Abstract
Reactive oxygen species produced by phagocytosing neutrophils are essential for innate host defense against invading microbes. Previous observations revealed that antibody-catalyzed ozone formation by human neutrophils contributed to the killing of bacteria. In this study, we discovered that 4 amino acids themselves were able to catalyze the production of an oxidant with the chemical signature of ozone from singlet oxygen in the water-oxidation pathway, at comparable level to antibodies. The resultant oxidant with the chemical signature of ozone exhibited significant bactericidal activity in our distinct cell-free system and in human neutrophils. The results also suggest that an oxidant with the chemical signature of ozone produced by neutrophils might potentiate a host defense system, when the host is challenged by high doses of infectious agents. Our findings provide biological insights into the killing of bacteria by neutrophils.
Keywords: host defense, singlet oxygen, neutrophil, chronic granulomatous disease
Neutrophils are one of the professional phagocytes, which ingest microorganisms into intracellular compartments called phagosomes, and destroy them. The production of reactive oxygen species (ROS) by phagocytosing neutrophils is essential for innate host defense against invading microbial pathogens. The phagocytosing neutrophils undergo a burst of oxygen consumption that is caused by the reduced NADPH oxidase, ultimately leading to the formation of hypochlorous acid (HOCl), singlet oxygen (1O2), and hydroxyl radical (•OH). However, a clear scenario of how the ROS kill microbes has not yet emerged (1). Recently, it has been proposed that neutrophils produce ozone, which likely contributes to the bactericidal and inflammatory activity of neutrophils (2–4), although the validity of this model is still a matter of debate (5, 6). In this model, antibodies can catalyze the production of ozone from singlet oxygen and water, but the precise mechanism of how antibodies achieve this reaction remains uncertain.
Chronic granulomatous disease (CGD) is characterized by a defect in ROS formation, leading to recurrent, often life-threatening bacterial and fungal infections, and granuloma formation in multiple organs. The disease is caused by a genetic mutation in 1 of 4 components of NADPH oxidase (gp91-phox, p47-phox, p67-phox, and p22-phox) of the superoxide (O2•−)-generating phagocytes (7). Patients with a defect in the gp91-phox component, which is the most common type of CGD (≈60%), are reported to exhibit a more severe clinical course than those with a defect in the p47-phox component (8). Recently, we experienced a patient with a rare variant type of CGD carrying a defect in the gp91-phox component. Despite the genetic defect, his granulocytes could produce significant amounts of singlet oxygen, but very little superoxide. Thus, neutrophils from this CGD patient would provide a useful model system in humans. In this study, we investigated the biological importance of ozone produced by human neutrophils by using the variant CGD neutrophils and our distinct in vitro assay system.
Results
Ozone Production by Immunoglobulins and Amino Acids in the Cell-Free System.
In our current study, we explored the mechanism by which antibodies produce ozone from singlet oxygen and water. We previously established a cell-free system, in which 6-formylpterin (6FP), a potent xanthine oxidase inhibitor, produces singlet oxygen without superoxide formation under UVA radiation in aqueous solutions (9). Using this system, we found that the portion of F(ab′)2 of antibodies, albumin, and chemotactic peptide, formyl-methionyl-leucyl-phenylalanine (FMLP), had the potential to generate an oxidant with the chemical signature of ozone, as intact antibodies (IgG) did, as denoted by the oxidation reaction of indigo carmine to isatin sulfonic acids, detected by a spectrophotometric assay (Fig. 1A). However, an inhibitory peptide of caspases, benzyloxycarbonyl-Val-Ala-Asp(OMe)-fluoromethylketone (zVAD-fmk), did not produce an oxidant with the chemical signature of ozone (Fig. 1B). Furthermore, the addition of catalase, which catalyzes the decomposition of H2O2 to H2O and O2, did not affect the generation of an oxidant with the chemical signature of ozone by IgG in this system (Fig. 1C). These results substantiate the previous observation by Wentworth et al. (2) and further suggest that the ozone generation brought about by antibodies is not attributable to the antigen-binding activity of antibodies. We further examined what components contribute to ozone production in this system. Surprisingly, among various water-soluble amino acids applied, 4 amino acids [tryptophan (Trp), methionine (Met), cysteine (Cys), and histidine (His)] exhibited catalytic activity sufficient for the conversion of singlet oxygen to an oxidant with the chemical signature of ozone in a dose-dependent manner (Fig. 1 D and E). Scavengers of singlet oxygen, sodium azide and edaravone (10), significantly abrogated the ability of these amino acids to catalyze the reaction (Fig. 1F), whereas catalase had no effects on the reaction (Fig. 1G), suggesting the specificity of this assay system. To further verify the amino acid-catalyzed ozone generation, we performed HPLC assay for detection of isatin sulfonic acid (Fig. 2 A and B) and 4-carboxybenzaldehyde (Fig. 2 C and D). The administration of Met to 6FP under UVA radiation successfully converted indigo carmine (Fig. 2A) to isatin sulfonic acid (Fig. 2B). Moreover, another detector of ozone, vinylbenzoic acid (Fig. 2C), was oxidized to 4-carboxybenzaldehyde (Fig. 2D), supporting the formation of an oxidant with the chemical signature of ozone by amino acids. The amino acids-catalyzed ozone generation was further confirmed by measuring 18O incorporation from the reaction solvent H218O into isatin sulfonic acid during indigo carmine oxidation by using mass spectral analysis. In a control experiment, where normal H216O was used in the presence of Met and 6FP with UVA irradiation, the mass peak 226 was detected, suggesting that isatin sulfonic acid was produced in the system (Fig. 2E). Mass spectral profile with H218O revealed that the additional mass peak 230, which is characteristic of ozone (2), was observed when Met and 6FP in a reaction mixture containing H218O were irradiated with UVA (Fig. 2G), whereas the mass peak 226 and 228 alone were observed in the absence of Met (Fig. 2F). These results demonstrate that the oxidant-carrying chemical signature of ozone was produced in our amino acids-mediated water oxidation pathway.
