Ware JE, Kosinski M, Bayliss MS, McHorney CA, Rogers WH, Raczek A. Comparison of methods for the scoring and statistical analysis of SF-36® health profiles and summary measures: Summary of results from the Medical Outcomes Study. Medical Care. 1995;33:AS264–AS279. [PubMed] [Google Scholar]
- Wisniewski SR, Belle SH, Coon DW, Marcus SM, Ory MG, Burgio LD, et al. The Resources for Enhancing Alzheimer’s Caregiver Health (REACH): Project design and baseline characteristics. Psychology and Aging. 2003;18:375–384. doi: 10.1037/0882-7974.18.3.375. [DOI] [PMC free article] [PubMed] [Google Scholar]