Fig. 1.
Ozone production by immunoglobulins and amino acids in the cell-free system. Indigo carmine was irradiated with UVA in the presence of 6FP. An oxidant with the chemical signature of ozone produced by the addition of immunoglobulins or amino acids converted indigo carmine to isatin sulfonic acids. Loss of indigo carmine was monitored by measuring its absorbance at 610 nm. (A) Effect of Ig, the portion of F(ab)′2 of antibodies, albumin, or FMLP in the presence of 6FP on ozone production. The data represent mean values ± SD (n = 3; *, P < 0.05, paired t test). (B) Effect of zVAD-fmk in the presence of 6FP on ozone production. The experiments were performed at least 3 times, and representative data are shown. (C) Effect of catalase in the presence of 6FP on IgG-mediated ozone production. The experiments were performed at least 3 times, and representative data are shown. (D) Effect of water-soluble amino acids in the presence of 6FP on ozone production. Representative data are shown. (E) Dose–response curves. Increasing concentrations of Trp, Met, Cys, or His (1 μM to 2 mM) were added to the reaction in the presence of 6FP. (F) Effect of scavengers of singlet oxygen, sodium azide, and edaravone on amino acid (Trp, Met, Cys, or His)-mediated ozone production. The data represent mean values ± SD (n = 3; *, P < 0.05; **, P < 0.01; paired t test). (G) Effect of catalase on amino acid (Trp, Met, Cys, or His)-mediated ozone production. The data represent mean values ± SD (n = 3).
Fig. 2.
HPLC and mass spectral analysis of ozone production in the cell-free system. (A and B) HPLC analysis. Indigo carmine was added to 6FP and Met with (B) or without (A) UVA irradiation. Arrows indicate a peak of indigo carmine (A) and isatin sulfonic acid (B). (C and D) HPLC analysis. Vinylbenzoic acid was added to 6FP and Met with (D) or without (C) UVA irradiation. Arrows indicate a peak of vinylbenzoic acid (C) and 4-carboxybenzaldehyde (D). (E–G) Mass spectral analysis. Indigo carmine was added to 6FP in a reaction mixture containing H216O in the presence of Met (E), H218O in the absence (F) or presence (G) of Met and irradiated with UVA. Note the presence of the mass peak 230 in G.
Ozone Produced by Amino Acids Exhibits Bactericidal Activity in the Cell-Free System.
We next investigated whether an oxidant with the chemical signature of ozone produced by amino acids killed bacteria in our cell-free system. Bactericidal studies were performed on catalase-positive bacteria, Escherichia coli, NIHJ-JC2. In this experiment, to increase solubility in water, we used a variant of 6FP, 2-(N,N-dimethylaminomethyleneamino)-6-formyl-3-pivaloylpteridine-4-one (6FP-tBu-DMF), which generates singlet oxygen to a similar extent to 6FP (9). Viable E. coli were nearly undetectable with the addition of 6FP-tBu-DMF and amino acids (Trp or Met) after a 2-h irradiation, whereas 6FP-tBu-DMF alone had little effect on the viability of E. coli even with a 2-h irradiation (Fig. 3A). The administration of IgG together with 6FP-tBu-DMF exhibited a similar profile to these amino acids (data not shown). These results provide evidence to support the key role of these amino acids in bactericidal activity. The viability of E. coli was not affected by the addition of Arg or Phe, which had failed to exhibit catalytic activity for the generation of an oxidant with the chemical signature of ozone (Fig. 3B). Given that hydrogen peroxide (H2O2), which is highly bactericidal, is also the ultimate product of the water-oxidation pathway, it is rational to speculate that H2O2 might mediate the bactericidal activity observed. Quantification of H2O2 toxicity against E. coli revealed that H2O2 levels on treatment with 6FP-tBu-DMF and amino acids were ≈80 μM, which was completely repressed by catalase treatment (Fig. 4A). However, H2O2 levels required to kill 50% of the bacteria were ≈1–10 mM (Fig. 4B), suggesting that H2O2 is unlikely to be the principal contributor to the killing activity in this system.
Fig. 3.
Ozone produced by amino acids kills bacteria in the cell-free system. E. coli were incubated with or without 6FP-tBu-DMF and amino acids under UVA irradiation for 2 h. (A) Effect of Trp or Met on the survival of E. coli. The addition of both 6FP-tBu-DMF and amino acids exhibited strong bactericidal activity after a 2-h irradiation. (B) Effect of Arg or Phe on the survival of E. coli. Note that the amino acids showed no effects on bactericidal activity.
Fig. 4.
H2O2 levels in the cell-free system. (A) H2O2 levels generated by 6FP-tBu-DMF and Trp after 2-h irradiation. Note that H2O2 production was completely repressed by catalase treatment. The data represent mean values ± SD (n = 3). (B) Concentration-dependent toxicity of H2O2 on the viability of E. coli. Increasing concentrations of H2O2 (0–103 mM) were added to the E. coli. The experiments were performed at least 3 times, and representative data are shown.
Ozone Production by Amino Acids in Human Neutrophils.
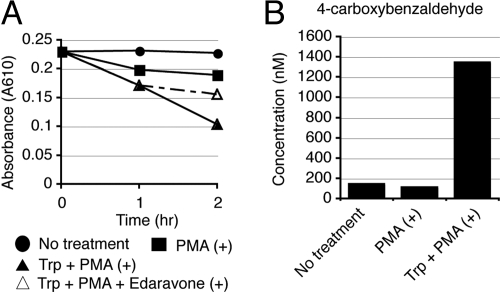
Next, we examined whether human neutrophils really have the functional capacity to produce ozone. We have identified a patient with a rare variant type of CGD carrying a defect in the gp91-phox component, whose granulocytes could produce significant amounts of singlet oxygen, but very little superoxide. Thus, neutrophils from this CGD patient should provide a useful model to allow the testing of our hypothesis in human neutrophils, because it is difficult to determine whether the amino acid-catalyzed generation of ozone actually occurs in vivo by using healthy human neutrophils. Extracellular superoxide production was measured by the superoxide dismutase (SOD)-inhibitable reduction of ferricytochrome c. Neutrophils from a healthy control released substantial amounts of superoxide in response to stimulation with either phorbol myristate acetate (PMA) or opsonized zymosan (OZ). In sharp contrast, neutrophils from this CGD patient did not release detectable superoxide in response to either stimulus (Fig. 5A). We next used the fluorescent dye dihydrorhodamine (DHR) 123 in a flow cytometric assay to detect ROS. PMA-stimulated neutrophils from a healthy control revealed a significant increase in DHR fluorescence, which was counteracted by pretreatment with diphenyleneiodonium (DPI), an inhibitor of NADPH-oxidase (Fig. 5B). In contrast, neutrophils from the gp91-phox-mutated variant CGD patient exhibited a moderate fluorescence increase after PMA stimulation, reminiscent of the partial DHR response reported in p47-phox-deficient CGD patients (Fig. 5B) (11). To further verify the results, we used 2 chemiluminescent probes, 2-methyl-6-phenyl-3,7-dihydroimidazo[1,2-a]pyrazin-3-one (CLA) and trans-1-(2′-methoxyvinyl)pyrene (MVP), which were developed to specifically detect superoxide and singlet oxygen, respectively (10, 12–14). The pretreatment of control neutrophils with DPI abolished the CLA chemiluminescence, suggesting the specificity of this probe for superoxide detection (Fig. 5C). The treatment of control neutrophils with 4-aminobenzoic acid hydrazide (ABAH), an inhibitor of myeloperoxidase (MPO), abrogated the MVP chemiluminescence (Fig. 5C). The CLA chemiluminescence in the variant CGD neutrophils was nearly undetectable, whereas the MVA chemiluminescence in the CGD neutrophils was approximately half of that in healthy control neutrophils, suggesting that the variant CGD neutrophils produced very small amounts of superoxide, but had the ability to produce singlet oxygen to some extent (Fig. 5C). To verify our results in the cell-free system, an oxidation reaction of indigo carmine was carried out on neutrophils from the variant CGD patient. Spectrophotometric assay revealed that the addition of Trp to PMA-stimulated neutrophils led to the successful conversion of indigo carmine to isatin sulfonic acids (Fig. 6A). A scavenger of singlet oxygen, edaravone, partially suppressed the reaction (Fig. 6A). HPLC analysis revealed that the PMA-stimulated CGD neutrophils with Trp administration produced 4-carboxybenzaldehyde from vinylbenzoic acid (Fig. 6B), substantiating the production of an oxidant with the chemical signature of ozone from singlet oxygen in human neutrophils.
Fig. 5.
Production of singlet oxygen with very little superoxide in a variant type of gp91-phox-deficient CGD neutrophils. (A) SOD-inhibitable reduction of ferricytochrome c in control and CGD neutrophils. Superoxide release was analyzed in unstimulated, OZ-stimulated, or PMA-stimulated neutrophils. (B) DHR assay in neutrophils from a healthy control (Top and Middle) and a CGD patient (Bottom). In Middle the pretreatment of control neutrophils with DPI, an inhibitor of NADPH-oxidase, is revealed. Fluorescence intensity is shown on the logarithmic x axis, and the cell count is shown on the y axis. (C) Superoxide (Upper) and singlet oxygen (Lower) release from control and CDG neutrophils. Neutrophils were incubated with CLA for superoxide detection or MVP for singlet oxygen detection, and luminescence was monitored every 30 s for 30 min. Some control neutrophils were pretreated with DPI for the CLA (Upper) or with ABAH, an inhibitor of MPO, for the MVA (Lower).
Fig. 6.
Ozone production in a variant type of gp91-phox-deficient CGD neutrophils. (A) Effect of Trp on ozone production in activated CGD neutrophils. Indigo carmine was incubated with unstimulated or PMA-stimulated CGD neutrophils. Trp was added to PMA-stimulated CGD neutrophils to analyze ozone production. Loss of indigo carmine was monitored by measuring its absorbance at 610 nm. Note that a scavenger of singlet oxygen, edaravone, partially suppressed the reaction. The experiments were performed at least 3 times, and representative data are shown. (B) HPLC analysis of 4-carboxybenzaldehyde in CGD neutrophils. PMA-stimulated CGD neutrophils with Trp administration produced 4-carboxybenzaldehyde from vinylbenzoic acid.
Ozone Produced by Amino Acids Augments Bactericidal Activity of Human Neutrophils.
Finally, we examined the bactericidal activity of neutrophils from the variant CGD patient. A bactericidal assay revealed that the CGD neutrophils were able to partially kill E. coli in a condition whereby the ratio of neutrophils to E. coli was 1:1, although the killing activity was less than that of healthy neutrophils (Fig. 7A). In the variant CGD neutrophils, the administration of amino acids, Trp and Met, augmented the bactericidal activity of neutrophils, which was more evident when the ratio of E. coli to neutrophils was high (>5:1) (Fig. 7B and data not shown). These results suggest that the formation of an oxidant with the chemical signature of ozone catalyzed by amino acids facilitate the bactericidal action of the variant CGD neutrophils. This beneficial effect of amino acids on bactericidal activity was also observed in healthy neutrophils, when higher doses of E. coli were added to neutrophils (Fig. 7C and data not shown). These results are indicative of a general role for amino acid-catalyzed ozone in the bactericidal action of human neutrophils. To examine the effect of H2O2 on the killing activity of neutrophils, we measured the H2O2 concentration by using a highly sensitive and stable H2O2 probe, N-acetyl-3,7,–2-phenylethylamine dihydroxyphenoxazine (15). In contrast to healthy neutrophils, the H2O2 level in the CGD neutrophils was negligible, indicating that H2O2 is unlikely to be relevant to the killing activity (Fig. 7D).
Fig. 7.
Ozone produced by amino acids augments the bactericidal activity of neutrophils. (A) Bactericidal activity of CGD and healthy control neutrophils (PMN). E. coli were incubated with CGD or control neutrophils for 2 h. (B and C) Effect of Trp on the bactericidal activity of CGD (B) and healthy control (C) neutrophils. CGD or control neutrophils were challenged with increasing amounts of E. coli at a ratio of 1:1, 1:5, or 1:10 in the presence or absence of Trp. The data represent mean values ± SD (n = 3; *, P < 0.05; **, P < 0.01; paired t test). (D) H2O2 levels produced by CGD and healthy control neutrophils. The experiments were performed at least 3 times, and representative data are shown.
Discussion
In this study, we showed that 4 amino acids, by themselves, were able to catalyze the production of an oxidant with the chemical signature of ozone from singlet oxygen in the water-oxidation pathway, comparably to antibodies. The resultant oxidant with the chemical signature of ozone exhibited significant bactericidal activity in our cell-free system and in human neutrophils. Ozone production by neutrophils is still a debatable issue. However, considering the findings of this study, where distinct model systems were exploited, we favor the proposal by Wentworth and colleagues (2–4) that antibodies can catalyze ozone generation in neutrophils. Our results further suggested the hypothesis that amino acids themselves exhibited catalytic activity to convert singlet oxygen and water to an oxidant with the chemical signature of ozone, and amino acid-catalyzed oxidant with the chemical signature of ozone showed bactericidal activity in human neutrophils.
What is the biological importance of ozone generated by neutrophils in host defense? MPO catalyzes the reaction to produce hypochlorous acid (HOCl) from hydrogen peroxide (H2O2) and chloride ion (Cl−) (16). MPO deficiency is the most common congenital neutrophil defect. Despite the important role for HOCl in killing microorganisms, MPO-deficient individuals are usually healthy, in sharp contrast to CGD patients (17, 18). However, some MPO-deficient patients revealed an increased susceptibility to infections with bacteria and fungi, particularly those caused by Candida albicans (17, 19, 20). In agreement with these facts, MPO-deficient mutant mice, which failed to produce HOCl and subsequent singlet oxygen, showed an increased susceptibility to pneumonia and death when challenged by high doses of bacteria and fungi, although they were generally healthy under normal conditions (21, 22). In human neutrophils we examined, the bactericidal activity induced by the addition of amino acids was prominent when larger numbers of E. coli were added to neutrophils (E. coli/neutrophils = 5:1 or 10:1), reminiscent of the MPO deficiency. Thus, ozone produced by neutrophils might potentiate a host defense system when the host is challenged by high doses of infectious agents.
Our current study further suggests the potential therapeutic role for amino acid-catalyzed oxidant with the chemical signature of ozone in infectious diseases. ROS have been playing a central role in photodynamic therapy (PDT) for cancer (23), where light and certain chemicals (photosensitizer) are used. The photosensitizer transfers energy from light to molecular oxygen to generate free radicals/radical ions or singlet oxygen. The ROS that are generated by PDT can kill tumor cells directly, damage the tumor-associated vasculature, and activate an immune response against tumor cells. PDT is now being applied to the treatment of many other diseases, including targeting of microorganisms (24). With an increase in antibiotic resistance, the development of new antimicrobial strategies is expected. In our distinct cell-free system, unexpectedly, the use of a potential photosensitizer, 6FP-tBu-DMF, and UVA, which produced singlet oxygen that is toxic, failed to kill bacteria (Fig. 3A). However, the addition of Trp or Met to 6FP-tBu-DMF and UVA, which produced an oxidant with the chemical signature of ozone, dramatically reduced the rate of viable bacteria (Fig. 3A). These results suggest that our study may contribute to the improvement of antimicrobial PDT.
Materials and Methods
Reagents.
Edaravone, human IgG, and human F(ab)′2 were kind gifts from Mitsubishi Pharma. 6FP was obtained from Sankyo Kasei Kogyo. 6FP-tBu-DMF was synthesized in our laboratories at the Institute of Advanced Energy, Kyoto University (9). CLA was purchased from Tokyo Kasei Kogyo; MVP, DHR, and the Amplex Red H2O2 kit were from Molecular Probes. Indigo carmine, gelatin, BSA, dextran, trisodium citrate dihydrate, acetonitrile, and sodium azide were from Nakalai; water-soluble amino acids were from Wako; heart infusion agar was from Nissui; Percoll was from GE Healthcare; zVAD-fmk was from the Peptide Institute; and H218 O (> 97% H2O) was from Cambridge Isotope Laboratories. Other chemicals, such as zymosan, FMLP, PMA, SOD, DPI, ABAH, catalase, tetrabutylammoniumhydrogen sulfate (TBA), isatin sulfonic acid, vinylbenzoic acid, and 4-carboxybenzaldehyde, were purchased from Sigma–Aldrich.
Human CGD Patient.
The human CGD patient was a 25-year-old male with gp91-phox deficiency. Mutation analysis revealed a G-to-A point mutation at nucleotide 252 in exon 3, which produces an aberrant splicing site (25).
Preparation of Neutrophils.
Human neutrophils were isolated from peripheral blood of healthy adult volunteers and the CGD patient by sedimentation through 2-step Percoll gradients, as described (26). Healthy volunteers and the patient provided written informed consent for participation in an institutional review board-approved protocol at Kyoto University Hospital.
Ozone Production in the Cell-Free System.
A solution of indigo carmine (30 μM) and 6FP (40 μM) was irradiated for 4 min at 5 mW/cm2 by using a UVA radiation apparatus (XX-15BLB 625 nm; UVP) in the presence or absence of human immunoglobulins [IgG and F(ab)′2] (5 mg/mL), BSA (5 mg/mL), FMLP (100 μM), zVAD-fmk (100 μM), or 19 water-soluble amino acids (1 mM) except for tyrosine. In this reaction, indigo carmine was converted to isatin sulfonic acids by ozone. Loss of indigo carmine was monitored by measuring its absorbance at 610 nm with a spectrometer (DU800; Beckman Coulter). For the dose–response reaction, increasing concentrations of Trp, Met, Cys, or His (1 μM to 2 mM) were added to the reaction in the presence of 6FP. Sodium azide (1 mM), edaravone (40 μM), and catalase (2,000 units/mL) were added to the reaction in the presence of 6FP to examine the effects on ozone production by IgG (5 mg/mL), Trp, Met, Cys, and His (1 mM). As a control, a sample without amino acids was analyzed. For HPLC analysis, indigo carmine (100 μM) or vinylbenzoic acid (30 μM) was mixed with 6FP (40 μM) and Met (1 mM) with or without UVA irradiation for 4 min. The samples were subjected to HPLC analysis.
HPLC Analysis for the Detection of Isatin Sulfonic Acid and 4-Carboxybenzaldehyde.
The conversion of indigo carmine to isatin sulfonic acid and the oxidation of vinylbenzoic acid to 4-carboxybenzaldehyde were considered as evidence of ozone formation (6). Samples were analyzed on a reverse-phase C18 HPLC column eluting with 70% 50 mM phosphate buffer (pH 7.2) containing 10 mM TBA and 30% acetonitrile with an L6000 Hitachi HPLC system (indigo carmine, RT = 12.0 min; isatin sulfonic acid, RT = 5.1 min; vinylbenzoic acid, RT = 15.3 min; 4-carboxybenzaldehyde, RT = 6.4 min) (3, 5). Peak areas were converted to concentrations by comparison to standard curves.
Assay for Measuring 18O Isotope Incorporation into Isatin Sulfonic Acid During Indigo Carmine Oxidation by Amino Acid-Catalyzed Water Oxidation.
An aliquot of indigo carmine (150 μM) in phosphate buffer (50 mM, pH 7.4) containing H218O (> 97% H2O) was added to a solution of 6FP (40 μM) in the presence or absence of Met (600 μM) in phosphate buffer (50 mM, pH 7.4) containing H218O (>97% H2O). The solution was irradiated for 4 min at 5 mW/cm2 by using an UVA radiation apparatus. Production of isatin sulfonic acid was determined by LC to confirm that reaction had been successful before mass spectral analysis. LC conditions were a reverse-phase C18 HPLC column and acetonitrile/water (10 mM ammonium acetate) (20:80) mobile phase at 1 mL/min (isatin sulfonic acid, RT = 2.1 min). MS was measured by using negative ion electrospray MS on a Waters Quattro micro API mass spectrometer. The raw data were extracted into Waters MassLynx version 4.0 format for presentation.
Bactericidal Assay in the Cell-Free System.
E. coli NIHJ-JC2 (5 × 106/mL) were incubated with or without 6FP-tBu-DMF (40 μM) and amino acids (Trp, Met, Arg, or Phe) (1 mM) under UVA irradiation (5 mW/cm2) for 2 h. Samples were removed at 60 and 120 min and suspended in water. An aliquot of the suspension was plated on a pour plate made with heart infusion agar. After a 24-h incubation at 37 °C, the colonies formed were counted.
H2O2 Production in the Cell-Free System and Human Neutrophils.
H2O2 production was measured by using a H2O2 probe, N-acetyl-3,7-dihydroxyphenoxazine (Amplex Red), including horseradish peroxidase (27). A solution of 6FP-tBu-DMF (40 μM) and Trp (1 mM), or PMA (50 ng/ml)-stimulated neutrophils (5 × 106 cells), were incubated with 50 μM Amplex Red for 2 h at 37° C. Fluorescence was measured by a fluorometric microplate reader (Fluoroskan Ascent; Labsystems) with excitation and emission wavelengths of 544 and 590 nm, respectively. The amount of H2O2 production was calculated according to the standard curve of H2O2. To confirm the specificity of this assay, catalase (2,000 units/mL) was added to the reaction before incubation with Amplex Red.
Bactericidal Effect of H2O2.
E. coli (5 × 106/mL) was incubated with increasing concentrations of H2O2 (0–103 mM) for 2 h. Samples were removed at 2 h, and the bactericidal assay was performed as described above.
Superoxide Release from Neutrophils.
Superoxide production was assessed by the SOD-inhibitable reduction of ferricytochrome c as described (28).
Flow Cytometric DHR Assay.
Neutrophils (5 × 105 cells) were loaded with 2 μM DHR for 5 min at 37 °C. After that, the cells were stimulated with 50 ng/ml PMA for 15 min at 37 °C and analyzed by flow cytometry. As a negative control, the pretreatment of neutrophils from a healthy control with 10 μM DPI, an inhibitor of NADPH oxidase, was performed before DHR loading.
Chemiluminescence Assay.
The productions of superoxide and singlet oxygen of neutrophils stimulated with PMA were examined by using chemiluminescence with an O2•−-specific probe, CLA, and an 1O2-specific probe, MVP, respectively. After mixing the neutrophils (2 × 106 cells) with 2.5 μM CLA or 40 μM MVP, the mixture was mounted on a luminescence reader (Aloka BLR-301), and the luminescence was monitored every 30 s for 30 min. As a negative control, the pretreatment of neutrophils from a healthy control with 10 μM DPI or 100 μM ABAH, an inhibitor of MPO, was performed.
Ozone Production of CGD Neutrophils.
Indigo carmine (30 μM) was incubated with unstimulated or PMA (50 ng/mL)-stimulated CGD neutrophils (1 × 106/mL) in the presence or absence of 1 mM Trp for 2 h at 37 °C. Edaravone (40 μM) was added to the reaction to examine the effect on ozone production. The loss of indigo carmine was monitored as described above. Vinylbenzoic acid (100 μM) was incubated with unstimulated or PMA (50 ng/mL)-stimulated CGD neutrophils (1 × 106/mL) in the presence or absence of 1 mM Trp for 2 h at 37 °C. The samples were subjected to HPLC analysis as described above.
Bactericidal Assay of Human Neutrophils.
Bactericidal activity of human neutrophils was determined by a standard technique (29). Briefly, the reaction mixture contained 2.5 × 106 neutrophils, 2.5 × 106 (PMN:E. coli = 1:1), or 1.25 × 107 (1:5), or 2.5 × 107 (1:10) E. coli cells, 10% human AB serum, 0.1% gelatin, and HBSS. The mixture was incubated with or without 1 mM Trp at 37 °C. Samples were removed at 60 and 120 min, and the bactericidal assay was performed as described above.
Statistical Analysis.
Data are expressed as the mean ± SD. P <0.05 by the paired Student's t test was considered significant.
Acknowledgments.
We thank Dr. Hiroyuki Nunoi for genetic analysis of the CGD patient and Dr. Harry L Malech for helpful discussions. This work was supported by the Mitsubishi Pharma Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Williams R. Killing controversy. J Exp Med. 2006;203:2404. doi: 10.1084/jem.20311fta. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wentworth P, Jr, et al. Evidence for antibody-catalyzed ozone formation in bacterial killing and inflammation. Science. 2002;298:2195–2199. doi: 10.1126/science.1077642. [DOI] [PubMed] [Google Scholar]
- 3.Babior BM, Takeuchi C, Ruedi J, Gutierrez A, Wentworth P., Jr Investigating antibody-catalyzed ozone generation by human neutrophils. Proc Natl Acad Sci USA. 2003;100:3031–3034. doi: 10.1073/pnas.0530251100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nieva J, Wentworth P., Jr The antibody-catalyzed water oxidation pathway: A new chemical arm to immune defense? Trends Biochem Sci. 2004;29:274–278. doi: 10.1016/j.tibs.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 5.Kettle AJ, Clark BM, Winterbourn CC. Superoxide converts indigo carmine to isatin sulfonic acid: Implications for the hypothesis that neutrophils produce ozone. J Biol Chem. 2004;279:18521–18525. doi: 10.1074/jbc.M400334200. [DOI] [PubMed] [Google Scholar]
- 6.Kettle AJ, Winterbourn CC. Do neutrophils produce ozone? An appraisal of current evidence. Biofactors. 2005;24:41–45. doi: 10.1002/biof.5520240105. [DOI] [PubMed] [Google Scholar]
- 7.Malech HL. Phagocyte oxidative mechanisms. Curr Opin Hematol. 1993;1:123–132. [Google Scholar]
- 8.Weening RS, Adriaansz LH, Weemaes CM, Lutter R, Roos D. Clinical differences in chronic granulomatous disease in patients with cytochrome b-negative or cytochrome b-positive neutrophils. J Pediatr. 1985;107:102–104. doi: 10.1016/s0022-3476(85)80626-0. [DOI] [PubMed] [Google Scholar]
- 9.Yamada H, et al. Photodynamic effects of a novel pterin derivative on a pancreatic cancer cell line. Biochem Biophys Res Commun. 2005;333:763–767. doi: 10.1016/j.bbrc.2005.05.185. [DOI] [PubMed] [Google Scholar]
- 10.Sommani P, et al. Effects of edaravone on singlet oxygen released from activated human neutrophils. J Pharmacol Sci. 2007;103:117–120. doi: 10.1254/jphs.sc0060170. [DOI] [PubMed] [Google Scholar]
- 11.Vowells SJ, et al. Genotype-dependent variability in flow cytometric evaluation of reduced nicotinamide adenine dinucleotide phosphate oxidase function in patients with chronic granulomatous disease. J Pediatr. 1996;128:104–107. doi: 10.1016/s0022-3476(96)70437-7. [DOI] [PubMed] [Google Scholar]
- 12.Nakano M, Sugioka K, Ushijima Y, Goto T. Chemiluminescence probe with cypridina luciferin analog, 2-methyl-6-phenyl-3,7-dihydroimidazo[1,2-a]pyrazin-3-one, for estimating the ability of human granulocytes to generate O2−. Anal Biochem. 1986;159:363–369. doi: 10.1016/0003-2697(86)90354-4. [DOI] [PubMed] [Google Scholar]
- 13.Posner GH, et al. A chemiluminescent probe specific for singlet oxygen. Biochem Biophys Res Commun. 1984;123:869–873. doi: 10.1016/0006-291x(84)90311-5. [DOI] [PubMed] [Google Scholar]
- 14.Teixeira MM, Cunha FQ, Noronha-Dutra A, Hothersall J. Production of singlet oxygen by eosinophils activated in vitro by C5a and leukotriene B4. FEBS Lett. 1999;453:265–268. doi: 10.1016/s0014-5793(99)00728-0. [DOI] [PubMed] [Google Scholar]
- 15.Yamashita K, et al. 6-Formylpterin intracellularly generates hydrogen peroxide and restores the impaired bactericidal activity of human neutrophils. Biochem Biophys Res Commun. 2001;289:85–90. doi: 10.1006/bbrc.2001.5956. [DOI] [PubMed] [Google Scholar]
- 16.Klebanoff SJ. Myeloperoxidase: Friend and foe. J Leukocyte Biol. 2005;77:598–625. doi: 10.1189/jlb.1204697. [DOI] [PubMed] [Google Scholar]
- 17.Parry MF, et al. Myeloperoxidase deficiency: Prevalence and clinical significance. Ann Intern Med. 1981;95:293–301. doi: 10.7326/0003-4819-95-3-293. [DOI] [PubMed] [Google Scholar]
- 18.Suzuki K, Muso E, Nauseef WM. Contribution of peroxidases in host-defense, diseases, and cellular functions. Jpn J Infect Dis. 2004;57:S1–S2. [PubMed] [Google Scholar]
- 19.Lehrer RI, Cline MJ. Leukocyte myeloperoxidase deficiency and disseminated candidiasis: The role of myeloperoxidase in resistance to Candida infection. J Clin Invest. 1969;48:1478–1488. doi: 10.1172/JCI106114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nguyen C, Katner HP. Myeloperoxidase deficiency manifesting as pustular candida dermatitis. Clin Infect Dis. 1997;24:258–260. doi: 10.1093/clinids/24.2.258. [DOI] [PubMed] [Google Scholar]
- 21.Aratani Y, et al. Severe impairment in early host defense against Candida albicans in mice deficient in myeloperoxidase. Infect Immun. 1999;67:1828–1836. doi: 10.1128/iai.67.4.1828-1836.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aratani Y, et al. Differential host susceptibility to pulmonary infections with bacteria and fungi in mice deficient in myeloperoxidase. J Infect Dis. 2000;182:1276–1279. doi: 10.1086/315843. [DOI] [PubMed] [Google Scholar]
- 23.Dolmans DE, Fukumura D, Jain RK. Photodynamic therapy for cancer. Nat Rev Cancer. 2003;3:380–387. doi: 10.1038/nrc1071. [DOI] [PubMed] [Google Scholar]
- 24.Maisch T. Antimicrobial photodynamic therapy: Useful in the future? Lasers Med Sci. 2007;22:83–91. doi: 10.1007/s10103-006-0409-7. [DOI] [PubMed] [Google Scholar]
- 25.Roos D, et al. Mutations in the X-linked and autosomal recessive forms of chronic granulomatous disease. Blood. 1996;87:1663–1681. [PubMed] [Google Scholar]
- 26.Moriguchi T, et al. Studies of the functions of polymorphonuclear leukocytes obtained from human bone marrow. Acta Hematol Jpn. 1990;53:668–677. [Google Scholar]
- 27.Zhou M, Panchuk-Voloshina N. A 1-step fluorometric method for the continuous measurement of monoamine oxidase activity. Anal Biochem. 1997;253:169–174. doi: 10.1006/abio.1997.2392. [DOI] [PubMed] [Google Scholar]
- 28.Asagoe K, et al. Down-regulation of CXCR2 expression on human polymorphonuclear leukocytes by TNF-α. J Immunol. 1998;160:4518–4525. [PubMed] [Google Scholar]
- 29.Johnston RB, Jr, et al. The role of superoxide anion generation in phagocytic bactericidal activity. Studies with normal and chronic granulomatous disease leukocytes. J Clin Invest. 1975;55:1357–1372. doi: 10.1172/JCI108055. [DOI] [PMC free article] [PubMed] [Google Scholar]